Abstract
Wheat is undoubtedly one of the world's major food sources since the dawn of Near Eastern agriculture and up to the present day. Morphological, physiological, and genetic modifications involved in domestication and subsequent evolution under domestication were investigated in a tetraploid recombinant inbred line population, derived from a cross between durum wheat and its immediate progenitor wild emmer wheat. Experimental data were used to test previous assumptions regarding a protracted domestication process. The brittle rachis (Br) spike, thought to be a primary characteristic of domestication, was mapped to chromosome 2A as a single gene, suggesting, in light of previously reported Br loci (homoeologous group 3), a complex genetic model involved in spike brittleness. Twenty-seven quantitative trait loci (QTLs) conferring threshability and yield components (kernel size and number of kernels per spike) were mapped. The large number of QTLs detected in this and other studies suggests that following domestication, wheat evolutionary processes involved many genomic changes. The Br gene did not show either genetic (co-localization with QTLs) or phenotypic association with threshability or yield components, suggesting independence of the respective loci. It is argued here that changes in spike threshability and agronomic traits (e.g. yield and its components) are the outcome of plant evolution under domestication, rather than the result of a protracted domestication process. Revealing the genomic basis of wheat domestication and evolution under domestication, and clarifying their inter-relationships, will improve our understanding of wheat biology and contribute to further crop improvement.
Keywords: Brittle rachis, plant evolution under domestication, quantitative trait loci, Triticum turgidum ssp.dicoccoides, wheat domestication
Introduction
The Neolithic transition from hunter-gatherer lifestyle to sedentary agrarian societies, beginning ∼10 000 years ago, was a crucial turning point in human history (Childe, 1951). Wheat (Triticum spp.) is one of the Neolithic founder crops, domesticated alongside other cereals—einkorn wheat (Triticum monococcum L.) and barley (Hordeum vulgare L.)—as well as pulses—pea (Pisum sativum L.), lentil (Lens culinaris Medikus), chickpea (Cicer arietinum L.), and bitter vetch (Vicia ervilia L. Willd.)—in the Near-Eastern Fertile Crescent ∼10 000 years ago (Lev-Yadun et al., 2000). Ample phytogeographical, molecular, archeobotanical, and genetic evidence points to a small ‘core area’ in southeastern Turkey and northern Syria as the cradle of agriculture (Lev-Yadun et al., 2000; Salamini et al., 2002; Abbo et al., 2006). Today, wheat is the world's most important food crop, providing about one-fifth of the calories consumed by man, with ∼620 Mt produced annually worldwide (http://faostat.fao.org). Wild emmer wheat [Triticum turgidum ssp. dicoccoides (körn.) Thell] is the allotetraploid (2n=4x= 28; genome BBAA) progenitor of domesticated tetraploid (2n=4x=28; BBAA) durum wheat [T. turgidum ssp. durum (Desf.) MacKey] and hexaploid (2n=6x=42; BBAADD) bread wheat (Triticum aestivum L.).
Plant domestication is generally thought to have involved a suite of complex morphological, physiological, and genetic changes referred to as ‘domestication syndrome’ (Hammer, 1984). The advent of molecular markers has enabled the dissection of these complex traits, via analysis of quantitative trait loci (QTLs). The genetic basis of the domestication syndrome has been studied in wheat (Peng et al., 2003) and several other crop plants, including rice (Oryza sativa L.; Lee et al., 2005), maize (Zea mays L.; White and Doebley, 1998), sorghum [Sorghum bicolor (L.) Moench; Paterson et al., 1995], pearl millet [Pennisetum glaucum (L.) R. Br.; Poncet et al., 2000], sunflower (Helianthus annuus L.; Wills and Burke, 2007), common bean (Phaseolus vulgaris L.; Koinange et al., 1996), soybean (Glycine max L.; Liu et al., 2007), and tomato (Solanum lycopersicum L.; Doganlar et al., 2002). These (and other) studies have indicated that a few gene clusters with large effects account for most of the variation associated with the differences between domesticated forms and their wild progenitors. Furthermore, QTLs of major effects show extensive synteny across genera of the Gramineae (Paterson et al., 1995) and Solanaceae (Doganlar et al., 2002) species with respect to domestication and crop evolution loci.
The major domestication trait in wheat is modification of the seed dispersal mode (by way of reduced spikelet shattering at maturity), which is considered a key feature in preventing yield losses (Fig. 1). Although the initial modification of wild plants and their adoption as potential crops was undoubtedly the crucial step in the birth of agriculture, evolution under domestication has taken place since the Neolithic period, through modern plant breeding to the present day (Ladizinsky, 1998a). Other domestication-related and crop evolution traits include: glume reduction (easier threshing), changes in plant architecture, changes in ear and kernel size, loss of seed dormancy, lower grain protein and mineral concentrations, and increased grain carbohydrate content (Harlan et al., 1973).
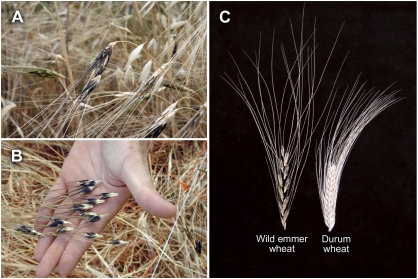
Fig. 1.
(A) Wild emmer wheat (Triticum turgidum ssp. dicoccoides) in its natural habitat in Israel, with a mature disarticulating spike. (B) Spikelets of wild emmer wheat collected from the soil surface. (C) Brittle rachis spike of wild emmer wheat and non-brittle spike of domesticated durum wheat.
Recently, a recombinant inbred line (RIL) population derived from a cross between domesticated durum wheat and wild emmer was used for QTL analysis of drought response traits (Peleg et al., 2009b) and grain nutrient concentrations (Peleg et al., 2009a). In the current study, the same RIL population was used to (i) determine the chromosome locations and phenotypic effects of domestication syndrome traits; and (ii) study the relationships of these traits/QTLs with wheat productivity and quality. The results shed light on the genetic bases of processes associated with wheat domestication syndrome and evolution under domestication.
Materials and methods
Plant material and growth conditions
A population of 152 F6 RILs was developed by single-seed descent from a cross between a durum wheat cultivar (Langdon; LDN hereafter) and a wild emmer accession (G18-16) (Peleg et al., 2008). The RIL population was tested in the field under two environmental conditions at the experimental farm of The Hebrew University of Jerusalem in Rehovot, Israel (31°54′ N, 34°47′ E; 54 m above sea level). The soil at this location is brown-red degrading sandy loam (Rhodoxeralf) composed of 76% sand, 8% silt, and 16% clay. Seeds were disinfected (3.6% sodium hypochloric acid, for 10 min) and placed for vernalization on a moist germination paper for 3 weeks in a dark cold room (4 °C), followed by 3 days of acclimation at room temperature. Seedlings were then transplanted into an insect-proof screenhouse protected by a polyethylene top. Water was applied via a drip irrigation system during the winter months (Dec-Apr) to mimic the natural pattern of rainfall in the east Mediterranean region. Two irrigation regimes were applied: well-watered (WW; 750 mm) and water-limited (WL; 350 mm), using a split-plot factorial (RIL×irrigation regime) block design with irrigation regimes in main plots and genotypes in subplots. The trial was replicated three times with 75 cm long plots, each containing five plants. Plants were treated with pesticides to avoid development of pathogens or insect pests and the plots were weeded by hand once a week.
