Abstract
Background and Objectives
Simvastatin's properties are suggestive of a potential pathophysiologic role in pulmonary hypertension. The objectives of this study were to investigate changes of pulmonary pathology and gene expressions, including endothelin (ET)-1, endothelin receptor A (ERA), inducible nitric oxide synthase (NOS2), endothelial nitric oxide synthase (NOS3), matrix metalloproteinase (MMP) 2, tissue inhibitor of matrix metalloproteinases (TIMP) and caspase 3, and to evaluate the effect of simvastatin on monocrotaline (M)-induced pulmonary hypertension.
Materials and Methods
Six week old male Sprague-Dawley rats were treated, as follows: control group, subcutaneous (sc) injection of saline; M group, sc injection of M (60 mg/kg); and simvastatin group, sc injection of M (60 mg/kg) plus 10 mg/kg/day simvastatin orally.
Results
On day 28, right ventricular hypertrophy (RVH) significantly decreased in the simvastatin group compared to the M group. Similarly, right ventricular pressure significantly decreased in the simvastatin group on day 28. From day 7, the ratio of medial thickening of the pulmonary artery was significantly increased in the M group, but there was no significant change in the simvastatin group. The number of muscular pulmonary arterioles was significantly reduced in the simvastatin group. On day 5, gene expressions of ET-1, ERA, NOS2, NOS3, MMP and TIMP significantly decreased in the simvastatin group.
Conclusion
Administration of simvastatin exerted weak inhibitory effects on RVH and on the number of muscular pulmonary arterioles, during the development of M-induced pulmonary hypertension in rats. Simvastatin decreased gene expressions on day 5.
Keywords: Hypertension, pulmonary; Gene expression; Monocrotaline; Simvastatin
Introduction
Pulmonary arterial hypertension includes a group of diseases characterized by progressive increase in the pulmonary vascular resistance, leading to right ventricular failure and premature death. Pulmonary endothelial dysfunction, characterized by impaired production of vasodilators and over-expression of vasoconstrictors, has been implicated in the pathogenesis of the disease.1),2) Despite intensive efforts, the molecular mechanism by which endothelial vasomediators contribute to pulmonary hypertension, has not been fully elucidated. Pulmonary vascular remodeling is characterized by endothelial cell injury, infiltration of smooth muscle cells in the subintima, and thickening of the medial layer in proximal vessels.3) It is usually secondary to the high pulmonary blood flow or pressure, typically seen in various congenital heart disease.4),5) To date, nitric oxide (NO) and endothelin-1 (ET-1) have been identified as major endothelium-dependent vasomediators. NO is a potent endogenous vasodilator produced in the lung and other tissues by endothelial nitric oxide synthase (NOS3).6)
Monocrotaline (M) has been reported to injure the endothelium of pulmonary arteries and to induce progressive pulmonary hypertension in rats, even after a single subcutaneous (sc) injection.7) Simvastatin, is a 3-hydroxyl-3-methylglutaryl coenzyme A reductase inhibitor (HMG CoA inhibitor), and is efficacious in both experimental and clinical pulmonary hypertension.8-12) However, in this animal model, the effect of simvastatin on M-induced pulmonary hypertension has not been established. It has been suggested that simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension.13)
The objectives of this study were to investigate the changes produced by simvastatin treatment in pulmonary pathology and gene expressions of ET-1, endothelin receptor A (ERA), inducible nitric oxide synthase (NOS2), NOS3, matrix metalloproteinase (MMP) 2, tissue inhibitor of matrix metalloproteinases (TIMP) and caspase-3 genes, in the M-induced pulmonary hypertension rat model.
Materials and Methods
Materials
Six-week-old male Sprague-Dawley rats, weighing approximately 250-300 g, were used for this study. All rats were housed in climate-controlled conditions, with a 12 hours light/12 hours dark cycle, and had free access to chow and water.
