Abstract
In order to ascertain the stomatal and photosynthetic responses of mustard to ethylene under varying N availability, photosynthetic characteristics of mustard grown with optimal (80 mg N kg−1 soil) or low (40 mg N kg−1 soil) N were studied after the application of an ethylene-releasing compound, ethephon (2-chloroethyl phosphonic acid) at 40 days after sowing (DAS). The availability of N influenced ethylene evolution and affected stomatal conductance and photosynthesis. The effect of ethylene was smaller under deficient N where plants contained higher glucose (Glc) sensitivity, despite high ethylene evolution even in the absence of ethephon, potentially because the plants were less sensitive to ethylene per se. Ethephon application at each level of N increased ethylene and decreased Glc sensitivity, which increased photosynthesis via its effect on the photosynthetic machinery and effects on stomatal conductance. Plants grown with sufficient-N and treated with 200 μl l−1 ethephon exhibited optimal ethylene, the greatest stomatal conductance and photosynthesis, and growth. These plants made maximum use of available N and exhibited the highest nitrogen-use efficiency (NUE).
Keywords: Ethephon, ethylene, glucose, nitrogen-use efficiency, photosynthesis
Introduction
Photosynthesis is the main driving force for carbon gain and crop productivity, which is controlled by several intrinsic and extrinsic factors at both the cellular and the organ levels (Lawlor, 2001). Among various factors influencing photosynthesis, plant hormones are important in the regulation of photosynthesis and growth processes (Brenner and Cheikh, 1995; Arteca, 1997). Ethylene as a plant hormone influences many aspects of plant growth and photosynthesis (Abeles et al., 1992; Fiorani et al., 2002; Pierik et al., 2006; Acharya and Assmann, 2009). Ethephon (2-chloroethyl phosphonic acid) induces ethylene release and is used commercially to hasten fruit ripening and fruit abscission, but is also known to affect several cellular, developmental, and stress response processes related to photosynthesis (Fiorani et al., 2002; Khan et al., 2008; Acharya and Assmann, 2009; Wilkinson and Davies, 2010). The involvement of ethylene in regulating stomatal and photosynthetic responses is not clear. Contradictory claims have been made on the effects of ethylene on stomatal opening and photosynthesis. Studies have shown that ethylene mediates auxin-induced stomatal opening in Vicia faba (Merritt et al., 2001) and modulates abscisic acid (ABA)-induced stomatal closure in Arabidopsis thaliana (Tanaka et al., 2005). By contrast, Pallas and Kays (1982) and Madhavan et al. (1983) have shown stomatal closure by ethylene. Desikan et al. (2006) have reported that the exogenous application of ethephon induces stomatal closure in Arabidopsis, and ethylene-mediated stomatal closure is dependent on H2O2 generated by NADPH oxidase AtrbohF. Ethephon application has also been shown either to increase photosynthesis (Buhler et al., 1978; Grewal and Kolar, 1990; Subrahmanyam and Rathore, 1992; Grewal et al., 1993; Khan et al., 2000; Khan, 2004b) or decrease it (Kays and Pallas, 1980; Rajala and Peltonen-Sainio, 2001; Khan, 2005). Ethylene modifies stomatal conductance and photosynthesis depending on ethylene production and sensitivity (Pierik et al., 2006).
The thylene response also depends on the availability of mineral nutrients (Tari and Szen, 1995; Lynch and Brown, 1997; Borch et al., 1999; Kim et al., 2008; Khan et al., 2008; Garnica et al., 2009; Jung et al., 2009). Recently, Benlloch- Gonźalez et al. (2010) have reported that potassium deficiency increases ethylene production and reduces stomatal responsiveness. Nitrogen (N) availability influences photosynthesis by affecting the photosynthetic machinery (Marschner, 1995; Nobel, 2009), stomatal conductance (Chapin et al., 1988; Dodd et al., 2003; Hossain et al., 2010), and ethylene production (Lynch and Brown, 1997; Khan et al., 2008). Earlier, it was reported that ethephon application increased photosynthesis and growth of mustard under high N levels (Khan et al., 2000, 2008), but the interactive effect of ethylene and N on the photosynthetic response has not been worked out in detail. The reason for the increase in photosynthesis and growth with ethephon and N may be due to direct ethylene-mediated changes in ribulose 1,5-bisphosphate carboxylase (Rubisco), carboxylation efficiency (CE), and an indirect effect of ethylene on stomatal aperture. Therefore, the reported study was undertaken to observe the effects of ethephon-induced ethylene on stomatal conductance, photosynthesis, and growth of mustard under variable N levels.
Materials and methods
Plant material and growth conditions
Seeds of mustard (Brassica juncea L. Czern & Coss.) cultivar Varuna were sown in 23 cm diameter earthen pots filled with 5 kg of reconstituted soil (sand:clay:peat; 70:20:10 by dry weight) in a greenhouse of the Botany Department, Aligarh Muslim University, Aligarh, India under natural day/night conditions with a day and night temperature of 24/18±3 °C and relative humidity of 68±6%. Available soil nitrate-N was 100 mg N kg−1 soil. Nitrogen at a concentration of 0, 40, and 80 mg N kg−1 soil was added as urea. Urea was added as the source of the added N taking into consideration that urea is hydrolysed to ammonium carbonate and then, by bacterial action, it is converted to nitrate within 1 or 2 d (Harper, 1984). A high soil phosphorus (P) status was maintained by the addition of 30 mg P kg−1 soil so that this nutrient may not influence the ethephon effects since ethephon on hydrolysis releases ethylene and P. Two plants per pot were maintained and the watering schedule was adjusted throughout the duration of the experiment in order to avert leaching. Ethephon (2-chloroethyl phosphonic acid) at 0, 100, and 200 μl l−1 concentration was sprayed on foliage at 40 days after sowing (DAS; pre-flowering stage) together with 0.5% surfactant teepol. In the control treatment, plants were sprayed with an equal amount of deionized water and 0.5% teepol. The treatments were arranged in a factorial randomized design. The number of replicates for each treatment was six. The volume of the spray was 50 ml per pot and was done with a hand sprayer. At 10 d after spray, i.e. at 50 DAS, one plant from each pot was selected for the determination of photosynthetic characteristics, ethylene evolution, and Rubisco activity. The remaining one plant was used for the determination of leaf area, plant dry mass, leaf N and glucose (Glc) content. The plants were uprooted; all leaves were harvested and used for leaf area determination and, subsequently, the leaves and the rest of the plant were dried separately and their combined dry mass was recorded as plant dry mass. The dried leaf was used for the determination of leaf N and Glc content.