Phenotypic measurements
Brittle rachis (Br) was qualitatively scored (as brittle versus non-brittle spike) at maturity for each individual plot. Each plot was harvested as soon as >50% of the plants reached maturity to minimize seed dispersal. All spikes were harvested, oven-dried (35 °C for 48 h), and weighed. A subsample of the harvested spikes from each plot (four spikes) was weighed and threshed to calculate thousand kernel weight (TKW), kernel number per spike (KNSP), and spike harvest index (SPHI; the proportion of kernel weight per spike weight).
Statistical analysis of phenotypic data
The JMP® ver.8.0 statistical package (SAS Institute, Cary, NC, USA) was used for statistical analyses. All phenotypic variables were tested for normal distribution. A factorial model was employed for the analysis of variance (ANOVA), with RILs and blocks as random effects and the trial as a fixed effect. Broad sense heritability estimate (H2) was calculated for each trait across two environmental conditions using variance components estimated based on ANOVA:
| (1) |
where: 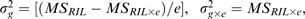 , e is the number of environments, and MS is the mean square.
, e is the number of environments, and MS is the mean square.
Genetic analyses
A genetic linkage map of 2317 cM was previously developed for the 152 RIL mapping population based on 197 SSRs (simple sequence repeats) and 493 DArT (diversity array technology) markers (Peleg et al., 2008). Several DArT markers that were represented in that map by clone ID numbers were renamed with the prefix ‘wPt’, ‘rPt’, or ‘tPt’, corresponding to wheat, rye (Secale cereale L.), or Triticale (× Triticosecale Wittmark), respectively, followed by a number. A skeleton map, comprised of 307 markers scattered along the 14 chromosomes of tetraploid wheat at an average spacing of 7.5 cM, was used for QTL mapping. χ2 analysis was performed to test for deviation of Br from the expected segregation ratio of 1:1 in the RIL population. Linkage analysis and map construction were performed based on the evolutionary strategy algorithm included in the MultiPoint package (Mester et al., 2003), as described by Peleg et al. (2008).
QTL analysis was performed with the MultiQTL package using the general interval mapping for the RIL-selfing population as described by Peleg et al. (2009b). To examine genotype by environment (G×E) interaction, the two-environment QTL model was compared against a submodel assuming an equal effect of all environments, using 5000 permutation tests (such a comparison was not applicable in the case of a two-QTL solution). The effect of epistatic interaction was examined for each trait by comparison of H0 (ϵ=0, i.e. additive effects of the QTL) and H1 (ϵ≠0), i.e. assuming epistasis). Correspondence between QTLs of different traits was determined using the hypergeometric probability function (Larsen and Marx, 1985) according to Paterson et al. (1995):
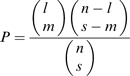 |
(2) |
where n is the number of comparable intervals, m is the number of ‘matches’ (QTLs of two traits with >50% overlap of their confidence intervals) declared between QTLs, l is the number of QTLs found in the larger sample, and s is the number of QTLs found in the smaller sample.
Results
Phenotypic diversity among RILs
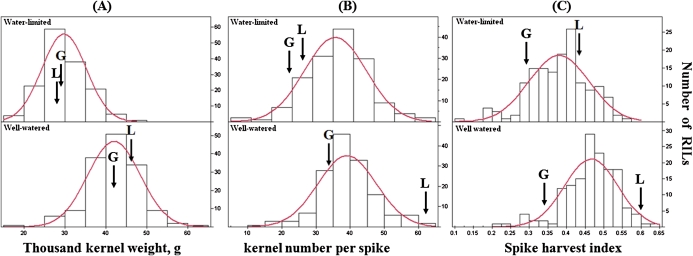
Frequency distributions of the continuous phenotypic traits among the RILs under each environmental condition are presented in Fig. 2. All variables exhibited normal distribution under each of the environments. Transgressive segregation was common among all traits. Broad-sense heritability estimates, indicating the proportion of phenotypic variance attributable to genetic effect, were calculated for TKW (H2=0.54), SPHI (H2=0.66), and KNPS (H2=0.73). Correlation analysis showed significant positive associations between SPHI and TKW (r=0.59, P <0.0001, and r=0.58, P <0.0001, for the WL and WW environments, respectively), as well as between SPHI and KNSP (r=0.71, P <0.0001, and r=0.61, P <0.0001 for WL and WW, respectively) (Fig. 3).
Fig. 2.
Phenotypic distribution of (A) thousand kernel weight, (B) kernel number per spike, and (C) spike harvest index, in 152 recombinant inbred lines (RILs) under water-limited and well-watered conditions. Data are means of three replicated plots. Arrows indicate the values of the parental lines Langdon (L) and G18-16 (G).
Fig. 3.
Correlation between wheat domestication-related traits in 152 in recombinant inbred lines of the cross between Langdon and G18-16, grown under two environmental conditions: water-limited and well-watered.
Rachis brittleness factor
The wild parent (G18-16) of this population is characterized by a Br spike, while the domesticated parent (LDN) has a non-fragile spike. The 152 RILs exhibited a segregation of 66 lines with brittle spikes (wild type) and 86 with non-brittle spikes (domesticated type). This segregation pattern fits the theoretical expected 1:1 ratio (χ2=2.6, P=0.11) for a RIL population, thus suggesting the involvement of a single gene in spike brittleness. The Br locus was mapped, using the MultiPoint package, on the long arm of chromosome 2A between markers XtPt-3136 and XwPt-4855 (Fig. 4).
Fig. 4.
Likelihood intervals for QTLs associated with grain yield (GY; adapted from Peleg et al., 2009b) and domestication syndrome-related traits: thousand kernel weight (TKW), kernel number per spike (KNSP), and threshability (SPHI), in recombinant inbred lines of the cross between Langdon and G18-16. QTLs expressed under water-limited (WL) and well-watered (WW) conditions are marked. The spike brittle rachis (Br) gene was mapped as a single trait.