Pulmonary hypertension was induced by the sc injection of 60 mg/kg monocrotaline (M) (Sigma Chemicals, St. Louis, MO, USA), dissolved in 0.5 N HCl solution. The rats were grouped, as follows: control group (n=18), sc injection of saline (0.1 mL/kg); M group (n=36), sc injection of M; simvastatin group (n=36), sc injection of M plus 10 mg/kg/day simvastatin by gavage, during all experimental days. The rats were sacrificed after 1 day, 5, 7, 14 and 28 days. Lung tissues were removed and immediately frozen at -70℃ for enzyme analysis, post-fixed in 10% formalin and processed routinely for paraffin embedding. All protocols were approved by the Institutional Review Board of the School of Medicine of Ewha Womans University.
Methods
Organ weights
The rats were weighed and observed for general appearance during the study period. At the scheduled time, the animals were sacrificed and the hearts and lungs were rapidly removed. The wet weights of the right ventricle (RV), left ventricle and septum (LV+S) were measured, and the ratio of organ weight to body weight was calculated. The RV to LV+S ratio {RV/(LV+S)} was used as an index of RVH.
Estimation of mean right ventricular pressure
The animals were placed in the supine position and instrumented with an arterial pressure line (Physiological Pressure Transducer, MLT1199; AD Instruments, Oxfordshire, UK). Hemodynamic parameters were recorded at baseline, and after 5, 7, 14 and 28 days. The catheter was placed in the RV to estimate the mean right ventricular pressure (RVP).
Morphometric analysis of the pulmonary arteries
Following gentle perfusion through trachea, the lung tissue was fixed with 10% buffered formalin for 24 hours, and then embedded in paraffin. Hematoxylin-eosin and Victoria blue stains were performed on 3 um-thick sections to evaluate the histopathologic changes of pulmonary blood vessels. More than 20 images of pulmonary arterioles (25-100 µm diameter) per tissue section were captured at a magnification of ×400, using the microscopic digital camera, and were analyzed using an image analysis program (analySIS, Olympus Soft Imaging Solutions, Singapore). The external diameter (D) and medial thickness on either side (M1 and M2) were measured along the shortest diameter. The medial wall thickness was expressed, as follows: % wall thickness={(M1+M2)/2/D}×100.
The dimension of pulmonary arteries (i.e., the distance between both sides of the outer elastic layer) and the thickness of the medial layer (i.e., the distance between the inner and outer elastic layers) were measured. The percent wall thickness of the medial layer of arterioles was determined by dividing the medial wall thickness by the dimension. In addition, the number of pulmonary arterioles accompanied by respiratory bronchioles and present in the alveolar wall was determined. A total number of 20 randomly selected microscopic fields per tissue section were assessed at a magnification ×200.14)
RNA extraction and cDNA synthesis
Total RNA was extracted by using the TRIzol Reagent™ (Invitrogen, Carlsbad, CA, USA), according to the Trizol method protocol, and re-suspended in diethyl pyrocarbonate water. The final RNA amount was spectrophotometrically determined at 260/280 nm. Quality was assessed as the absence of smear of 18 S and 28 S bands, analyzed with Bio analyzer 2100 (Agilent). RNA samples were stored at -70℃ until used. cDNAs were synthesized from 1 ug of total RNA, according to the manufacture's protocol (Hight Capacity RNA-to-cDNA kit, Appllied Biosystems, USA).