Measurement of ethylene
Ethylene evolution was measured by cutting 0.5 g leaf material into small pieces that were placed in 30 ml tubes containing moist paper to minimize evaporation from the tissue and were stoppered with secure rubber caps and placed in the light for 2 h under the same conditions used for plant growth. Earlier experiments showed that 2 h incubation time was adequate for ethylene detection without the interference of wound-induced ethylene, which began after 2 h of leaf incubation (data not shown). A 1 ml gas sample from the tubes was withdrawn with a hypodermic syringe and assayed on a gas chromatograph (Nucon 5700, New Delhi, India) equipped with a 1.8 m Porapack N (80–100 mesh) column, a flame ionization detector, and data station. Nitrogen was used as the carrier gas. The flow rates of nitrogen, hydrogen and oxygen were 30, 30, and 300 ml min−1, respectively. The detector was at 150 °C. Ethylene identification was based on the retention time and quantified by comparison with the peaks from standard ethylene concentration.
Determination of Rubisco activity
Rubisco activity was determined spectrophotometrically by monitoring NADH oxidation at 30 °C at 340 nm (Usuda, 1985). Leaf samples were homogenized in a chilled mortar with ice-cold extraction buffer containing 0.25 M TRIS-HCl (pH 7.8), 0.05 M MgCl2, 0.0025 M EDTA, and 37.5 mg dithiothreitol (DTT). The homogenate was centrifuged at 10 000 g at 4 °C for 10 min. The resulting supernatant was used for the enzyme assay. The reaction mixture contained 100 mM TRIS-HCl (pH 8.0), 40 mM NaHCO3, 10 mM MgCl2, 0.2 mM NADH, 4 mM ATP, 0.2 mM EDTA, 5 mM DTT, and 1 U of 3-phosphoglycerate kinase. The activity was estimated after the addition of enzyme extract and 0.2 mM ribulose-1,5-bisphosphate (RuBP). Estimation of protein was done according to Bradford (1976) using bovine serum albumin as the standard.
Photosynthetic characteristics
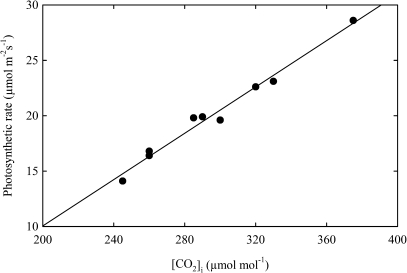
The measurements of photosynthetic rate (PN), stomatal conductance (gs), and intercellular CO2 concentration ([CO2]i) were done with an infrared gas analyser (Li-Cor 6200, Nebraska) at a light saturating intensity on a sunny day when the photosynthetically active radiation was ∼680 μmol m−2 s−1, the air temperature was ∼23 °C, and the relative humidity was ∼74% on the fully expanded top leaf of the main axis of the plant. Care was taken to use leaves of the same age for the measurement of photosynthesis in control and treated plants. Carboxylation efficiency was calculated with the help of graph plotted from the values of PN and [CO2]i (Farquhar and Sharkey, 1982) (Fig. 1). Water use efficiency (WUE) was calculated as the ratio of photosynthesis to gs to avoid effects of small differences in vapour pressure between measurements (Von Cammerer and Farquhar, 1981).
Fig. 1.
Linear regression between photosynthetic rate (PN) and intercellular CO2 concentration [CO2]i drawn to calculate carboxylation efficiency (CE) of mustard (Brassica juncea L.) plants sprayed with 0, 100 or 200 μl l−1 ethephon at 40 DAS and grown with soil-applied 0, 40, and 80 mg N kg−1 soil at 50 DAS (** significant at P <0.01).
Determination of glucose content
Leaf glucose content was determined with the method of Krishnaveni et al. (1984) and using glucose as the standard. Leaf extract was prepared by extracting dried leaf in 80% ethanol. The extract was heated in a water bath at 60 °C for 10 min and then cooled. The samples were then centrifuged at 1500 g for 1 min. The supernatant was used for Glc determination. The reaction mixture consisted of 25 mg O-dianisidine, 1 ml methanol, 49 ml of 0.1 M phosphate buffer (pH 6.5), 5 mg of peroxidase, and 5 mg of glucose oxidase. The reaction was started by adding 0.5 ml of the extract to 1 ml of the reaction mixture in a test tube. The tubes were incubated at 35 °C for 40 min and the reaction was terminated by addition of 2 ml of 6 N HCl. The colour intensity was read at 540 nm.
Determination of NUE
Nitrogen-use efficiency of the plants was calculated as an increase in plant N at a respective level of soil N compared with the control. Plant N concentration was determined in acid-peroxide digested material using the method of Lindner (1944). Soil nitrate-N was determined with the phenol disulphonic acid method as described earlier (Khan et al., 2008).
Determination of growth
Leaf area was measured with a leaf area meter (LA 211, Systronics, New Delhi, India). Plant dry mass was determined by drying plants in an oven at 80 °C until a constant weight was reached.
