Abstract
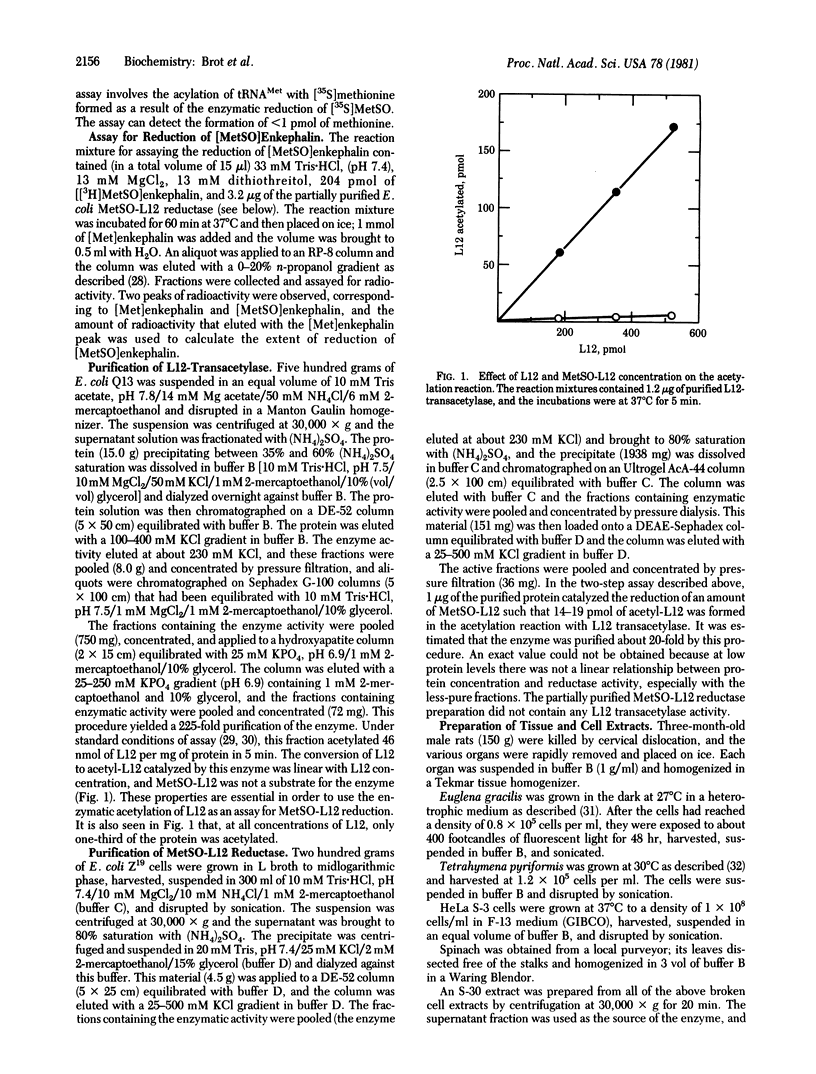
An enzyme that catalyzes the reduction of methionine sulfoxide residues in ribosomal protein L12 has been partially purified from Escherichia coli extracts. Methionine sulfoxide present in oxidize [Met]enkephalin is also reduced by the purified enzyme. The enzyme is different from a previously reported E. coli enzyme that catalyzes the reduction of methionine sulfoxide to methionine [Ejiri, S. I., Weissbach, H. & Brot, N. (1980) Anal. Biochem. 102, 393--398]. Extracts of rat tissues, Euglena gracilis, Tetrahymena pyriformis, HeLa cells, and spinach also can catalyze the reduction of methionine sulfoxide residues in protein.