Major characteristics of the detected QTLs
Twenty-seven significant QTLs, scattered across 13 chromosomes of the tetraploid wheat (no QTLs were detected on chromosome 1A), were detected for domestication-related traits under the two environmental conditions (Table 1). Higher phenotypic values (i.e. domesticated type) were associated in 12 QTLs (44%) with the wild allele (G18-16) and in 15 QTLs (56%) with the domesticated allele (LDN). Ten QTLs exhibited G×E interaction, of which four QTLs were detected under both environments with different effects and six QTLs were detected under one environment, whereas the remaining 17 QTLs (67%) showed no interaction with environmental conditions (Table 1). No significant two-locus epistasis was found between any of the QTLs controlling any of the traits.
Table 1.
Summary of QTLs associated with domestication related traits detected in tetraploid wheat (Langdon×G18-16 recombinant inbred line population) under two environmental conditions: well-watered (WW) and water-limited (WL)
| Trait | No. of QTLs | LOD | Favourable allele |
Environment |
|||
| G18-16 | LDN | WL+WW | WL | WW | |||
| Spike harvest index | 6 | 5.5–10.7 | 2 | 4 | 6 | – | – |
| Thousand kernel weight | 12 | 3.3–12.0 | 8 | 4 | 6 | 3 | 3 |
| Kernel number per spike | 9 | 4.3–14.4 | 2 | 7 | 9 | – | – |
| Total | 27 | 12 | 15 | 21 | 3 | 3 | |
QTLs detected for each trait
Detailed biometric parameters of QTLs detected for each of the traits are as follows.
Spike harvest index
A total of six significant QTLs were associated with SPHI, with LOD scores ranging between 5.5 and 10.7, explaining 1.8–13.0% of the variation (Tables 1, 2). Higher SPHI was conferred by the G18-16 allele at two loci (2B and 7B) and by the LDN allele at four loci (4A1, 4A2, 4B, and 5A). Two QTLs (2B and 7B) showed significant G×E interaction with a similar trend across environments.
Table 2.
Biometric parameters of QTLs affecting domestication-related traits in wheat under two environmental conditions: well-watered and water-limited
| Trait/chromosome | Position (cM) | Nearest marker | LODa | Water limited |
Well watered |
Favourable alleled | G×Ee (P) | ||
| PEVb | dc | PEV | d | ||||||
| Spike harvest index | |||||||||
| 2B | 116.2±17.6 | XwPt-1294 | 5.5*** | 0.105 | 0.044±0.031 | 0.018 | 0.006±0.001 | G | ** |
| 4A1 | 31.8±20.1 | Xgwm610 | 10.7*** | 0.130 | –0.046±0.030 | 0.117 | –0.027±0.025 | L | – |
| 4A2 | 82.1±17.8 | XwPt-11573 | 10.7*** | 0.130 | –0.010±0.030 | 0.117 | –0.021±0.020 | L | – |
| 4B | 61.4±11.7 | Xgwm857 | 8.8*** | 0.062 | –0.040±0.012 | 0.129 | –0.050±0.010 | L | NS |
| 5A | 16.4±15.0 | Xgwm154 | 7.1*** | 0.064 | –0.039±0.014 | 0.114 | –0.047±0.011 | L | NS |
| 7B | 36.6±18.3 | Xgwm537 | 6.2*** | 0.111 | 0.043±0.035 | 0.032 | –0.009±0.003 | G | * |
| Thousand kernel weight | |||||||||
| 1B | 35.8±17.9 | Xgwm1024 | 3.3* | – | – | 0.064 | –2.646±2.343 | L | WW |
| 2A | 137.2±15.2 | XtPt-3136 | 3.5* | – | – | 0.067 | 3.497±1.117 | G | WW |
| 2B | 134.4±11.0 | XwPt-0694 | 5.3*** | 0.068 | 2.551±0.545 | – | – | G | WL |
| 4A | 30.6±4.34 | Xgwm610 | 12.0*** | 0.141 | –3.799±0.829 | 0.166 | –5.556±1.034 | L | NS |
| 4B | 46.8±15.3 | Xgwm513 | 4. 9** | 0.041 | –1.965±0.884 | 0.066 | –3.193±1.240 | L | NS |
| 5A | 88.9±14.4 | Xwmc415a | 3.3* | – | – | 0.070 | 2.893±2.434 | G | WW |
| 5B | 55.0±10.5 | Xgwm371 | 7.0*** | 0.093 | 2.990±0.514 | – | – | G | WL |
| 6A | 92.3±15.8 | Xgwm786 | 4.6* | 0.080 | 2.806±0.984 | 0.018 | 1.041±1.574 | G | * |
| 6B1 | 81.2±19.0 | Xbarc136 | 9.5*** | 0.109 | –0.351±0.301 | 0.168 | –2.596±2.211 | L | – |
| 6B2 | 126.9±17.5 | XwPt-8554 | 9.5*** | 0.109 | 2.087±2.112 | 0.168 | 1.887±1.675 | G | – |
| 7A | 69.7±17.9 | XwPt-9555 | 4.9* | 0.045 | 2.051±0.915 | 0.051 | 2.894±1.309 | G | NS |
| 7B | 19.8±8.8 | Xgwm263 | 5.8*** | 0.118 | 3.403±0.605 | – | – | G | WL |
| Kernel number per spike | |||||||||
| 1B | 44.0±7.8 | Xgwm1028 | 7.9*** | 0.064 | 4.085±1.093 | 0.066 | 4.371±1.180 | G | NS |
| 2B | 56.3±1.7 | Xgwm410 | 14.4*** | 0.130 | –5.960±0.988 | 0.130 | –6.320±1.100 | L | NS |
| 3A | 108.2±2.1 | XwPt-1888 | 9.0*** | 0.047 | –3.435±0.979 | 0.099 | –5.445±1.023 | L | NS |
| 3B | 158.2±11.5 | XwPt-2491 | 5.4** | 0.054 | –3.654±1.295 | 0.041 | –3.311±1.402 | L | NS |
| 4A | 35.0±12.4 | Xgwm610 | 4.3* | 0.041 | –3.026±1.461 | 0.022 | –2.287±1.354 | L | NS |
| 4B | 5.4±5.1 | Xgwm930 | 7.9*** | 0.118 | –5.575±0.467 | 0.030 | –2.676±1.308 | L | * |
| 5A | 124.0±20.5 | Xgwm126 | 7.4*** | 0.049 | –3.593±1.076 | 0.083 | –4.949±1.136 | L | NS |
| 6A | 72.8±11.9 | Xgwm1150 | 5.1** | 0.023 | –2.150±1.324 | 0.076 | –4.744±1.067 | L | NS |
| 6B | 80.3±15.6 | Xbarc136 | 4.8** | 0.039 | 2.480±2.182 | 0.035 | 2.291±2.422 | G | NS |
*, **, ***, and NS indicate significance at P ≤0.05, 0.01, 0.001 or non-significant effect, respectively.