Statistical analysis
Data were analysed statistically and the standard error was calculated. Analysis of variance (ANOVA) was performed on the data using SPSS (ver. 10.0 Inc., USA) to determine the least significant difference (LSD) for significant data to identify difference in the mean of the treatment. The treatments means were separated by LSD test. Data are presented as mean ±SE (n=6). Linear regression was performed for the determination of CE and significance level was determined at P <0.01 (Fig. 1).
Results
The effect of ethephon spraying resulted in significantly higher values of photosynthetic and growth characteristics than water spray. Spray of 200 μl l−1 ethephon proved superior to water spray or to 100 μl l−1 ethephon spray. Deprivation of N reduced photosynthesis and growth and ethephon spray at each level of N increased these characteristics (Figs 2–6).
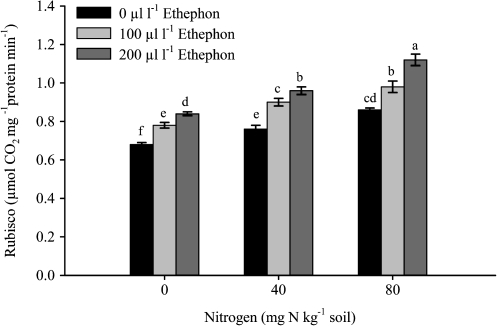
Fig. 2.
Ribulose1,5-bisphosphate carboxylase (Rubisco) activity of mustard (Brassica juncea L.) plants sprayed with 0, 100 or 200 μl l−1 ethephon at 40 DAS and grown with soil-applied 0, 40, and 80 mg N kg−1 soil at 50 DAS. Bars showing the same letter are not significantly different by LSD test at P <0.05 (n=6).
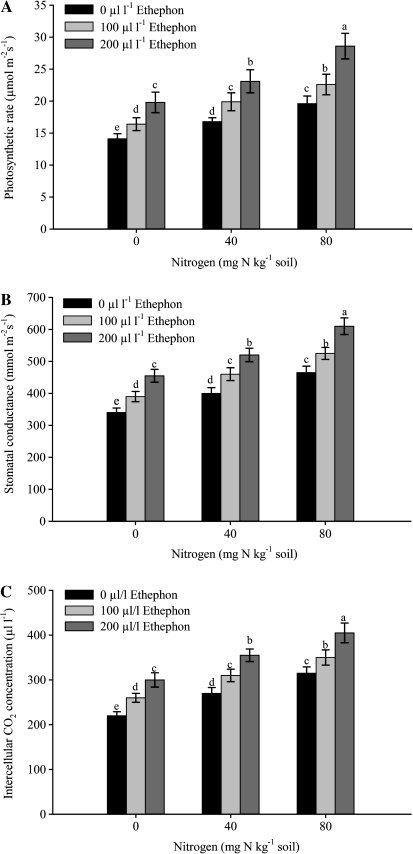
Fig. 3.
Photosynthetic rate (A), stomatal conductance (B), and intercellular CO2 concentration (C) of mustard (Brassica juncea L.) plants sprayed with 0, 100 or 200 μl l−1 ethephon at 40 DAS and grown with soil-applied 0, 40, and 80 mg N kg−1 soil at 50 DAS. Bars showing the same letter are not significantly different by LSD test at P <0.05 (n=6).
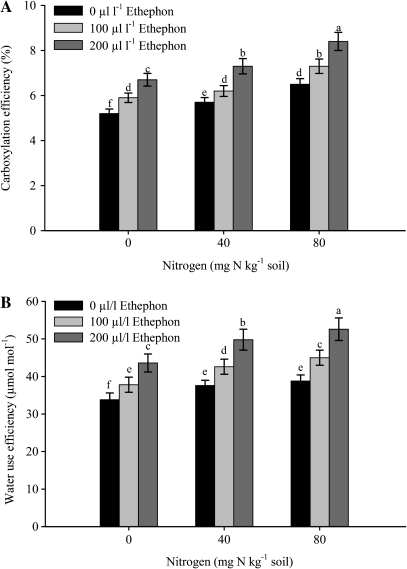
Fig. 4.
Carboxylation efficiency (A) and water use efficiency (B) of mustard (Brassica juncea L.) plants sprayed with 0, 100 or 200 μl l−1 ethephon at 40 DAS and grown with soil-applied 0, 40, and 80 mg N kg−1 soil at 50 DAS. Bars showing the same letter are not significantly different by LSD test at P <0.05 (n=6).
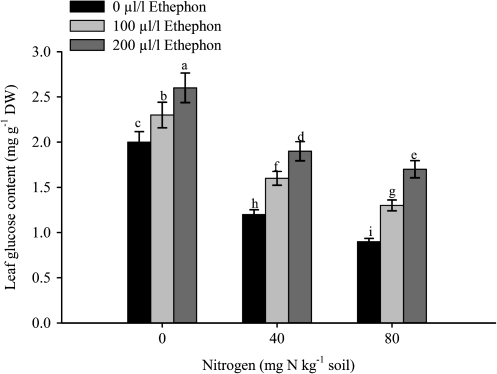
Fig. 5.
Leaf glucose content of mustard (Brassica juncea L.) plants sprayed with 0, 100 or 200 μl l−1 ethephon at 40 DAS and grown with soil-applied 0, 40, and 80 mg N kg−1 soil at 50 DAS. Bars showing the same letter are not significantly different by LSD test at P <0.05 (n=6).
