Abstract
The phytohormones ethylene and abscisic acid (ABA) play essential roles in the abiotic stress adaptation of plants, with both cross-talk of ethylene signalling and ABA biosynthesis and signalling reported. Any reciprocal effects on each other's biosynthesis, however, remain elusive. ACC synthase (ACS) acts as the key enzyme in ethylene biosynthesis. A pilot study on changes in ACS promoter activities in response to abiotic stresses revealed the unique involvement in abiotic stress responses of the only type 3 ACC synthase, ACS7, among all nine ACSs of Arabidopsis. Hence an acs7 mutant was characterized and its abiotic stress responses were analysed. The acs7 mutant germinated slightly faster than the wild type and subsequently maintained a higher growth rate at the vegetative growth stage. Ethylene emission of acs7 was merely one-third of that of the wild type. acs7 exhibited enhanced tolerance to salt, osmotic, and heat stresses. Furthermore, acs7 seeds were hypersensitive to both ABA and glucose during germination. Transcript analyses revealed that acs7 had elevated transcript levels of the stress-responsive genes involved in the ABA-dependent pathway under salt stress. The ABA level was also higher in acs7 following salt treatment. Our data suggest that ACS7 acts as a negative regulator of ABA sensitivity and accumulation under stress and appears as a node in the cross-talk between ethylene and ABA.
Keywords: Abiotic stresses, abscisic acid, AtACS7, cross-talk, ethylene
Introduction
Both ethylene and abscisic acid (ABA) are known to regulate not only developmental processes but also adaptive stress responses of plants (Morgan and Drew, 1997; Ma et al., 2006). For instance, ABA and ethylene have long been implicated in salt stress responses (Anderson et al., 2004; Wang et al., 2007; Cheng et al., 2009). Both the ABA-dependent and -independent pathways are involved in the response to salt stress and the accompanying osmotic challenge. Three types of cis-regulatory elements have been identified in the promoters of stress-responsive genes, including Dehydration Responsive Elements (DRE/CRT), ABA Responsive Elements (ABRE), and MYB/MYC Recognition Sequences (MYCRS/MYBRS) (Zhu, 2002). The ABA-dependent pathway regulates the expressions of stress-responsive genes, such as RD29A, RD29B, RAB18, and RD22 (Shinozaki et al., 2003), through CBF4 (a member of the DREB subfamily), MYC/MYB, and bZIP-type transcription factors, which bind to the DRE/CRT, MYCRS/MYBRS, and ABRE promoter elements, respectively (Zhu, 2002; Chinnusamy et al., 2004). Ethylene signalling has also been shown to play a role in salt tolerance (Achard et al., 2006; Cao et al., 2007). Upon abiotic stresses, ethylene binds to the endoplasmic reticulum (ER)-localized receptors ETR1, EIN4, and their homologues (Bleecker, 1999), resulting in the deactivation of CTR1 (the negative regulator of ethylene signalling) and activation of the positive regulator EIN2, eventually leading to a transcriptional cascade involving the EIN3/EIL and ERF transcription factors (Chen et al., 2005; Lin et al., 2009). The mutations of ETR1, EIN4, and EIN2 have all been shown to confer salt sensitivity (Cao et al., 2007), whereas the mutation of CTR1 confers salt tolerance (Achard et al., 2006).
A number of researches have revealed the intertwining nature of ethylene and ABA signalling pathways (Beaudoin et al., 2000; Benschop et al., 2007; Cutler et al., 2010). For instance, the expression of 9-CIS-EP-OXYCAROTENOID DIOXYGENASE 3 (NCED3), which encodes the key enzyme in ABA biosynthesis, is up-regulated in the ein2-1 mutant, and CYP707A2, a cytochrome P450 gene which encodes the key component of ABA catabolism, is down-regulated in etr1-1 (Cheng et al., 2009), suggesting that when ethylene signalling is impaired, ABA biosynthesis may be enhanced. Disruption of EIN2 alters the expression pattern of stress marker gene RD29B in response to salt stress (Wang et al., 2007).
Ethylene and ABA have also been implicated in heat stress tolerance (Larkindale et al., 2005; Clarke et al., 2009). Facing heat stress, plants produce heat shock proteins (HSPs) and modulate hormone signalling pathways (Foyer et al., 1997; Larkindale et al., 2005). HSPs are molecular chaperones that can protect cellular proteins against irreversible heat-induced denaturation and help in the refolding of heat-damaged proteins (Hong and Vierling, 2000; Hong et al., 2003). For instance, Hsp101, a member of the Clp/Hsp100 family, functions in re-solubilizing the protein aggregates formed under heat stress; while the Hsp70/DnaJ refolding complex helps refold the proteins to their native states (Gurley, 2000; Lee and Vierling, 2000). The expression of HSPs is known to be induced by heat shock transcription factors (HSFs). For instance, HsfA2 has been shown to sustain the transcript level of HSP genes and extend the duration of thermotolerance in Arabidopsis (Charng et al., 2007). Meanwhile, many hormones, including ethylene, ABA, and SA are involved in thermotolerance (Larkindale et al., 2005).
Apart from ethylene signalling, recent studies have started to reveal the importance of the ethylene biosynthesis pathway in modulating not only developmental growth but also stress adaptation (Yang and Hoffman, 1984; Tsuchisaka et al., 2009). The key enzyme in the ethylene biosynthesis pathway is ACC synthase (ACS), which converts S-adenosylmethionine (AdoMet) to 1-aminocyclopropane-1-carboxylic acid (ACC) (Yang and Hoffman, 1984). In Arabidopsis, there are nine authentic ACSs, including ACS1–2, ACS4–9, and ACS11. Each member has a distinct spatial and temporal expression pattern in the different stages of plant growth and development, and under various stresses (Wang et al., 2005). The catalytic core of ACS proteins is highly conserved, while the C-terminal regulatory domain varies. Based on the sequence of the C-terminal region, the ACS proteins are divided into three groups. Type 1 ACS proteins have the longest C-terminus with a single calcium-dependent protein kinase (CDPK) phosphorylation site and three mitogen-activated protein kinase (MAPK) phosphorylation sites, and type 2 ACS proteins have an intermediate length of the C-terminus with a single CDPK phosphorylation site. By contrast, type 3 ACS proteins have a very short C-terminus and no phosphorylation site (Chae and Kieber, 2005; Yoshida et al., 2005), with their specific function or regulatory mechanism almost completely unknown.