LOD scores that were found to be significant when comparing hypotheses H1 (there is a QTL in the chromosome) and H0 (no effect of the chromosome on the trait), using the 1000 permutation test (Churchill and Doerge, 1994).
Proportion of explained variance of the trait.
The adaptive effect of an allele calculated as one-half of the mean difference between homozygotes with and without the allele.
Favourable parental allele contributing to higher values; Langdon (L) and G18-16 (G).
Genotype×environment interaction, tested by comparing the model with a submodel in which both environments had an equal effect, using the 1000 permutation test. This test is not applicable when a QTL is specific for only one environment or in the case of the two-QTL model.
Thousand kernel weight
A total of 12 significant QTLs were associated with TKW, with LOD scores ranging between 3.3 and 12.0, explaining 1.8–16.6% of the variation (Tables 1, 2). Higher TKW was conferred by the G18-16 allele at eight loci (2A, 2B, 5A, 5B, 6A, 6B2, 7A, and 7B) and by the LDN allele at four loci (1B, 4A, 4B, and 6B1). Seven QTLs showed significant G×E interaction: one of them (6A) exhibited a similar trend across environments, three QTLs (2B, 5B, and 7B) were found only in the WL environment and the other three QTLs (1B, 2A, and 5A) only in the WW environment.
Kernel number per spike
A total of nine significant QTLs were associated with KNSP, with LOD scores ranging between 4.3 and 14.4, explaining 2.2–13.0% of the variation (Tables 1, 2). Higher KNSP was conferred by the G18-16 allele at two loci (1B and 6B) and by the LDN allele at seven loci (2B, 3A, 3B, 4A, 4B, 5A, and 6A). One QTL (4B) showed significant G×E interaction, with a similar trend across environments.
Discussion
Early agriculturalists are thought to have been highly conscious of their initial selected stocks for early attempts at plant manipulation (Abbo et al., 2009, 2011a, b). Subsequent to the initial domestication event, selection by man has continued to change crop plants, which by definition partly involves correlated responses to selection, some of which may have been unintentional (Ladizinsky, 1998b). Thus, crop species have gone through dramatic morphological and physiological changes ever since domestication began. The accumulated genetic change achieved by farmers over the last 10 000 years is estimated to be far greater than that achieved by breeders in the last 100 years (Simmonds, 1979). Genetic analysis of the factors controlling these domestication syndrome traits is not only important from an evolutionary perspective; they also have broad economic and societal consequences for the simple reason that the yield potential of our staple crops is a function of their respective evolutionary histories (e.g. Abbo et al., 2003, 2009).
Rachis brittleness factor
The wild Near Eastern cereals (barley, wheat, and rye) are characterized by a brittle inflorescence, which leads to shattering of the dispersal units (i.e. spikelets) upon maturity (Fig. 1). This seed dispersal mechanism is essential under natural conditions, where the arrow-like morphology of the spikelet mediates their penetration though surface litter into the soil (Elbaum et al., 2007). In this context, the disarticulating (brittle) rachis (Br) character is of evolutionary significance because of its adaptive value as an effective seed dispersal mechanism. Under domestication, however, Br poses considerable difficulty for harvest operations, as it leaves only a few spikelets on the rachis to collect. Thus, a non-brittle rachis spike is thought to have been the first symptom of wheat domestication. Spikelet shattering in wheat has been reported to be a dominant character (Love and Craig, 1919). In the present study, Br was found to be controlled by a single gene, which mapped to the long arm of chromosome 2A (Fig. 4). Most previous genetic and cytogenetic analyses of rachis brittleness have shown that Br is controlled by loci on homoeologous group 3 of both hexaploid and tetraploid wheat. The wild-type seed dispersal mode was found to be governed by two dominant genes, Br-A2 and Br-A3, located on the short arm of chromosome 3A and 3B, respectively (Watanabe and Ikebata, 2000; Nalam et al., 2006). In the present study, however, location of a Br locus on chromosome 2A was in accordance with a previous report based on segregation in an F2:3 mapping population derived from a cross of the same durum wheat cultivar (LDN) with a different wild emmer accession (H52) (Peng et al., 2003). Furthermore, Dvořák et al. (2006) reported a brittle rachis phenotype of bread wheat (cv. Chinese Spring) with added and substituted wild T. monococcum chromosome 4Am, suggesting that a gene affecting spike rachis disarticulation is located on this chromosome. It can be hypothesized that the Br loci on chromosome 2A reported in the current and previous (Peng et al., 2003) studies, as well as other reported loci (Jantasuriyarat et al., 2004; Dvořák et al., 2006), are involved in regulation of the known Br genes in homoeologous group 3. On the other hand, since in two independent studies using different durum wheat×wild emmer populations, the Br gene was located to the same chromosome 2A interval, the existence of an independent major Br gene on chromosome 2 is suggested. A detailed analysis of the Br gene complex is under way.
The incipient Neolithic farmers must have used seeds of wild emmer in their first sowing attempts. If harvesting was performed after the ears began to shatter, non-brittle rachis mutants would have been rapidly adopted. However, if the early farmers harvested before spikelet dispersal to avoid loss of yield, a non-brittle spike mutant would not necessarily have been advantageous (Tanno and Willcox, 2006). Under this assumption, the emergence of non-shattering (domesticated) spike types in the cultivated fields is expected to have occurred at a later stage, depending on the harvest methods and/or intentional or unintentional selection exerted by the ancient farmers (Hillman and Davis, 1999; Zohary, 2004). Indeed, although a br mutation may establish itself within a few decades of repeated sowing and harvest of wild forms (Ladizinsky, 1998b), harvest of partially ripe spikes, or gathering disarticulated dispersal units (i.e. spikelets) from the ground, would have delayed the process significantly (Kislev et al., 2004). It is worth noting that spontaneous non-brittle mutants (partially or completely non-brittle types) have been recurrently found in several natural wild emmer populations in Israel (Kamn, 1974; ZP, personal observation). Thus, the possibility that the early farmers adopted such types for their first manipulation attempts and enjoyed the advent of non-brittle spike germplasm in their early manipulation attempts should not be overlooked (Abbo et al., 2011a).