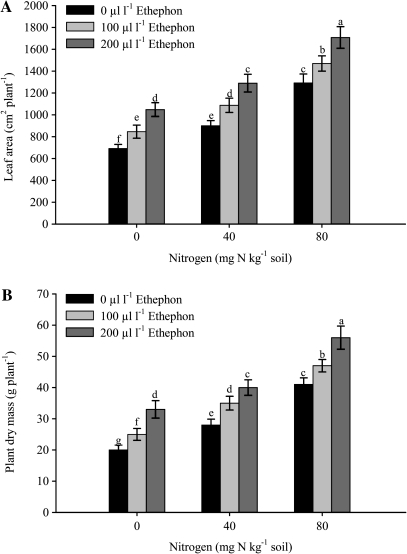
Fig. 6.
Leaf area (A) and plant dry mass (B) of mustard (Brassica juncea L.) plants sprayed with 0, 100 or 200 μl l−1 ethephon at 40 DAS and grown with soil-applied 0, 40, and 80 mg N kg−1 soil at 50 DAS. Bars showing the same letter are not significantly different by LSD test at P <0.05 (n=6).
The ethylene evolution of plants grown with deficient-N (0 mg N kg−1 soil) was higher than that of plants grown with sufficient-N (80 mg N kg−1 soil) or low-N (40 mg N kg−1 soil). The application of ethephon further increased ethylene evolution at each N level (Table 1). Maximum ethylene evolution was noted in plants sprayed with 200 μl l−1 ethephon and 0 mg N kg−1 soil. Ethylene evolution decreased by 45.7% in plants sprayed with 200 μl l−1 ethephon and grown with 80 mg N kg−1 soil, while a lower decrease of 37.1% was observed in plants sprayed with 200 μl l−1 ethephon and grown with 40 mg N kg−1 soil compared with 200 μl l−1 ethephon and 0 mg N kg−1 soil. Moreover, the ethylene evolved with 200 μl l−1 ethephon and 80 mg N kg−1 soil was higher than the ethylene released with the spray of 100 μl l−1 ethephon and 80 mg N kg−1 soil.
Table 1.
Ethylene (μl m−2 s−1) evolution in leaves of mustard (Brassica juncea L.) plants sprayed with 0, 100 or 200 μl l−1 ethephon at 40 DAS and grown with soil-applied 0, 40, and 80 mg N kg−1 soil determined at 50 DAS (n=6)
| N levels (mg N kg−1 soil) | Ethephon (μl l−1) |
||
| 0 | 100 | 200 | |
| 0 | 33.4±1.2 c | 41.7±1.4 b | 51.2±2.0 a |
| 40 | 19.5±0.8 g | 21.0±1.0 f | 32.2±1.6 c |
| 80 | 16.7±0.6 h | 22.8±0.8 e | 27.8±1.2 d |
Data showing the same letter are not significantly different by LSD test at P <0.05.
The maximal increase in photosynthetic characteristics was noted with spraying with 200 μl l−1 ethephon and plants receiving 80 mg N kg−1soil (Figs 2–4). The activity of Rubisco increased with the ethephon and N treatments. The maximum increase occurred with the combined treatment of 200 μl l−1 ethephon and 80 mg N kg−1 soil. The activity of Rubisco was enhanced by 33.3% with 200 μl l−1 and 80 mg N kg−1 soil compared with spraying with 200 μl l−1 ethephon on plants grown with 0 mg N kg−1 soil. The application of 200 μl l−1 ethephon on plants grown with 40 mg N kg−1 soil could result in an increase of 14.3% in the activity of Rubisco compared with 200 μl l−1 ethephon plus 0 mg N kg−1 soil. A lower concentration of ethephon (100 μl l−1) either with 40 or 80 mg N kg−1 soil proved to be less effective (Fig. 2). The application of 200 μl l−1 ethephon and 80 mg N kg−1 soil resulted in maximal photosynthesis, gs, and [CO2]i (Fig. 3A–C). Photosynthetic rate, gs, and [CO2]i were enhanced by 44.4, 34.1, and 35%, respectively in 200 μl l−1 ethephon and 80 mg N kg−1 soil over 200 μl l−1 ethephon and 0 mg N kg−1 soil. Spraying 200 μl l−1 ethephon on to plants treated with 40 mg N kg−1 soil could increase PN, gs, and [CO2]i by 16.7, 14.3, and 18.3%, respectively compared to spraying with 200 μl l−1 ethephon and treated with 0 mg N kg−1 soil. The similar treatment (200 μl l−1 ethephon, 80 mg N kg−1 soil) increased CE (Fig. 4A) and WUE (Fig. 4B) maximally. The increases in these characteristics with 200 μl l−1 ethephon and 80 mg N kg−1soil were 25.4% and 20.6% compared with 200 μl l−1 ethephon and 0 mg N kg−1soil. The increases in these characteristics with 200 μl l−1 ethephon and 40 mg N kg−1 soil were lower.
The maximal Glc content was found in plants receiving 0 mg N kg−1soil and no ethephon. The addition of N decreased leaf Glc content, whereas the application of ethephon increased the Glc content at each level of N (Fig. 5). Plants receiving 40 mg N kg−1 soil and sprayed with 200 μl l−1 ethephon decreased the Glc content by 26.9% compared with 200 μl l−1 ethephon and 0 mg N kg−1 soil. Glc content was decreased by 34.6% with the treatments of 200 μl l−1 ethephon and 80 mg N kg−1 soil compared to 200 μl l−1 ethephon and 0 mg N kg−1 soil.
Leaf area and plant dry mass were affected maximally by spraying with 200 μl l−1 ethephon when plants received 80 mg N kg−1 soil (Fig. 6A, B). The plants treated with 40 mg N kg−1 soil responded less to 200 μl l−1 ethephon spray. Leaf area and plant dry mass were increased by 62.9% and 69.7% with the treatments of 200 μl l−1ethephon and 80 mg N kg−1 soil compared with 200 μl l−1 ethephon and 0 mg N kg−1 soil. The plants receiving 40 mg N kg−1 soil and sprayed with 200 μl l−1 ethephon could increase leaf area and plant dry mass by 23.4% and 21.2%.