Arabidopsis has a single type 3 ACS, ACS7, which has a broad expression pattern during plant growth and development. In 5-d-old etiolated seedlings, ACS7 is expressed in the cotyledons, the elongation zone of the hypocotyls, and the vascular tissue in the root; in 5-d-old light-grown seedlings, ACS7 is expressed in the cotyledons, primary leaves, the embryonic root, and in the roots except the root tip; in the mature plants, ACS7 is expressed in the roots, younger leaves, inflorescence stem, and siliques (Tsuchisaka and Theologis, 2004). A recent survey on acs mutants has implicated the involvement of ACS7 in the determination of flowering time (Tsuchisaka et al., 2009). Moreover, the expression of ACS7 is regulated by several phytohormones and environmental factors, such as ethylene, ABA, GA3, light, and salt (Wang et al., 2005; Achard et al., 2006).
Although many efforts have gone into the investigation of the interaction between ethylene and ABA signalling in abiotic stress adaptation, the possible involvement of any ethylene biosynthesis gene in such cross-talk has yet to be explored. In a pilot study, the unique responses of the ACS7 promoter to various abiotic stresses were observed. Then a loss-of-function mutant of ACS7 was characterized, and its responses to abiotic stresses including salt, osmotic, and heat stress were systematically analysed. Surprisingly, the acs7 mutant showed enhanced tolerance to all the stresses tested. The acs7 mutant was then discovered to be hypersensitive to exogenous ABA. Consistently, the acs7 mutant showed hypersensitivity to high glucose, but not sucrose. Transcript analyses revealed that the acs7 mutant had elevated transcript levels of stress-responsive genes involved in the ABA-dependent pathway under salt stress. The endogenous ABA level was also higher in the acs7 mutant following salt stress. These results suggested that ACS7 acts as a negative regulator of ABA sensitivity and accumulation under abiotic stresses and might function as a molecular link between ethylene biosynthesis and the ABA-mediated abiotic stress signal pathway.
Materials and methods
Plant materials and growth conditions
Wild-type Arabidopsis thaliana (ecotype Wassilewskija-4) was obtained from the Arabidopsis Biological Resource Center (ABRC, The Ohio State University, Columbus, OH, USA). The T-DNA insertion line of AtACS7 (background Wassilewskija-4) was obtained from the Institut National de la Recherche Agronomique (INRA, Institut Jean Pierre Bourgin, Station Génétique et amélioration des plantes, UR254, F-78026 Versailles, France) (Samson et al., 2002).
Seeds were surface-sterilized in 10% (v/v) sodium hypochlorite for 2 min, washed 10 times with sterilized water, plated on to half-strength Murashige and Skoog (1/2 MS) medium [0.8% (w/v) agar, pH 5.7, 1% (w/v) sucrose], stratified at 4 °C for 2 d in the dark, and grown in a plant growth chamber [22/19 °C, 16/8 h light/dark, with a photosynthetic photon flux density (PPFD) of 90 μE m−2 s−1].
Verification of the acs7 mutant
The acs7 mutant lines were verified by PCR as described (http://signal.salk.edu/tdnaprimers.2.html). The primers used were T-DNA-specific primer (FLAG LB4) and ACS7 gene-specific primers (acs7LP and acs7RP).
The expression of ACS7 was analysed by RT-PCR using TIP41-LIKE (At4g34270) as a control. Primers used to amplify the transcription of TIP41-LIKE and ACS7 were rtTIP-F and rtTIP-R, and rtACS7-F and rtACS7-R. The sequences of primers are listed in Supplementary Table S1 at JXB online.
Phenotypic analyses
Four-day-old, vertically-grown wild-type and acs7 seedlings were transferred to new plates and allowed to grow either vertically or horizontally for phenotypic analyses. The plates were then photographed daily, and additional root growth was measured with Image J (National Institutes of Health; http://rsb.info.nih.gov/ij/download.html).
To study the response of the wild-type and acs7 to ethylene, 4-d-old seedlings were transferred to medium supplemented with a series of concentrations of ACC (ethylene precursor) for further observation.
The germination assay was performed on medium supplemented with 2 μM ABA, 150 mM NaCl or 300 mM mannitol. The percentage of seed germination (defined by the emergence of the radicle) was scored over time.
To evaluate salt stress tolerance, 5-d-old seedlings were transferred to medium supplemented with 150 mM NaCl, and the survival rate (defined by the death of the shoot apical meristem as observed with a stereoscope) was scored daily.
To evaluate heat stress tolerance, plates of 4-d-old seedlings were transferred to a dark incubator set at 43 °C for 3 h, then back to normal growth conditions, and afterwards the percentage of seedlings with cotyledon chlorosis was scored daily.
To evaluate their glucose- and sucrose-sensitivity, seeds were sown on medium supplemented with 6% glucose or sucrose, and afterwards the plates were photographed daily. After being germinated on 6% glucose for 10 d, the number of seedlings with true leaves was scored. Error bars ±SD.
50 μM Nordihydroguaiaretic acid (NDGA) (Sigma) was used as the inhibitor of ABA biosynthesis (Han et al., 2004; Liu et al., 2009).
All phenotypic analyses were performed at least in triplicate. The level of significance was evaluated by Student's t test.
Gene expression analyses
Real-time RT-PCR was used to compare the expression of genes of interest in the wild type and acs7 following various stress treatments. For the salt-stress treatment, 9-d-old seedlings were transferred to medium supplemented with 175 mM NaCl and harvested after 3, 6, 12, and 24 h of treatment, respectively. For the heat-stress treatment, 9-day-old seedlings were transferred to a dark incubator set at 43 °C and harvested after 0.5, 1, and 2 h of treatment, respectively.
RNA extraction and cDNA synthesis were as described by Liu et al. (2010). Total RNA was extracted, and residual genomic DNA was digested with RNase-free DNase I. The absence of genomic DNA was confirmed. First strand cDNA was synthesized from 2.0 μg of total RNA using AMV reverse transcriptase (Promega, Madison, WI, USA). cDNA samples diluted 10-fold were used as templates.
Real-time RT-PCR analysis was performed using SYBR Green Perfect mix (TaKaRa, Dalian, China) on an iQ5 (Bio-Rad, California, USA) (Liu et al., 2010). All reactions were performed under the following conditions: 95 °C for 2 min; 40 cycles of 95 °C for 10 s, and 56 °C for 30 s. All reactions were done at least in triplicate. TIP41-LIKE was used as an internal control. All primers used are listed in Supplementary Table S1 at JXB online.