Spike threshability
The emergence of the free-threshing character represents an advanced step in Triticum evolution under domestication. Early farmers most probably selected for a low degree of glume tenacity and free-threshing habit, which improved the efficiency of post-harvest processing. Free-threshing wheat has thinner glumes and chaff that allow easy release of the naked kernels. A major regulatory gene (Q-factor), located on the long arm of chromosome 5A (Sears, 1954; Faris et al., 2003; Simons et al., 2006), governs the free-threshing character with pleiotropic effects on other important domestication characters such as rachis fragility, glume shape and tenacity, and spike morphology (reviewed by Simons et al., 2006). Two threshability genes have been reported on group 2 chromosomes: the Tg gene controlling glume toughness (Sears, 1954; Simonetti et al., 1999; Jantasuriyarat et al., 2004) and a non-orthologous gene, sog, controlling soft glume (Sood et al., 2009). In the current study, threshability (i.e. the proportion of threshed grain from a spike; SPHI) was conferred by six QTLs (2B, 4A1, 4A2, 4B, 5A, and 7B), with the domesticated allele being favourable in four cases (4A1, 4A2, 4B, and 5A). The QTL on chromosome 2B is in accordance with previous studies (Simonetti et al., 1999; Jantasuriyarat et al., 2004) and was designated Tg2. Interestingly, the wild allele (derived from the wild parent G18-16) conferred higher threshability in this QTL (Table 2). The QTL on chromosome 5A corresponds to the known Q gene (Simonetti et al., 1999; Jantasuriyarat et al., 2004; Simons et al., 2006). An additional four QTLs affecting threshability, which were not previously reported, were detected on chromosomes 4A (two QTLs), 4B, and 7B. Simonetti et al. (1999) and Jantasuriyarat et al. (2004) reported a QTL affecting threshability on chromosome 6A which was not found in the present study. These results suggest that in addition to the major tough glume genes on chromosomes 2 (Tg and sog) and 5A (Q-gene), additional genes are involved in determining emmer wheat threshability, as reflected by the newly detected QTLs on chromosomes 4A, 4B, and 7B.
Yield components
Some students of plant domestication refer to grain size, which is a major yield component, as a marker of domestication, and take the gradual change over millennia as an indication of a gradual, slowly evolving domestication process. Willcox (2004) pointed out the difficulty in progressing with selection for larger grain size and, similarly, Purugganan and Fuller (2011, p. 181) suggested that ancient farmers were unable to distinguish Br from br plants growing in their fields. Therefore, combining the documented changes in grain weight and the proportion of Br versus br in archeobotanical remains led several authors (e.g. Allaby, 2010; Purugganan and Fuller, 2011, and references therein) to suggest that plant domestication was a millennia-long, protracted process. Here, on the other hand, the changes in agronomic traits (e.g. kernel number and size) are viewed as a reflection of plant evolution under domestication processes rather than as evidence for the duration of the presumed domestication process. This is because under any sowing and reaping regime (i.e. under domestication), such genes for quantitatively inherited agronomic traits are, by definition, subjected to selection.
QTLs conferring yield components—KNSP and TKW—were identified in the current study on 10 chromosomes of tetraploid wheat (Table 2). KNSP was conferred by nine QTLs (1B, 2B, 3A, 3B, 4A, 4B, 5A, 6A, and 6B), with the wild allele being favourable in two cases (Tables 1, 2). Nine QTL conferring KNSP were mapped in previous studies (1A, 1B, 2A, 2B, 3B, 4B, 6A, 7A, and 7B) (Huang et al., 2004; Marza et al., 2006), with five of them corresponding to the present results. Kernel weight, measured as TKW, is positively associated with agronomic yield and is phenotypically a very stable yield component, with relatively high heritability values. In the current study, the heritability estimate value of TKW was 0.54. Furthermore, larger kernel weight benefits seedling vigour, and may consequently promote yield increase. TKW was conferred by 12 QTLs (1B, 2A, 2B, 4A, 4B, 5A, 5B, 6A, 6B1, 6B2, 7A, and 7B; Tables 1, 2). Investigation of different mapping populations led to the discovery of numerous QTLs associated with TKW, which were located on all 14 chromosomes of tetraploid wheat (Ammiraju et al., 2001; Börner et al., 2002; Groos et al., 2003; Peng et al., 2003; Elouafi and Nachit, 2004; Quarrie et al., 2005; Narasimhamoorthy et al., 2006; Sun et al., 2009). TKW is under complex polygenic control, and alleles having both positive and negative effects on this trait have been mapped using chromosome substitution [LDN(DIC)] lines (Elias et al., 1996). Furthermore, grain weight and size also depend on environmental constraints (e.g. water availability and temperatures during grain filling). Thus, the large number of QTLs affecting TKW found in the current study is reasonable. Seven out of the 12 detected QTLs were found to be sensitive to environment, as they were detected under the WW (1B, 2A, and 5A) or WL (2B, 5B, and 7B) environment or showed significant G×E interaction (6A) (Table 2).
KNSP and TKW are established sequentially during plant development. The potential number of grains is determined at early stages of growth, whereas grain weight is fixed during grain filling. Phenotypically, these two traits showed a positive association (Fig. 3). In three genomic regions, QTLs conferring TKW were co-localized with QTLs conferring KNSP (1B, 5A, and 7B; Fig. 4), falling short of the 0.05 significant level (Table 3) (Larsen and Marx, 1985; Paterson et al., 1995).
Table 3.
Genotypic association among grain yield (GY) and domestication-related traits: thousand kernel weight (TKW), kernel number per spike (KNPS) and spike harvest index (SPHI).
Values indicate the number of corresponding QTLs with >50% overlap between their confidence intervals, out of the total number of QTLs detected for each trait (indicated in parentheses).
| TKW (12) | KNSP (9) | SPHI (6) | |
| GY (6) | 3* | 1 | 4** |
| TKW (12) | 3 (P=0.06) | 4** | |
| KNSP (9) | 1 |
* and ** indicate significance at P ≤0.05 and 0.01, respectively.
Grain yield
Securing grain yield (GY) has been a major priority for farmers throughout the history of agriculture. While yield stability is thought to have been more important than yield maximization in ancient times, maximal yield is a prime goal of modern farming systems (Abbo et al., 2010). In a previous study of the same RIL population, GY was conferred by six QTLs (2B, 2B, 4A, 4B, 5A, and 7B) (Peleg et al., 2009b). Most GY QTLs were derived from the domesticated allele (LDN) which is the expected outcome of strong selection pressure for GY per se (Ladizinsky, 1998a). In three genomic regions, QTLs conferring higher GY were co-localized with QTLs conferring improved TKW (2B, 4B, and 7B; Fig. 4; Table 3). The likelihood that such an association would occur by chance is P=0.04 (Larsen and Marx, 1985; Paterson et al., 1995). This was further supported by a positive phenotypic correlation between GY and TKW (r=0.60, P=0.0001, and r=0.53, P=0.0003 for the WL and WW treatment, respectively; Fig. 3). Four out of the six QTLs conferring SPHI were significantly co-localized with GY QTLs (Fig. 4; Table 3). These findings were further supported by strong positive correlations between these traits (r=0.89, P=0.0001, and r=0.78, P=0.0001 for the WL and WW treatment, respectively). Thus, although the time frame for the selection in favour of these traits by farmers cannot be identified, the combination of selection for free-threshing habit and increased seed size was also accompanied by improved GY.