NUE was significantly affected by ethephon and N treatments. NUE of plants sprayed with 200 μl l−1 ethephon and 80 mg N kg−1 soil was about two-times higher than plants receiving 200 μl l−1 ethephon and 40 mg N kg−1 soil (Table 2). The effect of 100 μl l−1 ethephon plus 40 or 80 mg N kg−1 soil was less.
Table 2.
N-use efficiency (mg plant N mg−1 N applied kg−1 soil) of plants sprayed with 100 or 200 μl l−1 ethephon at 40 DAS
| N levels (mg N kg−1 soil) | N-use efficiency (%) |
||
| Ethephon spray (μl l−1) | |||
| 0 | 100 | 200 | |
| 40 | 5.9 | 7.9 | 11.6** |
| 80 | 11.7 | 15.8 | 27.5** |
N-use efficiency was calculated at 50 DAS as an increase in plant N uptake at 40 and 80 mg N kg−1 soil over 0 mg N kg−1 soil (n=6). ** indicates significant (P <0.01) effect of ethephon over control at each N level.
Discussion
Photosynthetic rate, gS, and the growth of plants responded positively and maximally to 200 μl l−1 ethephon and sufficient-N (80 mg N kg−1 soil). These values were lower in plants grown with 0 or 40 mg N kg−1 soil, but the application of ethephon (100 or 200 μl l−1) resulted in a similar increase in photosynthesis and growth.
The enhancement in photosynthesis by ethephon application was mediated through ethylene-induced changes in various photosynthetic characteristics, and was also associated with an increase in gS and intercellular CO2, indicating that ethylene may also have increased photosynthesis by increasing the availability of CO2. This led to the uncoupling of gS and [CO2]i: both increased together whereas usually increasing [CO2]i decreases gS (Morison, 1998; Pozo et al., 2005; Levine et al., 2009; Nobel, 2009). Ethephon is a direct ethylene-releasing source when it is applied to plants and elicits responses identical to those induced by ethylene gas (Cooke and Randall, 1968; Edgerton and Blanpied, 1968). Mustard shows the typical ethylene and N responses, and ethephon application has been found to increase photosynthesis (Grewal and Kolar, 1990; Subrahmanyam and Rathore, 1992; Grewal et al., 1993; Khan et al., 2000, 2008; Khan, 2004b). Khan (2004a) showed a strong positive correlation between 1-aminocyclopropane carboxylic acid synthase, a rate-limiting enzyme in ethylene biosynthesis, with photosynthesis of mustard cultivars differing in photosynthetic capacity.
Based on the results reported here it may be said that ethephon application to plants grown with variable N levels induced stomatal and photosynthetic responses, and there was a significant interaction between ethylene, N availability, and photosynthetic characteristics. The reports on the interaction of ethylene and N are available in the literature. Baker and Corey (1990) found equal ethylene evolution and shoot dry mass in tomato plants with urea and nitrate nutrition. Nitrogen influences the quantity, structure, and composition of the photosynthetic apparatus and hence plays a crucial role in determining the photosynthetic capacity of the plant in both natural and agricultural environments (Abrol et al., 1999; Kumar et al., 2002). In crops, Rubisco content increases linearly with N uptake and leaf N (Sage et al., 1987; Makino et al., 1997; Nakano et al., 1997).
The present work suggests that ethephon application at each level of N increases ethylene and influences stomatal, photosynthetic, and growth responses. Ethylene increased the stomatal conductance of plants, thereby increasing the diffusion of CO2 and thus photosynthesis (Fig. 3). Ethylene can alter the rate of photosynthesis by affecting the diffusion rate of CO2 from the atmosphere to the intercellular cavities by influencing stomatal aperture (Pierik et al., 2006; Acharya and Assmann, 2009; Wilkinson and Davies, 2010). In addition, ethylene could stimulate photosynthesis by increasing the allocation of N to the photosynthetic machinery. There is probably some signalling response chain at the guard cells that is directly affected by ethylene. The application of 200 μl l−1 ethephon and sufficient-N (80 mg N kg−1 soil) evolved optimal ethylene, and maximally increased stomatal conductance and photosynthetic responses.
The increase in N availability resulted in the increase in photosynthesis but decreased ethylene evolution. At 0 mg N kg−1 soil, stomatal conductance and photosynthesis were low despite high ethylene evolution. It may thus be suggested that plants were less sensitive to ethylene under deficient N.
Plants grown with 0 mg N kg−1 soil showed increased Glc sensitivity that resulted in the inhibition of Rubisco activity and photosynthesis. The increase in Glc content under N deficiency has been reported (Boussadia et al., 2010), which represses photosynthetic genes and enzymes (Ehness et al., 1997). High endogenous Glc concentration results in the stronger repression of Rubisco mRNA levels. Moreover, the inability to perceive ethylene results in increased sensitivity to Glc. This increased sensitivity to Glc results in negative effects on Rubisco content and photosynthetic capacity (Tholen et al., 2007). The application of ethephon to deficient-N plants increased Glc content, ethylene evolution, and photosynthesis. Endogenous Glc concentrations are often positively correlated with ethylene production in rice, and external sugar application in this species significantly stimulates ethylene production (Kobayashi and Saka, 2003). Glc sensitivity to inhibit photosynthesis in plants unable to perceive ethylene decreases with ethephon application. Seneweera et al. (2003) suggested that ethylene production promoted growth under circumstances where leaf Glc concentration was high. The developmental arrest by high sugar levels can be overcome by applying the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (Zhou et al., 1998). Ethylene reduces the negative feedback of carbohydrates on photosynthetic gene expression (Tholen et al., 2007). As the availability of N increases from 0 to 80 mg N kg−1 soil, ethylene evolution and Glc content decreases. This results in reducing the negative effects of Glc on Rubisco and photosynthesis. Recently, it has been reported that the ethylene response factor TERF1 enhances Glc sensitivity in tobacco through activating the expression of sugar-related genes (Li et al., 2009).