Histochemical GUS staining
Histochemical GUS staining of homozygous transgenic lines harbouring pACS:GUS fusion genes under various stresses was performed as described previously (Wang et al., 2005; Liu et al., 2010). Images were recorded with a scanner (EPSON 1260). Three independent replicates with at least eight seeds and seedlings each were used to give the typical results.
Measurements of ethylene emission
Ethylene emission of the wild type and acs7 was determined in 3-d-old etiolated seedlings by gas chromatography (Agilent 6890N) as described by Li et al. (2009).
ABA determination
Nine-day-old seedlings of the wild type and acs7 were treated with 175 mM NaCl for 24 h. The seedlings, with or without salt treatment, were ground into powder in liquid nitrogen. Quantification of ABA was carried out with LC-MS at the National Center for Plant Gene Research (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China) as previously described by Fu et al. (2011).
Measurements of endogenous proline and soluble sugar content
The leaves of 9- and 19-d-old seedlings were harvested, weighed, and ground into powder in liquid nitrogen. Proline and sugar contents were determined as described by Li et al. (2004).
Results
Identification of an acs7 knockout mutant
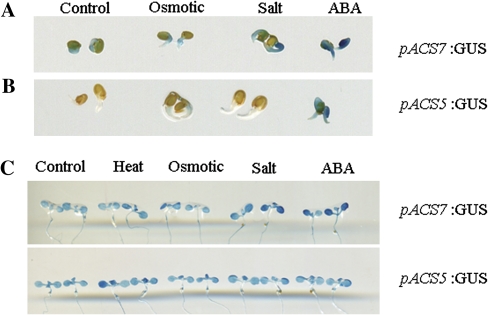
Previous analyses on the promoter activities of AtACS genes had revealed that, at any given growth stage, there is at least one ACS expressed (Tsuchisaka and Theologis, 2004), and that the expression of several ACSs can be induced by various hormones and stress conditions (Wang et al., 2005). As a pilot study, the promoter activities of the three types of ACSs in response to various abiotic stress conditions were surveyed (data not shown), in which the uniqueness of ACS7, the only type 3 ACS in Arabidopsis, was revealed. ACS7 was expressed in germinating seeds, and its promoter activity was elevated further following both salt and ABA treatment (Fig. 1A). By contrast, GUS staining was barely detected in other ACS promoter-GUS lines, such as pACS5:GUS during germination, except following the ABA treatment (Fig. 1B). Similarly, enhanced promoter activities were observed in 9-d-old pACS7:GUS seedlings following heat, osmotic, salt, and ABA treatments, while only a slightly enhanced activity was observed in pACS5:GUS lines following heat stress (Fig. 1C).
Fig. 1.
The unique expression pattern of ACS7 under various stress treatments. The seeds and 9-d-old seedlings of pACS7:GUS and pACS5:GUS lines were exposed to 43 °C, 300 mM mannitol, 150 mM NaCl, and 20 μM ABA for 3 h, respectively, before histochemical GUS staining. (A) ACS7 was expressed in germinating seeds, and the expression was further induced by both salt and ABA. (B) By contrast, promoter activity of ACS5 was barely detected during germination, except following the ABA treatment. (C) The expression of ACS7 was enhanced following heat, osmotic, salt, and ABA treatments in 9-d-old seedlings, while the expression of ACS5 was only increased following heat treatment. There were three independent replicates with at least eight seeds and seedlings each to give the typical results. (This figure is available in colour at JXB online.)
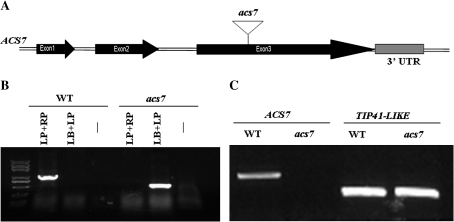
To explore the possible involvement of ACS7 in abiotic stress adaptation, a FLAG T-DNA insertion line (ecotype Wassilewskija-4) was obtained with a T-DNA insertion in the third exon of the ACS7 gene (Fig. 2A). The homozygous mutant was confirmed by PCR (Fig. 2B) and RT-PCR (Fig. 2C). This line turned out to be the same acs7 line, acs7-1, used before (Tsuchisaka et al., 2009).
Fig. 2.
Molecular characterization of the acs7 mutant. (A) The T-DNA was inserted into the third exon of the ACS7 gene. The black boxes represent exons and the lines represent introns. (B) Homozygous mutants were identified by PCR using primers annealing to genomic DNA (LP and RP) and left border of the T-DNA (LB). (C) The knockout of ACS7 was confirmed in acs7 by RT-PCR using TIP41-LIKE as a control.
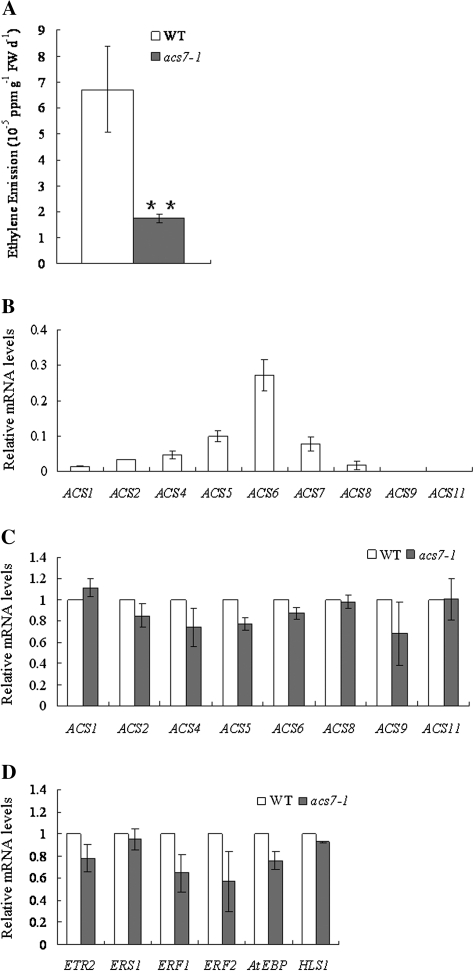
In 3-d-old etiolated seedlings, ethylene emission of acs7 was reduced to approximately one-third compared with the wild type (Fig. 3A), suggesting that AtACS7 may have a major contribution to the production of ethylene in growing etiolated seedlings.
Fig. 3.
The transcript levels of several ACSs and ethylene-responsive genes were repressed in acs7, with lower ethylene emission. (A) acs7 had lower ethylene emission. Data were means (±SD) of three experiments and the level of significance was evaluated by Student's t test. (B) Transcript levels of ACSs in the wild type were normalized to the internal control, TIP41-LIKE. (C) Transcript levels of ACSs in acs7. (D) Transcript levels of ethylene-responsive genes in acs7. In (C) and (D), the transcript level of each gene was set to 1 in the wild type. There were three biological replicates with at least three technical repeats for each gene. Error bars ±SD.