Gene expression analysis by real time reverse transcription-polymerase chain reaction
Real-time quantitative polymerase chain reaction (PCR) was performed in triplicate in 384-well plates. The 384-well high-throughput analysis was performed, using the ABI Prism 7900 Sequence Detection System Software (Applied Biosystems, CA, USA) and white colored 384-well plates (ABgene, Hamburg, Germany), for intensification of the fluorescent signals by a factor of three. The system operates using a thermal cycler and a laser that is directed via fiber optics to each of the 384 sample wells. The fluorescence emission from each sample is then collected by a charge-coupled device-camera and the quantitative data were analyzed using the Sequence Detection System Software (SDS version 2.0, Applied Biosystems, Carlsbad, CA, USA). The reaction mixtures contained 10 pmol/uL of each primer and 2X SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA), which includes the HotStarTaqt DNA-Polymerase in an optimized buffer, the dNTP mix (with dUTP additive), the SYBRs Green I fluorescent dye, and ROX dye as the passive reference. Each of the 384-well real-time quantitative PCR plates included serial dilutions (1, 1/2 and 1/4) of cDNA, which were used to generate relative standard curves for genes.15)
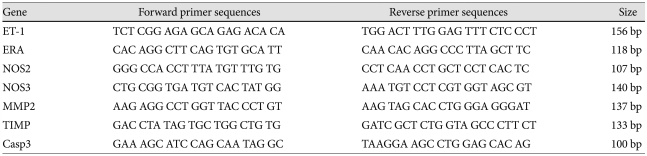
The resulting first-strand of cDNA was normalized by the glyceraldehyde 3-phosphate dehydrogenase gene. The normalized cDNA was the used as a template for the PCR procedure. The specific primers for rat ET-1 were 5'-TCTCGGA GAG CAGAGACACA-3' (forward) and 5'-TGGACTTTG GAGTTTCTCCCT-3' (reverse). The specific primers for ERA were 5'-CACAGGCTTCAGTGTGCAT T-3' (forward) and 5'-CAACACAGGCCCTTAGCTTC-3' (reverse). The specific primers for NOS2 were 5'-GGGCCACCTTTATG TTTGTG-3' (forward) and 5'-CCTCAACCTGCTCCTC ACTC-3' (reverse). The specific primers for NOS3 were 5'-CT GCGGTGATGTCACTATGG-3' (forward) and 5'-AAAT GTCCTCGTGGTAGGGT-3' (reverse). The specific primers for MMP2 were 5'-AAGAGGCCTGGTTACCCTGT-3' (forward) and 5'-AAGTAGCACCTGGGAGGGAT-3' (reverse). The specific primers for TIMP were 5'-GACCTA TAGTGCTGGCTGTG-3' (forward) and 5'-GATCGCTCT GGTAGCCCTTCT-3' (reverse). And finally, the specific primers for caspase 3 were 5'-GAAAGCATCCAGTAGGC-3' (forward) and 5'-TAAGGAAGCCTGGAGCACAG-3' (reverse) (Table 1) (Fig. 1).
Table 1.
Gene sequences of primer of RT-PCR
RT-PCR: reverse transcription-polymerase chain reaction, ET-1: endothelin-1, ERA: endothelin receptor A, NOS2: inducible nitric oxide synthase, NOS3: endothelial nitric oxide synthase, MMP2: matrix metalloproteinase 2, TIMP: tissue inhibitor of matrix metalloproteinases, Casp3: Caspase 3
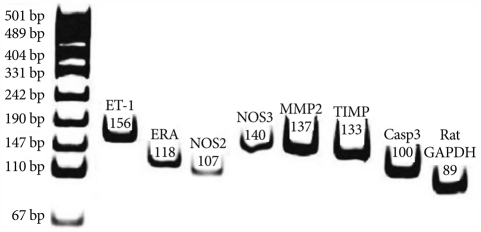
Fig. 1.