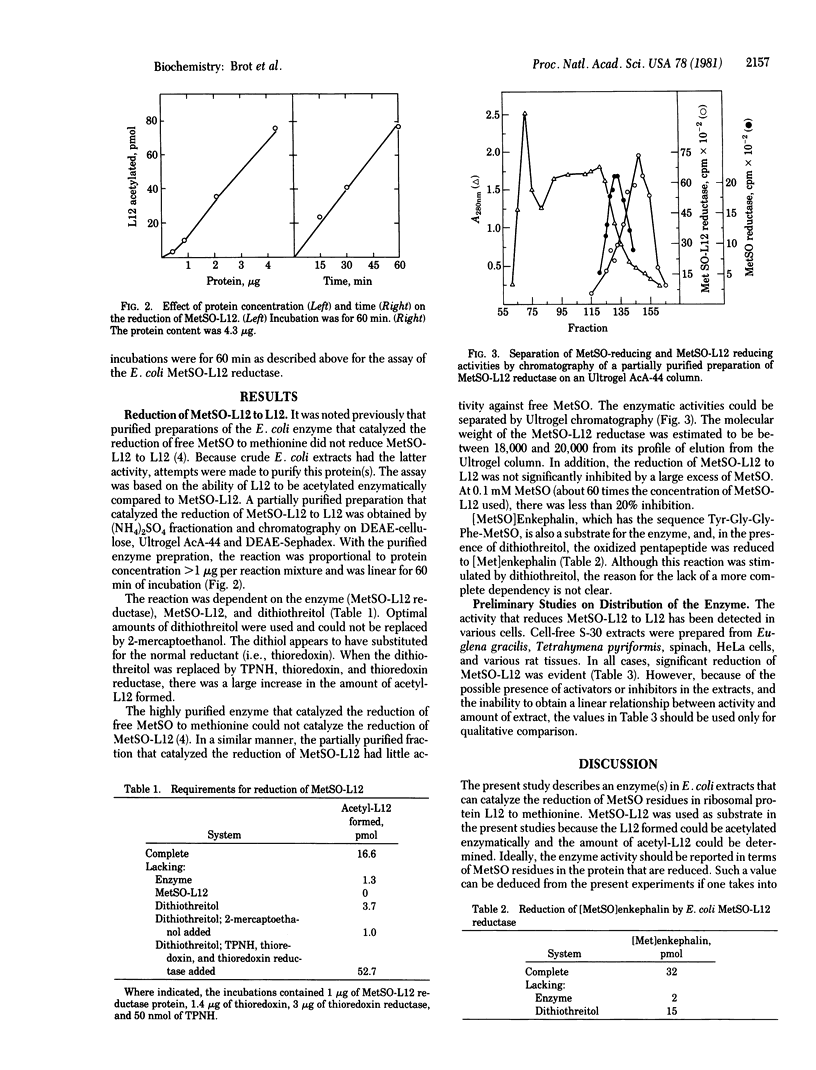
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brot N., Marcel R., Cupp L., Weissbach H. The enzymatic acetylation of ribosomal bound protein L 12 . Arch Biochem Biophys. 1973 Apr;155(2):475–477. doi: 10.1016/0003-9861(73)90140-9. [DOI] [PubMed] [Google Scholar]
- Brot N., Marcel R., Yamasaki E., Weissbach H. Further studies on the role of 50 S ribosomal proteins in protein synthesis. J Biol Chem. 1973 Oct 25;248(20):6952–6956. [PubMed] [Google Scholar]
- Brot N., Weisbach H. The enzymatic acetylation of E. coli ribosomal protein L 12 . Biochem Biophys Res Commun. 1972 Nov 1;49(3):673–679. doi: 10.1016/0006-291x(72)90464-0. [DOI] [PubMed] [Google Scholar]
- Caldwell P., Luk D. C., Weissbach H., Brot N. Oxidation of the methionine residues of Escherichia coli ribosomal protein L12 decreases the protein's biological activity. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5349–5352. doi: 10.1073/pnas.75.11.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. M., Weissbach H. The effect of amino acid starvation on nucleoside uptake and RNA synthesis in Tetrahymena. J Biol Chem. 1980 May 25;255(10):4691–4697. [PubMed] [Google Scholar]
- Carlsson F. H., Louw A. I. The oxidation of methionine and its effect of the properties of cardiotoxin VII1 from Naja melanoleuca venom. Biochim Biophys Acta. 1978 Jun 21;534(2):322–330. doi: 10.1016/0005-2795(78)90015-6. [DOI] [PubMed] [Google Scholar]
- DEDMAN M. L., FARMER T. H., MORRIS C. J. Studies on pituitary adrenocorticotrophin. 3. Identification of the oxidation-reduction centre. Biochem J. 1961 Feb;78:348–352. doi: 10.1042/bj0780348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Mar E. G., Brodrick J. W., Geokas M. C., Largman C. Effect of oxidation of methionine in a peptide substrate for human elastases: a model for inactivation of alpha 1-protease inhibitor. Biochem Biophys Res Commun. 1979 May 28;88(2):346–350. doi: 10.1016/0006-291x(79)92054-0. [DOI] [PubMed] [Google Scholar]
- Doney R. C., Thompson J. F. The reduction of S-methyl-L-cysteine sulfoxide and L-methionine sulfoxide in turnip and bean leaves. Biochim Biophys Acta. 1966 Jul 27;124(1):39–49. doi: 10.1016/0304-4165(66)90311-4. [DOI] [PubMed] [Google Scholar]
- ERIKSSON S. PULMONARY EMPHYSEMA AND ALPHA1-ANTITRYPSIN DEFICIENCY. Acta Med Scand. 1964 Feb;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Ejiri S. I., Weissbach H., Brot N. Reduction of methionine sulfoxide to methionine by Escherichia coli. J Bacteriol. 1979 Jul;139(1):161–164. doi: 10.1128/jb.139.1.161-164.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejiri S. I., Weissbach H., Brot N. The purification of methionine sulfoxide reductase from Escherichia coli. Anal Biochem. 1980 Mar 1;102(2):393–398. doi: 10.1016/0003-2697(80)90173-6. [DOI] [PubMed] [Google Scholar]