Previously, it has been shown that the expression of several ACS genes is regulated by ethylene in a feed-forward fashion (Barry et al., 2000; Giovannoni, 2001; Alexander and Grierson, 2002; Argueso et al., 2007). The transcript levels of all other ACS genes in acs7 were therefore analysed. Indeed, most ACSs, especially the ones that could form a hetero-dimer with ACS7, had reduced transcript levels in acs7 (Fig. 3B, C; see Supplementary Table S2 at JXB online), further supporting a role for ACS7 in ethylene production. Consistently, the transcript levels of ethylene-responsive genes, including ETR2 (ETHYLENE RESPONSE 2), ERF1 (ETHYLENE RESPONSE FACTOR 1), ERF2 (ETHYLENE RESPONSE FACTOR 2), AtEBP (ETHYLENE RESPONSE FACTOR 72), and HLS1 (HOOKLESS 1) (Wang et al., 2002; Christians and Larsen, 2007), were lower in acs7 compared with that of the wild type (Fig. 3D).
Phenotypes of the acs7 mutant under normal growth conditions
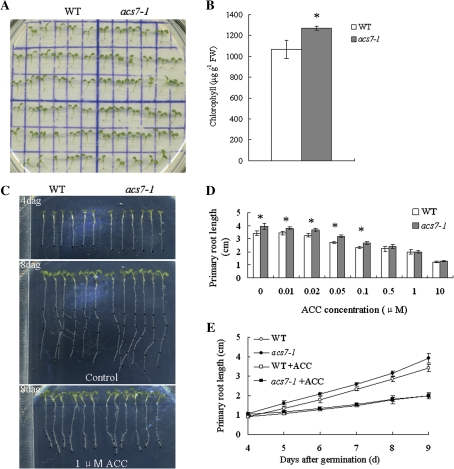
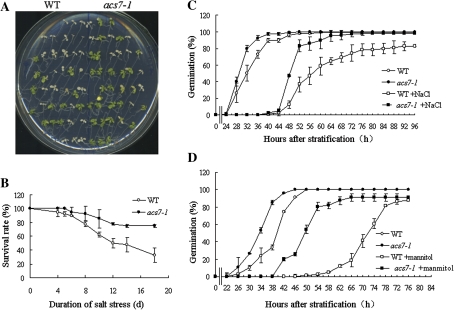
On plates, acs7 germinated slightly earlier than the wild type (see Supplementary Fig. S1 at JXB online), and had larger cotyledons and true leaves with higher chlorophyll level (Fig. 4A, B). The primary root of acs7 also grew faster than that of the wild-type (Fig. 4C). Since ethylene is known to regulate primary root elongation negatively (Rahman et al., 2001; Růzicka et al., 2007), both the wild-type and acs7 seedlings were subjected to a series of concentrations of ACC treatment, and their primary root elongation was recorded and compared with the untreated seedlings (Fig. 4C, D, E; see Supplementary Fig. S2 at JXB online). The difference in the primary root length between the wild type and acs7 was reduced by the application of ACC, suggesting that the accelerated primary root elongation was indeed due to the reduced endogenous ethylene level in the roots.
Fig. 4.
Phenotypes of acs7 seedlings. (A) Nine-day-old acs7 seedlings had larger cotyledons and true leaves. (B) Higher level of chlorophyll content was detected in acs7. (C) The acs7 seedlings had longer primary roots than the wild type, which could be restored by 1 μM ACC, the precursor of ethylene. (D) Four-day-old seedlings were treated with different concentrations of ACC for 5 d. The primary root length of the wild-type and acs7 seedlings was quantified. (E) Quantification of the primary root length of the wild-type and acs7 seedlings in (C). There were three independent replicates with six seedlings each. Error bars ±SD. The level of significance was evaluated by Student's t test. (This figure is available in colour at JXB online.)
Thermotolerance is enhanced in the acs7 mutant
Although ethylene has long been regarded as essential for abiotic stress responses (Morgan and Drew, 1997; Cao et al., 2007), few studies have focused on the impact of ethylene synthesis in stress adaptation. Hence, the responses of acs7 to abiotic stresses were evaluated in this study.
Previous studies have revealed the involvement of endogenous ethylene signalling in protecting plants from heat stress (Larkindale and Knight, 2002; Larkindale et al., 2005). Mutants in ethylene biosynthesis, however, have not been tested for their thermotolerance. To evaluate the response of acs7 to heat stress, 4-d-old acs7 and wild-type seedlings were exposed to 43 °C for 3 h in the dark (to avoid light-dependent oxidative damage). After being transferred back to normal growth conditions, the acs7 seedlings, having the lower percentage of cotyledon chlorosis (Fig 5A, B), were less damaged by heat, and were able to resume growth by producing new leaves (see Supplementary Fig. S3 at JXB online).
Fig. 5.
Enhanced thermotolerance was observed in acs7. (A) Four-day-old wild-type and acs7 seedlings were exposed to 43 °C for 3 h in the dark, and then transferred back to normal growth conditions. acs7 exhibited enhanced tolerance to heat stress. (B) The percentage of seedlings with cotyledon chlorosis was scored over time. The acs7 seedlings were less damaged by heat treatment. All analyses were performed at least in triplicate. Error bars ±SD. (This figure is available in colour at JXB online.)
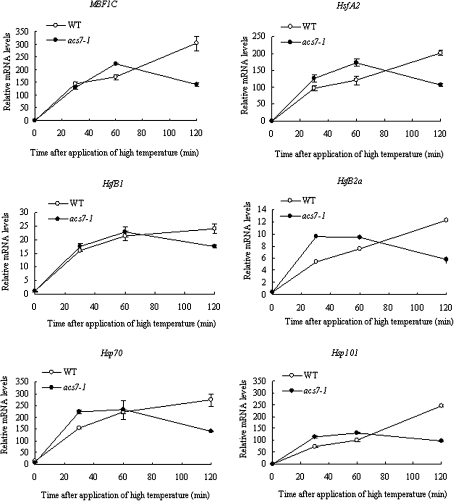
To analyse the molecular basis for the increased thermotolerance of the acs7 mutant, the expression of a number of heat stress-related genes was examined. These include heat shock transcription factors (HSFs) and heat shock proteins (HSPs), such as HsfA2, HsfB1, HsfB2a, Hsp70, and Hsp101 (Larkindale et al., 2005). Overall, these genes were induced more quickly in acs7 compared with the wild type (Fig. 6), indicating that the enhanced thermotolerance of acs7 at least partly resulted from the higher and earlier induction of HSFs and HSPs. In addition, the expression of multi-protein bridging factor 1c (MBF1c), a key regulator of thermotolerance upstream of ethylene signalling (Suzuki et al., 2008), was induced earlier in acs7 (Fig. 6), which might lead to earlier induction of the expressions of heat stress-responsive genes.