The increase in WUE with ethephon application also supports the positive effect of ethephon on photosynthesis, especially as gS also increased at higher WUE. To maximize WUE, stomatal opening must be synchronized with the capability for CO2 fixation (Nobel, 2009). WUE is associated with a higher Rubisco activity or rate of electron transport (Van den Boogard et al., 1995). The higher plant WUE was caused by a lower transpiration rate associated with a higher leaf area per unit plant dry mass. Maximally increased photosynthesis with 200 μl l−1 ethephon and sufficient-N led to the increase in WUE. This may be due to increase in binding of CO2 to the active site of Rubisco (Van den Boogard et al., 1995).
The greatest leaf area due to 200 μl l−1 ethephon and sufficient-N increased ground cover and had an important influence on WUE, reducing soil evaporation and increasing plant dry mass accumulation. The higher leaf area is correlated with ethephon-enhanced ethylene biosynthesis. The role of low ethylene concentration in inducing leaf growth (Lee and Reid, 1997; Hussain et al., 1999; Fiorani et al., 2002; Khan, 2005) and cell expansion (Rodriguez-Pousada et al., 1993) has been reported. Tholen et al. (2004) found that ethylene-insensitive genotypes of Arabidopsis, Nicotiana tabacum, and Petunia x hybrida had no increase in total leaf area compared to normal ethylene-sensitive control plants. In addition to studies on the effects of ethylene treatment, the role of ethylene during leaf development had been confirmed physiologically using ethylene inhibitors and genetically using ethylene-insensitive mutants or transgenic plants that did not express the key enzymes of ethylene biosynthesis (Bleecker et al., 1998; Oh et al., 1999). In our earlier studies, ethephon application was found to enhance ethylene evolution and leaf area (Khan, 2004b, 2005; Khan et al., 2008).
Plants grown with deficient or low-N exhibited lower NUE than plants grown with sufficient-N. The application of ethephon on these plants increased NUE. Van Sanford et al. (1989) and Bulman and Smith (1993) found positive effects of ethephon on NUE of winter wheat and barley. Khan et al. (2008) have shown that ethylene has a role in N metabolism and increases NR activity and N content in Brassica juncea. Ethylene has also been reported to enhance glutamine synthetase activity and mRNA level in Hevea brasiliensis (Pujade-Renaud et al., 1994). The low NUE of plants under low-N invested a lower amount of N to Rubisco and resulted in lower photosynthesis. Maximum NUE resulted from the application of 200 μl l−1 ethephon on plants grown with sufficient-N. These plants had ethylene evolution which maximally increased photosynthesis and made maximum use of the available N due to the enhancement of vegetative growth.
In conclusion, it may be said that ethylene increases photosynthesis and growth at all levels of N (deficient and sufficient) both via an increase in carboxylation efficiency and via an increase in gS and the availability of [CO2]i, thereby uncoupling the usual effect of increased [CO2]i to reduce gS. Increasing ethylene increases photosynthesis directly via Glc sensitivity, and increasing the availability of [CO2]i, and this occurs at all levels of N, although less sensitively at deficient N where stress ethylene production may have reduced gS and photosynthetic machinery sensitivity to ethylene although this remains to be tested.
Glossary
Abbreviations
- [CO2]i
intercellular CO2 concentration
- CE
carboxylation efficiency
- DAS
days after sowing
- Glc
glucose
- gs
stomatal conductance
- NUE
nitrogen-use efficiency
- PN
photosynthetic rate
- WUE
water use efficiency
References
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in plant biology. 2nd edn. San Diego: Academic Press; 1992. [Google Scholar]
- Abrol YP, Chatterjee SR, Kumar PA, Jain V. Improvement in nitrogen use efficiency: physiological and molecular approaches. Current Science. 1999;76:1357–1364. [Google Scholar]
- Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant Molecular Biology. 2009;69:451–462. doi: 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- Arteca RN. Plant growth substances: principles and applications. Delhi: CBS Publishers and Distributors; 1997. [Google Scholar]
- Baker AV, Corey KA. Ethylene evolution by tomato plants receiving nitrogen nutrition from urea. Horticultural Science. 1990;25:420–421. [Google Scholar]
- Benlloch-Gonźalez M, Romera J, Cristescu S, Harren F, Fournier JM, Benlloch M. K+ starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. Journal of Experimental Botany. 2010;61:1139–1145. doi: 10.1093/jxb/erp379. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1998;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell and Environment. 1999;22:425–431. [Google Scholar]
- Boussadia O, Steppeb K, Zgallaid H, Hadjc SBE, Brahama M, Lemeurb R, Labekee MCV. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’. Scientia Horticulturae. 2010;123:336–342. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilising the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenner ML, Cheikh N. Hormones in photosynthate partitioning and seed filling. In: Davies PJ, editor. Plant hormones. Dordrecht: Kluwer; 1995. pp. 649–670. [Google Scholar]
- Buhler B, Drumm H, Mohr H. Investigations on the role of ethylene in phytochrome-mediated photoporphogenesis. II. Enzyme levels and chlorophyll synthesis. Planta (Berlin) 1978;142:119–122. doi: 10.1007/BF00385129. [DOI] [PubMed] [Google Scholar]
- Bulman P, Smith DL. Yield and grain protein response of spring barley to ethephon and triadimefon. Crop Science. 1993;33:798–803. [Google Scholar]
- Chapin FS, Walter CHS, Clarkson DT. Growth response of barley and tomato to nitrogen stress and its control by abscisic acid water relations and photosynthesis. Planta. 1988;173:352–366. doi: 10.1007/BF00401022. [DOI] [PubMed] [Google Scholar]