Concluding remarks
Ever since its domestication, wheat has been one of the world's major food sources, and thus a better understanding of the genetic and physiological bases of its domestication is essential to further improving wheat production. In the current study, a cross between domesticated durum wheat and its direct progenitor wild emmer wheat was used for genetic dissection of domestication-related traits. The number of genes and mutations required for a critical domestication transition has been addressed in many studies. It has been suggested that in many cases, a single gene played a pivotal role in moving the population over the trajectory of a key domestication transition (e.g. Br, Q, Tg, and sog).
Recently, Fuller et al. (2010) have postulated that under the ancient ‘pre-domestication cultivation’ regime, the increased proportion of non-shattering domesticated spike (Br types) within the original ‘cultivated’ wild-type populations necessitated increased investment of human labour (see Fig. 1 in Fuller et al., 2010). In their model, the extra labour required to process the non-brittle types imposed selection pressure favouring the brittle spike (br) allele. Hence, a ‘slow rate of evolution during plant domestication’ was postulated based on allochronic data documenting the ratio of Br:br spikes in relevant Near Eastern archeobotanical remains (Purugganan and Fuller, 2011). Fuller et al. (2010) also suggested that the payback for the additional labour, caused by the slow and unintentional accumulation of the non-brittle genotypes in the cultivated fields, increased the reliability of the harvest, resulting in higher yield. As one possible explanation for the documented slow evolution of ‘fully domesticated’ cereal populations, Fuller et al. (2010) suggested that individual farmers (within the same communities) may have used different harvest methods; while some households used sickles which may have selected in favour of Br types, other households may have used beating sticks which selected against the Br allele (therein). Is it possible that farmers who kept using beating sticks were trying to avoid Fuller et al.’s (2010) extra labour ‘trap’?
The experimental wheat population used here can serve to test some of the assumptions underlying Fuller et al.’s (2010) model concerning the extra labour required to release the grains from Br wheat spikes. Although the time required to thresh the harvested materials was not routinely estimated, hands-on experience with threshing thousands of RILs, as well as domesticated and wild samples, suggests that threshing of wild-type dispersal units is more difficult and time-consuming than that required for non-brittle (non-free-threshing) spikes. Moreover, it is claimed that the highly efficient harvest of non-shattering spikes is likely to have facilitated rapid adoption of the Br mutant (upon its emergence) during the early cultivation attempts. The existence of exotic forms of domesticated hulled wheat [e.g. spelt wheat, T. aestivum L. ssp. spelta (L.) Thell.], whereas brittle spike landraces are unknown to us, provides indirect evidence for the central role of Br in wheat domestication. The rich stands of wild cereals in the oak–pistachio woodland belt of the Near Eastern Fertile Crescent enabled the Neolithic communities to continue with some of their foraging habits. Therefore, it is possible that cultivation of domesticated plants and gathering from their wild populations co-existed for a long time, not necessarily to replenish crops that failed due to drought or other reasons, as suggested by Tanno and Willcox (2006) and Willcox et al. (2008). Gathering of numerous plant species still exists today in many societies, as documented in Turkey by Ertuğ (2000). Such continued gathering from the wild may provide an alternative explanation to Tanno and Willcox's (2006) and Puragganan and Fuller's (2011) archeobotanical data, thereby putting the protracted domestication model into question. In addition, genetic data from the current study show that the Br gene does not overlap with QTLs conferring threshability (SPHI; Fig. 4). Indeed, among the RIL population used here, the plants showing the brittle rachis phenotype did not differ in SPHI, grain size, or yield from the non-shattering plants (Table 4), indicating the independence of the respective loci. The independent segregation of threshability and brittleness factors does not preclude the possibility that easily harvestable and threshable Br types evolved quite rapidly.
Table 4.
Contrasts between the group of recombinant inbred lines (RILs) carrying the Br wild allele (brittle rachis characteristics) and the group of RILs carrying the br allele (domesticated) under well-watered and water-limited environmental conditions.
| Trait | Environment | Mean value of Br group | Mean value of br group | P-value |
| GY | Well-watered | 57.63 | 56.74 | 0.87 |
| Water-limited | 27.58 | 28.87 | 0.24 | |
| SPHI | Well-watered | 0.47 | 0.47 | 0.94 |
| Water-limited | 0.37 | 0.38 | 0.51 | |
| TKW | Well-watered | 41.87 | 42.55 | 0.53 |
| Water-limited | 29.92 | 29.84 | 0.93 | |
| KNSP | Well-watered | 38.84 | 39.37 | 0.34 |
| Water-limited | 30.49 | 31.33 | 0.53 |
GY, grain yield; TKW, thousand kernel weight; KNSP, kernel number per spike; SPHI, spike harvest index.
Allelic differences identified via the analysis of hybrid progeny from a cross between a domesticated and a wild accession represent the total changes which have accumulated since domestication began and during subsequent selection under domestication. It is hypothesized that wheat domestication was a relatively short episode during which the non-brittle spike emerged, whereas the subsequent emergence of free threshing and changes in agronomic traits occurred throughout a prolonged (and still ongoing) process of evolution under domestication. Detailed characterization of brittle rachis and other traits related to wheat domestication and evolution (now in progress in our laboratory) will provide a critical test of the above hypotheses concerning the domestication of Near Eastern cereals.
Acknowledgments
This study was supported by The Israel Science Foundation grant #1089/04. ZP is indebted to the Israel Council for a higher education postdoctoral fellowship award. The authors thank A. Avneri, Y. Shkolnik. and R. Ben-David for their technical assistance.