Fig. 6.
The expression of heat stress-related genes, including HsfA2, HsfB1, HsfB2a, Hsp70, Hsp101, and MBF1c, were generally induced earlier and to a higher level in acs7 following the heat treatment. There were three biological replicates with at least three technical repeats for each gene. Error bars ±SD.
The acs7 mutant exhibits enhanced salt tolerance and accumulates more ABA under salt stress
It has been shown that 100 μM ACC treatment could enhance salt tolerance in Arabidopsis (Achard et al., 2006; Cao et al., 2007), and that mutants defective in ethylene signalling, such as etr1-1, ein4-1, and ein2-1, showed higher sensitivity to salt (Cao et al., 2007).
The salt stress tolerance of acs7 and the wild type was then analysed in two ways. Firstly, young seedlings were treated with 150 mM NaCl for a prolonged period of time, and their survival rate was scored over time. Compared with the wild-type, acs7 was much more tolerant (Fig. 7A, B). Secondly, germination kinetics was recorded over time for both the wild-type and mutant seeds, with or without 150 mM NaCl, or 300 mM mannitol. Although seed germination was postponed by salt and osmotic stress treatment in both the wild-type and acs7, the mutant still maintained higher germination rates over time (Fig. 7C, D). Combined, these data suggested that the absence of ACS7 enhanced salt tolerance in Arabidopsis.
Fig. 7.
acs7 exhibited enhanced salt and osmotic stress tolerance. (A) Five-day-old wild-type and acs7 seedlings were transferred to new medium supplemented with or without 150 mM NaCl and allowed to grow for 18 d. acs7 showed enhanced tolerance to salt stress. (B) Quantification of the survival rate over time. The seedlings were defined to be dead with the death of the shoot apical meristem. (C) Germination kinetics of the wild type and acs7 with or without 150 mM NaCl. (D) Germination kinetics of the wild type and acs7 with or without 300 mM mannitol. All analyses were performed at least in triplicate. Error bars ±SD. (This figure is available in colour at JXB online.)
The molecular basis for the elevated salt tolerance was then explored. Since salt stress adaptation is known to be mediated by multiple pathways and at both the transcriptional and post-transcriptional levels, the expressions of a number of marker genes, which are known to be salt stress-responsive at the transcript level, were monitored in a time-course study.
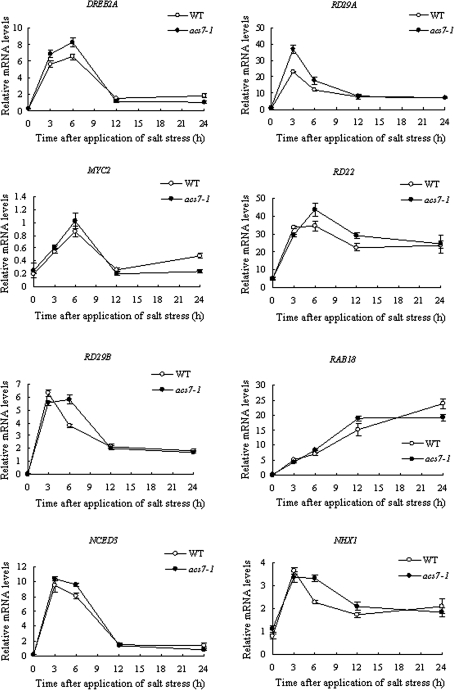
NHX1 encodes a tonoplast sodium/proton antiporter which is important for salt tolerance and ion homeostasis (Zhu, 2000; Yokoi et al., 2002). In unstressed seedlings, the transcript level of NHX1 was already higher in the mutant (Fig. 8). Expression of NHX1 in both the wild type and acs7 was quickly and significantly induced by salt, and acs7 maintained a high transcript level of NHX1 for a longer time. These results suggested that the salt tolerance observed in acs7 may be partly due to the enhanced osmotic tolerance mediated by NHX1.
Fig. 8.
The transcript levels of salt-responsive and ABA-related genes were induced earlier and to a higher level in acs7 by 175 mM NaCl. Three biological replicates with at least three technical repeats were done for each gene. Error bars ±SD.
The transcription factor DREB2A is known specifically to interact with the DRE/CRT cis-element to induce osmotic stress-responsive gene expression (Sakuma et al., 2006). When unstressed, no difference in DREB2A expression was observed between the wild type and acs7. Nevertheless, the induction of DREB2A expression by salt was clearly earlier in acs7 (Fig. 8), very likely leading to an earlier activation of salt stress-responsive genes downstream.
The expression of RD29A is both ABA-dependent and ABA-independent (the latter mediated by DREB2A). Transcript levels of RD29B, RAB18, MYC2, and RD22 are all known to be regulated mainly through ABA-dependent pathways (Zhu, 2002; Chinnusamy et al., 2004). In acs7, expression of these genes was overall more induced by salt stress compared with the wild type (Fig. 8). The expression level of NCED3, which encodes a rate-limiting enzyme in ABA biosynthesis, was also higher in acs7 following salt treatment (Fig. 8).
Moreover, in order to test whether ABA accumulation contributed to the salt tolerance of acs7, the endogenous ABA levels of acs7 and the wild type at the 24 h time point following 175 mM NaCl treatment were measured with LC-MS. The ABA level in acs7 was higher than the wild-type seedlings under salt stress (Table 1). Meanwhile, when nordihydroguaiaretic acid (NDGA), the ABA biosynthesis inhibitor (Han et al., 2004; Liu et al., 2009), was supplied, acs7 seedlings became even more sensitive to salt compared with the wild type (see Supplementary Fig. S4 at JXB online). Since it is widely accepted that ethylene has a positive role in salinity adaptation (Achard et al., 2006), such a phenotype could thus have resulted from a reduction of both endogenous ABA and endogenous ethylene in the mutant.
Table 1.