- Fox L., Erion J., Tarnowski J., Spremulli L., Brot N., Weissbach H. Euglena gracilis chloroplast EF-Ts. Evidence that it is a nuclear-coded gene product. J Biol Chem. 1980 Jul 10;255(13):6018–6019. [PubMed] [Google Scholar]
- Garner M. H., Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J Biol Chem. 1970 May 10;245(9):2371–2374. [PubMed] [Google Scholar]
- Jori G., Galiazzo G., Marzotto A., Scoffone E. Dye-sensitized selective photooxidation of methionine. Biochim Biophys Acta. 1968 Jan 22;154(1):1–9. doi: 10.1016/0005-2795(68)90252-3. [DOI] [PubMed] [Google Scholar]
- Jori G., Galiazzo G., Marzotto A., Scoffone E. Selective and reversibe photo-oxidation of the methionyl residues in lysozyme. J Biol Chem. 1968 Aug 25;243(16):4272–4278. [PubMed] [Google Scholar]
- Kido K., Kassell B. Oxidation of methionine residues of porcine and bovine pepsins. Biochemistry. 1975 Feb 11;14(3):631–635. doi: 10.1021/bi00674a026. [DOI] [PubMed] [Google Scholar]
- Koteliansky V. E., Domogatsky S. P., Gudkov A. T. Dimer state of protein L7/L12 and EF-G-dependent reactions of ribosomes. Eur J Biochem. 1978 Oct;90(2):319–323. doi: 10.1111/j.1432-1033.1978.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stein S., Udenfriend S. Separation of opioid peptides utilizing high performance liquid chromatography. Int J Pept Protein Res. 1979 May;13(5):493–497. doi: 10.1111/j.1399-3011.1979.tb01911.x. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- NEUMANN N. P., MOORE S., STEIN W. H. Modification of the methionine residues in ribonuclease. Biochemistry. 1962 Jan;1:68–75. doi: 10.1021/bi00907a011. [DOI] [PubMed] [Google Scholar]
- SCHACHTER H., DIXON G. H. Identification of the methionine involved in the active center of chymotrypsin. Biochem Biophys Res Commun. 1962 Sep 25;9:132–137. doi: 10.1016/0006-291x(62)90101-8. [DOI] [PubMed] [Google Scholar]
- SOURKES T. L., TRANO Y. Reduction of methionine sulfoxides by Escherichia coli. Arch Biochem Biophys. 1953 Feb;42(2):321–326. doi: 10.1016/0003-9861(53)90361-0. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975 Oct 7;14(20):4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- Truscott R. J., Augusteyn R. C. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977 May 27;492(1):43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Chemical modification studies on the Ca2+-dependent protein modulator of cyclic nucleotide phosphodiesterase. Biochemistry. 1977 Jun 14;16(12):2742–2749. doi: 10.1021/bi00631a024. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]