References
- Abbo S, Berger J, Turner NC. Evolution of cultivated chickpea: four bottlenecks limit diversity and constrain adaptation. Functional Plant Biology. 2003;30:1081–1087. doi: 10.1071/FP03084. [DOI] [PubMed] [Google Scholar]
- Abbo S, Gopher A, Peleg Z, Saranga Y, Fahima T, Salamini FS, Lev-Yadun S. The ripples of ‘The Big (agricultural) Bang’: the spread of early wheat cultivation. Genome. 2006;49:861–863. doi: 10.1139/g06-049. [DOI] [PubMed] [Google Scholar]
- Abbo S, Lev-Yadun S, Gopher A. Yield stability: an agronomic perspective on the origin of Near Eastern agriculture. Vegetation History and Archaeobotany. 2010;19:143–150. [Google Scholar]
- Abbo S, Lev-Yadun S, Gopher A. Origin of Near Eastern plant domestication: homage to Claude Levi-Strauss and ‘La Pensée Sauvage’. Genetic Resources and Crop Evolution. 2011a;58:175–179. [Google Scholar]
- Abbo S, Rachamim E, Zehavi Y, Zezak I, Lev-Yadun S, Gopher A. Experimental growing of wild pea in Israel and its bearing on Near Eastern plant domestication. Annals of Botany. 2011b;107:1399–1404. doi: 10.1093/aob/mcr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbo S, Saranga Y, Peleg Z, Kerem Z, Lev-Yadun S, Gopher A. Reconsidering domestication of legumes versus cereals in the ancient Near East. Quarterly Review of Biology. 2009;84:29–50. doi: 10.1086/596462. [DOI] [PubMed] [Google Scholar]
- Allaby R. Integrating the processes in the evolutionary system of domestication. Journal of Experimental Botany. 2010;61:935–944. doi: 10.1093/jxb/erp382. [DOI] [PubMed] [Google Scholar]
- Ammiraju JSS, Dholakia BB, Santra DK, Singh H, Lagu MD, Tamhankar SA, Dhaliwal HS, Rao VS, Gupta VS, Ranjekar PK. Identification of inter simple sequence repeat (ISSR) markers associated with seed size in wheat. Theoretical and Applied Genetics. 2001;102:726–732. [Google Scholar]
- Börner A, Schumann E, Fürste A, Cöster H, Leithold B, Röder M, Weber W. Mapping of quantitative trait loci determining agronomic important characters in hexaploid wheat (Triticum aestivum L.) Theoretical and Applied Genetics. 2002;105:921–936. doi: 10.1007/s00122-002-0994-1. [DOI] [PubMed] [Google Scholar]
- Childe VG. Man makes himself. New York and Toronto, The New American Library of World Literature. 1951 [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doganlar S, Frary A, Daunay M-C, Lester RN, Tanksley SD. Conservation of gene function in the Solanaceae as revealed by comparative mapping of domestication traits in eggplant. Genetics. 2002;161:1713–1726. doi: 10.1093/genetics/161.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořák J, Akhunov ED, Akhunov AR, Deal KR, Luo M-C Molecular characterization of a diagnostic DNA marker for domesticated tetraploid wheat provides evidence for gene flow from wild tetraploid wheat to hexaploid wheat. Molecular Biology and Evolotion. 2006;23:1386–1396. doi: 10.1093/molbev/msl004. [DOI] [PubMed] [Google Scholar]
- Elbaum R, Zaltzman L, Burgert I, Fratzl P. The role of wheat awns in the seed dispersal unit. Science. 2007;316:884–886. doi: 10.1126/science.1140097. [DOI] [PubMed] [Google Scholar]
- Elias EM, Steiger DK, Cantrell RG. Evaluation of lines derived from wild emmer chromosome substitutions. II. Agronomic traits. Crop Science. 1996;36:228–233. [Google Scholar]
- Elouafi I, Nachit MM. A genetic linkage map of the Durum× Triticum dicoccoides backcross population based on SSRs and AFLP markers, and QTL analysis for milling traits. Theoretical and Applied Genetics. 2004;108:401–413. doi: 10.1007/s00122-003-1440-8. [DOI] [PubMed] [Google Scholar]
- Ertuğ F. An ethnobotanical study in Central Anatolia (Turkey) Economic Botany. 2000;54:155–182. [Google Scholar]
- Faris JD, Fellers JP, Brooks SA, Gill BS. A bacterial artificial chromosome contig spanning the major domestication locus Q in wheat and identification of a candidate gene. Genetics. 2003;164:311–321. doi: 10.1093/genetics/164.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DQ, Allaby RG, Stevens C. Domestication as innovation: the entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeology. 2010;42:13–28. [Google Scholar]
- Groos C, Robert N, Bervas E, Charmet G. Genetic analysis of grain protein-content, grain yield and thousand-kernel weight in bread wheat. Theoretical and Applied Genetics. 2003;106:1032–1040. doi: 10.1007/s00122-002-1111-1. [DOI] [PubMed] [Google Scholar]
- Hammer K. Das Domestikationssyndrom. Kulturpflanze. 1984;32:11–34. [Google Scholar]
- Harlan JR, de Wet MJ, Price EG. Comparative evolution of cereals. Evolution. 1973;27:311–325. doi: 10.1111/j.1558-5646.1973.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Hillman G, Davis S. Domestication rate in wild wheats and barley under primitive cultivation. In: Anderson P, editor. Prehistory of agriculture: new experimental and ethnographic approaches. Los Angeles, CA: Institute of Archaeology University of California; 1999. pp. 70–102. [Google Scholar]
- Huang XQ, Kempf H, Ganal MW, Röder MS. Advanced backcross QTL analysis in progenies derived from a cross between a German elite winter wheat variety and a synthetic wheat (Triticum aestivum L.) Theoretical and Applied Genetics. 2004;109:933–943. doi: 10.1007/s00122-004-1708-7. [DOI] [PubMed] [Google Scholar]
- Jantasuriyarat C, Vales MI, Watson CJW, Riera-Lizarazu O. Identification and mapping of genetic loci affecting the free-threshing habit and spike compactness in wheat (Triticum aestivum L.) Theoretical and Applied Genetics. 2004;108:261–273. doi: 10.1007/s00122-003-1432-8. [DOI] [PubMed] [Google Scholar]
- Kamn A. Non-brittle types in a wild population of Triticum dicoccoides körn. in Israel. Israel Journal of Botany. 1974;23:43–58. [Google Scholar]
- Kislev ME, Weiss E, Hartmann A. Impetus for sowing and the beginning of agriculture: ground collecting of wild cereals. Proceedings of the National Academy of Sciences, USA. 2004;101:2692–2695. doi: 10.1073/pnas.0308739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinange EMK, Singh SP, Gepts P. Genetic control of the domestication syndrome in common bean. Crop Science. 1996;36:1037–1045. [Google Scholar]
- Ladizinsky G. Plant evolution under domestication. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998a. [Google Scholar]
- Ladizinsky G. How many tough-rachis mutants gave rise to domesticated barley? Genetic Resources and Crop Evolution. 1998b;45:411–414. [Google Scholar]
- Larsen RJ, Marx ML. An introduction to probability and its applications. Englewood Cliffs, NJ: Prentice Hall Inc; 1985. [Google Scholar]