ABA content was higher in acs7 under salt stress
| Treatment | ABA content (pg mg−1 FW) | |
| WT | acs7 | |
| Control | 1.16±0.03 | 1.08±0.06 |
| 175 mM NaCl | 7.39±0.26 | 8.14±0.31* |
Nine-day-old seedlings of the wild type and acs7 were treated with 175 mM NaCl for 24 h. The seedlings with or without salt treatment were ground into powder in liquid nitrogen and sampled for ABA determination. Data were means (±SD) of three experiments. The difference in ABA levels between acs7 and the wild type under salt stress was significant as evaluated by Student's t test.
Combined, our results suggested that the loss of ACS7 confers salt stress tolerance mainly by promoting the ABA-dependent stress-responsive pathway and by endogenous ABA accumulation.
The acs7 mutant is hypersensitive to ABA
To analyse the possible changes in ABA level and response in acs7 further, a series of physiological measurements were carried out, including the ABA-mediated inhibition of germination, accumulation of proline and soluble sugar, and glucose sensitivity (Zhu, 2002; León and Sheen, 2003; Carrari et al., 2004; Vinocur and Altman, 2005; Tuteja, 2007).
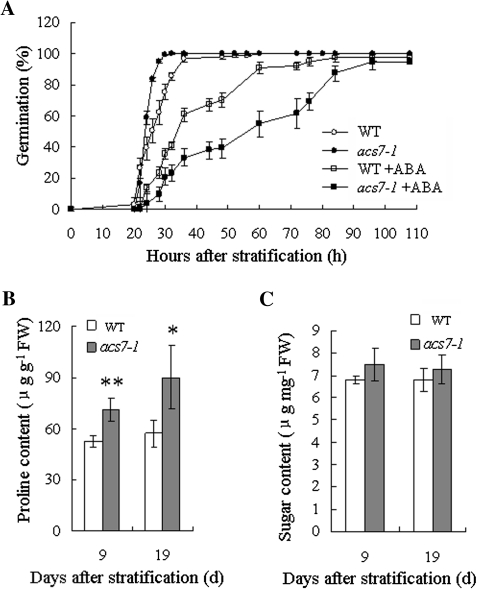
The germination sensitivity of acs7 seeds to exogenous ABA was evaluated first. Although no difference was observed in the final germination percentage between acs7 and the wild type, the germination kinetics was different; the acs7 seeds germinated faster than the wild type on control plates, nevertheless, 2 μM ABA clearly halted the germination of the mutant (Fig. 9A).
Fig. 9.
ABA related phenotypes in acs7. (A) acs7 was hypersensitive to exogenous ABA during germination. (B) Proline content was significantly higher in acs7. (C) Soluble sugar content was slightly higher in acs7. At least three independent repeats gave typical results. Error bars ±SD. The level of significance was evaluated by Student's t test.
The 9-d-old and 19-d-old seedlings of the wild type and acs7 under normal growth condition were collected, and measured for their proline and soluble sugar contents. Both metabolites were higher in acs7, especially proline (Fig. 9B, C). Consistently, the transcript level of P5CS1, which encodes the rate-limiting enzyme in the biosynthesis of proline, was increased in acs7 (see Supplementary Fig. S5 at JXB online).
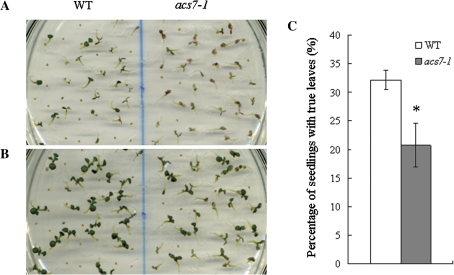
The germination assay on medium supplemented with high glucose gave consistent results. acs7 still germinated faster, yet immediately after radicle emergence, the seedling growth rate was strongly delayed by 6% glucose (Fig. 10A, B). On day 10, the percentage of the wild-type seedlings with true leaves was significantly higher than that of acs7 (Fig. 10C). Sucrose, however, does not have an impact on acs7 germination (see Supplementary Fig. S6 at JXB online).
Fig. 10.
acs7 was hypersensitive to high glucose. The seeds of the wild type and acs7 were sown on 1/2 MS medium supplemented with 6% glucose, and grown under normal growth conditions. The plates were photographed daily afterwards. (A) Accumulated anthocyanin was observed in 7-d-old acs7 seedlings. (B) High glucose inhibited the growth of 10-d-old acs7 seedlings. The experiment was performed in triplicate. (C) On 6% glucose, the growth rate of the wild type was higher than that of acs7. The number of seedlings with true leaves in (B) was scored. Error bars ±SD. The level of significance was evaluated by Student's t test. (This figure is available in colour at JXB online.)
The expression level of CYP707A2 was lower in the acs7 mutant, while genes involved in ABA biosynthesis (ABA1, ABA2, NCED3, ABA3, and AAO3) generally had slightly higher transcript levels (see Supplementary Fig. S5 at JXB online). Consistently, the expression levels of two ABA-responsive genes, RD29B and RAB18 (Saez et al., 2008), were also higher in acs7 (see Supplementary Fig. S5 at JXB online).
Discussion
ACC synthase acts as the rate-limiting enzyme in ethylene biosynthesis (Yang and Hoffman, 1984). In Arabidopsis, the expression of each AtACS gene is regulated by different developmental and environmental signals (Wang et al., 2002; Tsuchisaka and Theologis, 2004; Peng et al., 2005), indicating that AtACSs may have unique and overlapping function in the regulation of plant growth and stress response. In a recent effort to obtain a mutant with all nine ACSs knocked out, it was noticed that both the two octuple mutant lines were defective in seed setting, suggesting that the presence of ACS activity is required for plant survival (Tsuchisaka et al., 2009). Nevertheless, to our knowledge, the specific function of any single ACS in regulating abiotic stress adaptation has not been reported. Here, by analysing the acs7-1 mutant, the possible role of AtACS7 in plant growth and stress tolerance was explored, mainly through physiological measurements and transcript analysis.
The most significant phenotypic difference was that acs7 always appeared bigger than the wild type, having larger cotyledons and longer primary roots. Since ethylene has long been regarded as a negative regulator of vegetative growth, our observation was therefore explainable. Indeed, the root phenotype was successfully restored by an application of ACC to the mutant.
Although the ethylene level was lower in acs7, no difference was observed in the final germination percentage between acs7 and the wild type. Interestingly, the acs7 seeds germinated slightly faster than the wild type, and radicle emergence of acs7 was consistently about 4 h earlier than the wild type. Considering that both the dry and germinating seeds of acs7 were noticeably larger than the wild type (data not shown), it was inferred that the phenotype was very possibly due to the more efficient imbibitions of the mutant.