- Cooke AR, Randall DI. 2-Haloethanephosphonic acids as ethylene releasing agents for the induction of flowering in pineapples. Nature. 1968;218:974–975. doi: 10.1038/218974a0. [DOI] [PubMed] [Google Scholar]
- Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. The Plant Journal. 2006;47:907–916. doi: 10.1111/j.1365-313X.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Tan LP, He J. Do increases in xylem sap pH and/or ABA concentration mediate stomatal closure following nitrate deprivation? Journal of Experimental Botany. 2003;54:1281–1288. doi: 10.1093/jxb/erg122. [DOI] [PubMed] [Google Scholar]
- Edgerton LJ, Blanpied GD. Regulation of growth and fruit maturation with 2-chloroethanephosphonic acid. Nature. 1968;219:1064–1065. doi: 10.1038/2191064a0. [DOI] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch T. Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. The Plant Cell. 1997;9:1825–1841. doi: 10.1105/tpc.9.10.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. American Review of Plant Physiology. 1982;33:317–345. [Google Scholar]
- Fiorani F, Bogemann GM, Visser EJW, Lambers H, Voesenek LACJ. Ethylene emission and responsiveness to applied ethylene vary among Poa species that inherently differ in leaf elongation rates. Plant Physiology. 2002;129:1382–1390. doi: 10.1104/pp.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnica M, Houdusse F, Yvin JC, Garcia-Mina JM. Nitrate supply induces changes in polyamine content and ethylene production in wheat plants grown with ammonium. Journal of Plant Physiology. 2009;166:363–374. doi: 10.1016/j.jplph.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Grewal HS, Kolar JS. Response of Brassica juncea to chlorocholine chloride and ethrel sprays in association with nitrogen application. Journal of Agriculture Science. 1990;114:87–91. [Google Scholar]
- Grewal HS, Kolar JS, Cheema SS, Singh G. Studies on the use of growth regulators in relation to nitrogen for enhancing sink capacity and yield of gobhi-sarson (Brassica napus) Indian Journal of Plant Physiology. 1993;36:1–4. [Google Scholar]
- Harper JE. Uptake of nitrogen forms by roots and leaves. In: Hauck RD, editor. Nitrogen in crop production. American Society of Agronomy; 1984. pp. 165–170. [Google Scholar]
- Hossain MD, Musa MF, Talib J, Jol H. Effects of nitrogen, phosphorus and potassium levels on kenaf (Hibiscus cannabinus L.) growth and photosynthesis under nutrient solution. Journal of Agricultural Science. 2010;2:49–57. [Google Scholar]
- Hussain A, Black CR, Taylor IB, Roberts JA. Soil compaction: a role for ethylene in regulating leaf expansion and shoot growth in tomato. Plant Physiology. 1999;121:1227–1237. doi: 10.1104/pp.121.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Ji-Yul, Shin R, Schachtman DP. Ethylene mediates responses and tolerance to potassium deprivation in Arabidopsis. The Plant Cell. 2009;21:607–621. doi: 10.1105/tpc.108.063099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays SJ, Pallas JE. Inhibition of photosynthesis by ethylene. Nature. 1980;385:51–52. doi: 10.1104/pp.70.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA. Activity of 1-aminocyclopropane carboxylic acid synthase in two mustard (Brassica juncea L.) cultivars differing in photosynthetic capacity. Photosynthetica. 2004a;42:477–480. [Google Scholar]
- Khan NA. An evaluation of the effects of exogenous ethephon, an ethylene releasing compound, on photosynthesis of mustard (Brassica juncea) cultivars that differ in photosynthetic capacity. BMC Plant Biology. 2004b;4 doi: 10.1186/1471-2229-4-21. Article No. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA. The influence of exogenous ethylene on growth and photosynthesis of mustard (Brassica juncea) following defoliation. Scientia Horticulturae. 2005;105:499–505. [Google Scholar]
- Khan NA, Lone NA, Samiullah Response of mustard (Brassica juncea L.) to applied nitrogen with or without ethrel sprays under non-irrigated conditions. Journal of Agronomy and Crop Science. 2000;184:63–66. [Google Scholar]
- Khan NA, Mir MR, Nazar R, Singh S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biology. 2008;10:534–538. doi: 10.1111/j.1438-8677.2008.00054.x. [DOI] [PubMed] [Google Scholar]
- Kim Hye-Ji, Lynch JP, Brown KM. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant, Cell and Environment. 2008;31:1744–1755. doi: 10.1111/j.1365-3040.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Saka H. Relationship between ethylene evolution and sucrose content in excised leaf blades of rice. Plant Production Science. 2003;3:398–403. [Google Scholar]
- Krishnaveni S, Balasubramanian T, Sadasivam S. Sugar distribution in sweet stalk sorghum. Food Chemistry. 1984;15:229–232. [Google Scholar]
- Kumar PA, Parry MAJ, Mitchell RAC, Ahmad A, Abrol YP. Photosynthesis and nitrogen use efficiency. In: Foyer CH, Noctor G, editors. Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism. Advances in photosynthesis and respiration. Vol. 12. Dordrecht: Springer; 2002. pp. 23–34. [Google Scholar]
- Lawlor DW. Photosynthesis. Oxford: Bios Scientific Publishers; 2001. pp. 309–351. [Google Scholar]
- Lee SH, Reid DM. The role of endogenous ethylene in the expansion of Helianthus annuus leaves. Canadian Journal of Botany. 1997;75:501–508. doi: 10.1139/b97-054. [DOI] [PubMed] [Google Scholar]