- Lee S-J, Oh C-S, Suh J-P, McCouch SR, Ahn S-N. Identification of QTLs for domestication-related and agronomic traits in an Oryza sativa×O. rufipogon BC1F7 population. Plant Breeding. 2005;124:209–219. [Google Scholar]
- Lev-Yadun S, Gopher A, Abbo S. The cradle of agriculture. Science. 2000;288:1602–1603. doi: 10.1126/science.288.5471.1602. [DOI] [PubMed] [Google Scholar]
- Liu B, Fujita T, Yan Z-H, Sakamoto S, Xu D, Abe J. QTL mapping of domestication-related traits in soybean (Glycine max) Annals of Botany. 2007;100:1027–1038. doi: 10.1093/aob/mcm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love HH, Craig WT. The synthetic production of wild wheat forms. Journal of Heredity. 1919;10:51–64. [Google Scholar]
- Marza F, Bai G, Carver B, Zhou W. Quantitative trait loci for yield and related traits in the wheat population Ning7840×Clark. Theoretical and Applied Genetics. 2006;112:688–698. doi: 10.1007/s00122-005-0172-3. [DOI] [PubMed] [Google Scholar]
- Mester DI, Ronin YI, Hu Y, Peng J, Nevo E, Korol AB. Efficient multipoint mapping: making use of dominant repulsion-phase markers. Theoretical and Applied Genetics. 2003;107:1102–1112. doi: 10.1007/s00122-003-1305-1. [DOI] [PubMed] [Google Scholar]
- Nalam V, Vales M, Watson C, Kianian S, Riera-Lizarazu O. Map-based analysis of genes affecting the brittle rachis character in tetraploid wheat (Triticum turgidum L.) Theoretical and Applied Genetics. 2006;112:373–381. doi: 10.1007/s00122-005-0140-y. [DOI] [PubMed] [Google Scholar]
- Narasimhamoorthy B, Gill BS, Fritz AK, Nelson JC, Brown-Guedira GL. Advanced backcross QTL analysis of a hard winter wheat×synthetic wheat population. Theoretical and Applied Genetics. 2006;112:787–796. doi: 10.1007/s00122-005-0159-0. [DOI] [PubMed] [Google Scholar]
- Paterson AH, Lin Y-R, Li Z, Schertz KF, Doebley JF, Pinson SRM, Liu S-C, Stansel JW, Irvine JE. Convergent domestication of cereal cops by independent mutations at corresponding genetic loci. Science. 1995;269:1714–1718. doi: 10.1126/science.269.5231.1714. [DOI] [PubMed] [Google Scholar]
- Peleg Z, Cakmak I, Ozturk L, Yazici A, Jun Y, Budak H, Korol A, Fahima T, Saranga Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat×wild emmer wheat RIL population. Theoretical and Applied Genetics. 2009a;119:353–369. doi: 10.1007/s00122-009-1044-z. [DOI] [PubMed] [Google Scholar]
- Peleg Z, Fahima T, Krugman T, Abbo S, Yakir D, Korol AB, Saranga Y. Genomic dissection of drought resistance in durum wheat×wild emmer wheat recombinant inbred line population. Plant, Cell and Environment. 2009b;32:758–779. doi: 10.1111/j.1365-3040.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- Peleg Z, Saranga Y, Suprunova T, Ronin Y, Röder M, Kilian A, Korol A, Fahima T. High-density genetic map of durum wheat×wild emmer wheat based on SSR and DArT markers. Theoretical and Applied Genetics. 2008;117:103–115. doi: 10.1007/s00122-008-0756-9. [DOI] [PubMed] [Google Scholar]
- Peng J, Ronin Y, Fahima T, Röder MS, Li Y, Nevo E, Korol A. Domestication quantitative trait loci in Triticum dicoccoides, the progenitor of wheat. Proceedings of the National Academy of Sciences, USA. 2003;100:2489–2494. doi: 10.1073/pnas.252763199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncet V, Lamy F, Devos KM, Gale MD, Sarr A, Robert T. Genetic control of domestication traits in pearl millet (Pennisetum glaucum L., Poaceae) Theoretical and Applied Genetics. 2000;100:147–159. doi: 10.1007/s00122-002-0889-1. [DOI] [PubMed] [Google Scholar]
- Purugganan MD, Fuller DQ. Archaeological data reveal slow rates of evolution during plant domestication. Evolution. 2011;65:171–183. doi: 10.1111/j.1558-5646.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- Quarrie SA, Steed A, Calestani C, et al. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring×SQ1 and its use to compare QTLs for grain yield across a range of environments. Theoretical and Applied Genetics. 2005;110:865–880. doi: 10.1007/s00122-004-1902-7. [DOI] [PubMed] [Google Scholar]
- Salamini F, Ozkan H, Brandolini A, Schafer-Pregl R, Martin W. Genetics and geography of wild cereal domestication in the Near East. Nature Reviews Genetics. 2002;3:429–441. doi: 10.1038/nrg817. [DOI] [PubMed] [Google Scholar]
- Sears ER. The aneuploids of common wheat. Missouri Agricultural Experimental Station Research Bulletin. 1954;572:1–59. [Google Scholar]
- Simmonds NW. Principles of crop improvement. London: Longman; 1979. [Google Scholar]
- Simonetti MC, Bellomo MP, Laghetti G, Perrino P, Simeone R, Blanco A. Quantitative trait loci influencing free-threshing habit in tetraploid wheats. Genetic Resources and Crop Evolution. 1999;46:267–271. [Google Scholar]
- Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai Y-S, Gill BS, Faris JD. Molecular characterization of the major wheat domestication gene Q. Genetics. 2006;172:547–555. doi: 10.1534/genetics.105.044727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Kuraparthy V, Bai G, Gill B. The major threshability genes soft glume (sog) and tenacious glume (Tg), of diploid and polyploid wheat, trace their origin to independent mutations at non-orthologous loci. Theoretical and Applied Genetics. 2009;119:341–351. doi: 10.1007/s00122-009-1043-0. [DOI] [PubMed] [Google Scholar]
- Sun X-Y, Wu K, Zhao Y, Kong F-M, Han G-Z, Jiang H-M, Huang X-J, Li R-J, Wang H-G, Li S-S. QTL analysis of kernel shape and weight using recombinant inbred lines in wheat. Euphytica. 2009;165:615–624. [Google Scholar]
- Tanno KI, Willcox G. How fast was wild wheat domesticated? Science. 2006;311:1886. doi: 10.1126/science.1124635. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ikebata N. The effects of homoeologous group 3 chromosomes on grain colour dependent seed dormancy and brittle rachis in tetraploid wheat. Euphytica. 2000;115:215–220. [Google Scholar]
- White S, Doebley J. Of genes and genomes and the origin of maize. Trends in Genetics. 1998;14:327–332. doi: 10.1016/s0168-9525(98)01524-8. [DOI] [PubMed] [Google Scholar]
- Willcox G. Measuring grain size and identifying Near Eastern cereal domestication: evidence from the Euphrates valley. Journal of Archaeological Science. 2004;31:145–150. [Google Scholar]
- Willcox G, Fornite S, Herveux L. Early Holocene cultivation before domestication in northern Syria. Vegetation History and Archaeobotany. 2008;17:313–325. [Google Scholar]
- Wills DM, Burke JM. Quantitative trait locus analysis of the early domestication of sunflower. Genetics. 2007;176:2589–2599. doi: 10.1534/genetics.107.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary D. Unconscious selection and the evolution of domesticated plants. Economic Botany. 2004;58:5–10. [Google Scholar]