A previous study showed that 10-d-old Arabidopsis seedlings, pretreated with 100 μM ACC, had a higher survival rate after being exposed to 40 °C for 1 h (Larkindale and Knight, 2002). Furthermore, it was demonstrated that the growth of ein2-1 and etr1-1 was significantly compromised after a heat treatment at 45 °C for 1 h (Larkindale et al., 2005). These results suggest that ethylene signalling may have a positive role in heat stress tolerance. Therefore, it was interesting to find that the acs7 seedlings were more tolerant to heat. Since the induction of HSPs is regarded as one of the efficient (and classic) mechanisms for survival at high temperature, the expression of HsfA2, HsfB1, HsfB2a, Hsp70, and Hsp101 in acs7 during the heat treatment were then profiled. All these genes were induced earlier in acs7, consistent with the enhanced thermotolerance of acs7.
On the other hand, the involvement not only of ethylene but ABA as well has been implicated in heat stress tolerance. It was shown that ABA biosynthesis and, in particular, ABA signalling mutants were hypersensitive to heat stress, indicative of a positive role for ABA in thermotolerance (Larkindale et al., 2005). Hence the heat stress tolerance of acs7 might, at least partly, result from activated ABA synthesis or signalling pathways. The evidence needs to be explored.
Since previous studies have suggested a positive role of both exogenous ethylene and ethylene signalling in salt tolerance (Achard et al., 2006; Cao et al., 2007), it was surprising to see that the acs7 mutant were more tolerant to salt and osmotic stresses. As a validation for our experimental system, other ACS knock-out mutants were assayed in parallel (data not shown). Neither type 1 nor type 2 ACS mutants showed the elevated salt stress tolerance that was observed in acs7. Hence it is concluded that ACS7, rather than the other ACS genes, or ethylene signalling genes, has a unique (negative) function in mediating the salt stress response.
Given the importance of ABA in the abiotic stress adaptation, it was tested whether the ABA content or response was altered in acs7. It turned out that the ABA level in acs7 was higher than the wild type at the 24 h time point following salt treatment. The transcript analyses showed that, under salt treatment, stress-responsive genes involved in the ABA-dependent pathway were activated at the earliest time point tested (3 h after salt treatment) (Fig. 8), when changes in ABA levels might not have occurred. Meanwhile, the expression of NCED3, a key component of ABA biosynthesis, was not significantly altered compared with that of other stress-responsive genes. Finally, acs7 seeds were hypersensitive to exogenous ABA. Combined, our results suggested that not only the increased ABA accumulation, but also the enhanced ABA sensitivity, may have contributed to the salt tolerance of acs7.
Consistently, early development of the acs7 seedlings on glucose was strongly inhibited (Fig. 10). Genetic screens for glucose-insensitive mutants had shown them to be ABA biosynthesis mutants, such as aba2 and aba3, and the glucose-oversensitive mutants were shown to be ethylene signalling mutants, such as etr1, ein2, and ein3 (León and Sheen, 2003), therefore the germination hypersensitive phenotypes could be explained either way.
In conclusion, our data strongly suggest elevated endogenous ABA levels and enhanced ABA sensitivity in acs7 under salt stress, which may have directly resulted from the loss of ACS7 and reduced ethylene production. Therefore, what was observed might have been mostly the long-term effects of the loss of ACS7. The immediate consequences of losing ACS7 will be explored in future. Meanwhile, why and how the loss of ACS7, not the other ACSs, could lead to such changes in ABA synthesis, signalling, and turnover, demands further attention. The unique function of ACS7 may have arisen from its unique protein structure. Nevertheless, our study revealed the distinctive role of ACS7, the only type 3 ACS, in linking ethylene and ABA, two phytohormones essential for plant abiotic stress adaptation.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. acs7 germinated slightly yet consistently faster than the wild type.
Supplementary Fig. S2. The primary root length of wild-type and acs7 seedlings under a series of concentrations of ACC treatments.
Supplementary Fig. S3. acs7 resumed vegetative growth following heat stress.
Supplementary Fig. S4. NDGA, the inhibitor of ABA biosynthesis, suppressed the salt-tolerant phenotype of acs7.
Supplementary Fig. S5. The expression levels of ABA-related genes in acs7.
Supplementary Fig. S6. Response to sucrose of acs7 was normal.
Supplementary Table S1. Primers used for genotyping, RT-PCR, and real-time RT-PCR analyses.
Supplementary Table S2. The number of active homo- and hetero-isozymes was possibly decreased in acs7.
Acknowledgments
We would like to thank the two reviewers for their insightful criticism and suggestions. We gratefully acknowledge the Arabidopsis Biological Resource Center (ABRC) for providing the wild-type Arabidopsis thaliana (ecotype Wassilewskija-4) and the Institut National de la Recherche Agronomique (INRA) for providing the T-DNA insertion line of AtACS7. We wish to thank Dr. Hongwei Guo (Peking University, Beijing, China) for help with ethylene measurements, Jinfang Chu and Xiaohong Sun (National Center for Plant Gene Research, Beijing, China) for help with ABA measurements, and our laboratory members Ms Li Xiong and Mr Dong Xiao for discussions. This work was supported by the National Natural Science Foundation of China (NSFC) (Nos 90717105 and 90917003) to NNW, and partially supported by Specialized Research Fund for the Doctoral Program of Higher Education of China (SRFDP) (No 200800550017) to NNW and (No 20090031120027) to QG.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signalling pathways modulates defense gene expression and disease resistance in Arabidopsis. The Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso CT, Hansen M, Kieber JJ. Regulation of ethylene biosynthesis. Journal of Plant Growth Regulation. 2007;26:92–105. [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiology. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signalling cascades. The Plant Cell. 2000;12:1103–1115. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Millenaar FF, Smeets ME, van Zanten M, Voesenek LA, Peeters AJ. Abscisic acid antagonizes ethylene-induced hyponastic growth in Arabidopsis. Plant Physiology. 2007;143:1013–1023. doi: 10.1104/pp.106.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB. Ethylene perception and signalling: an evolutionary perspective. Trends in Plant Science. 1999;4:269–274. doi: 10.1016/s1360-1385(99)01427-2. [DOI] [PubMed] [Google Scholar]
- Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiology. 2007;143:707–719. doi: 10.1104/pp.106.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Fernie AR, Iusem ND. Heard it through the grapevine? ABA and sugar cross-talk: the ASR story. Trends in Plant Science. 2004;9:57–59. doi: 10.1016/j.tplants.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Chae HS, Kieber JJ. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends in Plant Science. 2005;10:291–296. doi: 10.1016/j.tplants.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiology. 2007;143:251–262. doi: 10.1104/pp.106.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. Ethylene signal transduction. Annals of Botany. 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Chiang MH, Hwang SG, Lin PC. Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signalling pathways. Plant Molecular Biology. 2009;71:61–80. doi: 10.1007/s11103-009-9509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. Journal of Experimental Botany. 2004;55:225–236. doi: 10.1093/jxb/erh005. [DOI] [PubMed] [Google Scholar]