- Levine LH, Richard JT, Wheeler RM. Super-elevated CO2 interferes with stomatal response to ABA and night closure in soybean (Glycine max) Journal of Plant Physiology. 2009;166:903–913. doi: 10.1016/j.jplph.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Li A, Zhang Z, Wang Xue-Chen, Huang R. Ethylene response factor TERF1 enhances glucose sensitivity in tobacco through activating the expression of sugar-related genes. Journal of Integrative Plant Biology. 2009;51:184–193. doi: 10.1111/j.1744-7909.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- Lindner RC. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiology. 1944;19:70–89. doi: 10.1104/pp.19.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. Ethylene and plant responses to nutritional stress. Physiologia Plantarum. 1997;100:613–619. [Google Scholar]
- Madhavan S, Chrmoinski A, Smith BN. Effect of ethylene on stomatal opening in tomato and carnation leaves. Plant and Cell Physiology. 1983;24:569–572. [Google Scholar]
- Makino A, Sato T, Nakano H, Mae T. Leaf photosynthesis, plant growth, and nitrogen allocation in rice under different irradiances. Planta. 1997;203:390–398. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. New York: Academic Press; 1995. pp. 229–299. [Google Scholar]
- Merritt F, Kemper A, Tallman G. Inhibitors of ethylene synthesis inhibit auxin-induced stomatal opening in epidermis detached from leaves of Vicia faba L. Plant and Cell Physiology. 2001;42:223–230. doi: 10.1093/pcp/pce030. [DOI] [PubMed] [Google Scholar]
- Morison JIL. Stomatal response to increased CO2 concentration. Journal of Experimental Botany. 1998;49:442–452. [Google Scholar]
- Nakano H, Makino A, Mae T. The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiology. 1997;115:191–198. doi: 10.1104/pp.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS. Physicochemical and environmental plant physiology. 2nd edn. San Diego: Academic Press; 2009. [Google Scholar]
- Oh SA, Park JH, Lee GL, Pack KH, Park SK, Nam HG. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. The Plant Journal. 1999;12:527–535. doi: 10.1046/j.1365-313x.1997.00527.x. [DOI] [PubMed] [Google Scholar]
- Pallas JE, Kays SJ. Inhibition of photosynthesis by ethylene: a stomatal effect. Plant Physiology. 1982;70:598–601. doi: 10.1104/pp.70.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. The janus face of ethylene: growth inhibition and stimulation. Trends in Plant Sciences. 2006;11:176–183. doi: 10.1016/j.tplants.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Pozo AD, Pérez P, Morcuende R, Alonso A, Carrasco-Martínez R. Acclimatory responses of stomatal conductance and photosynthesis to elevated CO2 and temperature in wheat crops grown at varying levels of nitrogen supply in a Mediterranean environment. Plant Science. 2005;169:908–916. [Google Scholar]
- Pujade-Renaud V, Clement A, Perrot-Rechenmann C, Prevot JC, Chrestin H, Jacob JL, Guern J. Ethylene induced increase in glutamine synthetase activity and mRNA levels in Hevea brasiliensis latex cells. Plant Physiology. 1994;105:127–132. doi: 10.1104/pp.105.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Peltonen-Sainio P. Plant growth regulator effects on spring cereal root and shoot growth. Agronomy Journal. 2001;93:936–943. [Google Scholar]
- Rodriguez-Pousida RA, De Rycke R, Dedondar A, Van Caeneghem W, Engler E, Van Montagu M, Van Der Straeten D. The Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene 1 is expressed during early development. The Plant Cell. 1993;5:897–911. doi: 10.1105/tpc.5.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW, Seemann JR. The nitrogen efficiency of C3 and C4 plants. III. Leaf nitrogen effect on activity of carboxylating enzymes in Chenopodium album L. and Amaranthus netroflexus L. Plant Physiology. 1987;85:355–359. doi: 10.1104/pp.85.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneweera S, Aben SK, Basra AS, Jones B, Conroy JP. Involvement of ethylene in the morphological and developmental response of rice to elevated atmospheric CO2 concentrations. Plant Growth Regulation. 2003;39:143–153. [Google Scholar]
- Subrahmanyam D, Rathore VS. Influence of ethylene on carbon-14 labeled carbondioxide assimilation and partitioning in mustard. Plant Physiology and Biochemistry. 1992;30:81–86. [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiology. 2005;138:2337–2343. doi: 10.1104/pp.105.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tari I, Szen L. Effect of nitrite and nitrate nutrition on ethylene production by wheat seedlings. Acta Phytopatholica et Entomologica Hungarica. 1995;30:99–104. [Google Scholar]
- Tholen D, Voesenek LACJ, Poorter H. Ethylene insensitivity does not increase leaf area or relative growth rate in Arabidopsis thaliana, Nicotiana tabacum and Petunia× hybrida. Plant Physiology. 2004;134:1803–1812. doi: 10.1104/pp.103.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Pons TL, Voesenek LACJ, Poorter H. Ethylene insensitivity results in the down-regulation of Rubisco expression and photosynthetic capacity in tobacco. Plant Physiology. 2007;144:1305–1315. doi: 10.1104/pp.107.099762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H. The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant and Cell Physiology. 1985;91:455–463. [Google Scholar]
- Van den Boogard R, Kostadirova S, Veneklaas EJ, Lambers H. Association of water use efficiency and nitrogen use efficiency with photosynthetic characteristics of two wheat cultivars. Journal of Experimental Botany. 1995;46:1429–1438. [Google Scholar]
- Van Sanford DA, Grove JH, Grabau J, MacKown CT. Ethephon and nitrogen use in winter wheat. Agronomy Journal. 1989;81:951–954. [Google Scholar]
- Von Cammerer S, Farquhar GD. Some relationship between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell and Environment. 2010;33:510–525. doi: 10.1111/j.1365-3040.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang J-C, Jones TL, Sheen J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proceedings of the National Academy of Sciences, USA. 1998;95:10294–10299. doi: 10.1073/pnas.95.17.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]