- Christians MJ, Larsen PB. Mutational loss of the prohibitin AtPHB3 results in an extreme constitutive ethylene response phenotype coupled with partial loss of ethylene-inducible gene expression in Arabidopsis seedlings. Journal of Experimental Botany. 2007;58:2237–2248. doi: 10.1093/jxb/erm086. [DOI] [PubMed] [Google Scholar]
- Clarke SM, Cristescu SM, Miersch O, Harren FJ, Wasternack C, Mur LA. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytologist. 2009;182:175–187. doi: 10.1111/j.1469-8137.2008.02735.x. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signalling network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum. 1997;100:241–254. [Google Scholar]
- Fu JH, Sun XH, Wang JD, Chu JF, Yan CY. Progress in quantitative analysis of plant hormones. Chinese Science Bulletin. 2011;56:355–366. [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Gurley WB. HSP101: a key component for the acquisition of thermotolerance in plants. The Plant Cell. 2000;12:457–460. doi: 10.1105/tpc.12.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SY, Kitahata N, Sekimata K, Saito T, Kobayashi M, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K, Yoshida S, Asami T. A novel inhibitor of 9- cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiology. 2004;135:1574–1582. doi: 10.1104/pp.104.039511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Lee U, Vierling E. Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiology. 2003;132:757–767. doi: 10.1104/pp.102.017145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proceedings of the National Academy of Sciences, USA. 2000;97:4392–4397. doi: 10.1073/pnas.97.8.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signalling pathways in the acquisition of thermotolerance. Plant Physiology. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiology. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Sheen J. Sugar and hormone connections. Trends in Plant Science. 2003;8:110–116. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Vierling E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiology. 2000;122:189–198. doi: 10.1104/pp.122.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wong WS, Zhu L, Guo HW, Ecker J, Li N. Phosphoproteomic analysis of ethylene-regulated protein phosphorylation in etiolated seedlings of Arabidopsis mutant ein2 using two-dimensional separations coupled with a hybrid quadrupole time-of-flight mass spectrometer. Proteomics. 2009;9:1646–1661. doi: 10.1002/pmic.200800420. [DOI] [PubMed] [Google Scholar]
- Li W, Li M, Zhang W, Welti R, Wang X. The plasma membrane-bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nature Biotechnology. 2004;22:427–433. doi: 10.1038/nbt949. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D. Recent advances in ethylene research. Journal of Experimental Botany. 2009;60:3311–3336. doi: 10.1093/jxb/erp204. [DOI] [PubMed] [Google Scholar]
- Liu D, Gong Q, Ma Y, et al. cpSecA, a thylakoid protein translocase subunit, is essential for photosynthetic development in Arabidopsis. Journal of Experimental Botany. 2010;61:1655–1669. doi: 10.1093/jxb/erq033. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi L, Ye N, Liu R, Jia W, Zhang J. Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytologist. 2009;183:1030–1042. doi: 10.1111/j.1469-8137.2009.02899.x. [DOI] [PubMed] [Google Scholar]
- Ma S, Gong Q, Bohnert HJ. Dissecting salt stress pathways. Journal of Experimental Botany. 2006;57:1097–1107. doi: 10.1093/jxb/erj098. [DOI] [PubMed] [Google Scholar]
- Morgan PW, Drew MC. Ethylene and plant responses to stress. Physiologia Plantarum. 1997;100:620–630. [Google Scholar]
- Peng HP, Lin TY, Wang NN, Shih MC. Differential expression of genes encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis during hypoxia. Plant Molecular Biology. 2005;58:15–25. doi: 10.1007/s11103-005-3573-4. [DOI] [PubMed] [Google Scholar]
- Rahman A, Amakawa T, Goto N, Tsurumi S. Auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots. Plant and Cell Physiology. 2001;42:301–307. doi: 10.1093/pcp/pce035. [DOI] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. The Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A, Rodrigues A, Santiago J, Rubio S, Rodriguez PL. HAB1–SWI3B interaction reveals a link between abscisic acid signalling and putative SWI/SNF chromatin-remodeling complexes in Arabidopsis. The Plant Cell. 2008;20:2972–2988. doi: 10.1105/tpc.107.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. The Plant Cell. 2006;18:1292–1309. doi: 10.1105/tpc.105.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Brunaud V, Balzergue S, Dubreucq B, Lepiniec L, Pelletier G, Caboche M, Lecharny A. FLAGdb/FST: a database of mapped flanking insertion sites (FSTs) of Arabidopsis thaliana T-DNA transformants. Nucleic Acids Research. 2002;30:94–97. doi: 10.1093/nar/30.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M. Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in plant Biology. 2003;6:410–417. doi: 10.1016/s1369-5266(03)00092-x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. Journal of Biological Chemistry. 2008;283:9269–9275. doi: 10.1074/jbc.M709187200. [DOI] [PubMed] [Google Scholar]
- Tsuchisaka A, Theologis A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiology. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Yu G, Jin H, Alonso JM, Ecker JR, Zhang X, Gao S, Theologis A. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics. 2009;183:979–1003. doi: 10.1534/genetics.109.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N. Mechanisms of high salinity tolerance in plants. Methods in Enzymology. 2007;428:419–438. doi: 10.1016/S0076-6879(07)28024-3. [DOI] [PubMed] [Google Scholar]
- Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Current Opinion in Biotechnology. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wang KL, Li H, Ecker JR. Ethylene biosynthesis and signalling networks. The Plant Cell. 2002;14, Supplement:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang NN, Shih MC, Li N. The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. Journal of Experimental Botany. 2005;56:909–920. doi: 10.1093/jxb/eri083. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu C, Li K, et al. Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Molecular Biology. 2007;64:633–644. doi: 10.1007/s11103-007-9182-7. [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology. 1984;35:155–189. [Google Scholar]
- Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. The Plant Journal. 2002;30:529–539. doi: 10.1046/j.1365-313x.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Nagata M, Saito K, Wang KL, Ecker JR. Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biology. 2005;5:14. doi: 10.1186/1471-2229-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiology. 2000;124:941–948. doi: 10.1104/pp.124.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.