Typical example of RT-PCR products are shown for the level of ET-1, ERA, NOS2, NOS3, MMP 2 and TIMP mRNA measured in the lung tissue. The RT-PCR products from the transcripts of ET-1, ERA, NOS2, NOS3, MMP2, TIMP, Casp 3 and GAPDH were 156 bp, 118 bp, 107 bp, 140 bp, 137 bp, 133 bp 100 and 89 bp, respectively. ET-1: endothelin-1, ERA: endothelin receptor A, NOS 2: inducible nitric oxide synthase, NOS 3: endothelial nitric oxide synthase, MMP-2: matrix metalloproteinase 2, TIMP: tissue inhibitor of matrix metalloproteinases, Casp3: caspase 3, GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
All primers were amplified using the same conditions. The thermal cycling conditions were 50℃ for 2 minutes and 95℃ for 10 minutes, followed by 40 cycles of 95℃ for 30 seconds, 60℃ for 30 seconds, and 72℃ for 30 seconds. In order to exclude the presence of unspecific products, the melting curve analysis of products was performed routinely after finishing amplification by high-resolution data collection, during an incremental temperature increase from 60℃ to 95℃, with a ramp rate of 0.21℃/second. We then converted, on the basis of the equation, the real-time PCR cycle numbers to gene amounts (ng). The real-time PCR analysis was performed on an Applied Biosystems Prism 7900 Sequence Detection System (PE Applied Biosystems).16)
Liver enzymes and lipid profiles
Liver enzymes, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and lipid profiles, such as total cholesterol, triglyceride, high density lipoprotein-cholesterol (HDL-C), and low density lipoprotein-cholesterol (LDL-C), were measured in each group.
Statistical analysis
Results were expressed as the mean±standard deviation. An unpaired two-tailed t-test and the Mann-Whitney test were used, and a p<0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS) for windows version 12.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Changes of the total body weight and heart weight
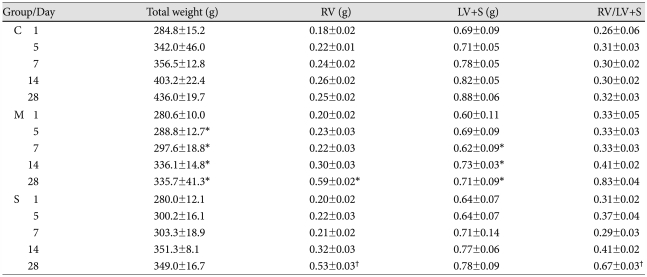
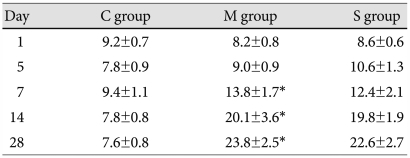
From day 5 until day 28, the total weight of the rats decreased significantly in the M group compared with the control group. On day 28, the RV weight significantly increased in the M group, and significantly decreased in the simvastatin group compared with the M group. From day 7, the LV and septum weight significantly decreased in the M group and there were no significant changes in the simvastatin group. On day 28, the RV/LV+S weight ratio significantly decreased in the simvastatin group, compared with the M group (Table 2).
Table 2.
Changes in the whole body weight, right ventricle weight, and left ventricle and septum weight, measured in each group on day 1, 5, 7, 14 and 28
*p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotalin, S: simvastatin, RV: right ventricle, S; septum, LV: left ventricle
Estimation of the right ventricular pressure
From day 5, the mean RVP significantly increased in the M group, compared with control group. On day 28, the RVP significantly decreased in the simvastatin group compared with the M group (Table 3).
Table 3.
Right ventricular pressure in each group (mmHg)
*p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin
Histologic study
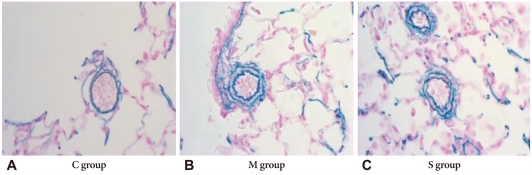
The basic pulmonary architecture was similar in each group. The predominant changes in the pulmonary vasculature occurred in the M group on day 7 and included the development of medial thickening in the pulmonary arterioles, compared with both the control group and the simvastatin group (Fig. 2).
Fig. 2.
Photographs of pulmonary arterioles in the three investigated groups (Victoria blue stain ×400). A: the muscular layer of pulmonary arterioles was normal in C group. B: the medial layer of pulmonary arterioles was progressively thickened after M injection in M group. C: the medial wall thickness after M injection was significantly attenuated in the simvastatin group. C: control group, M: monocrotaline group, S: simvastatin group.
Morphometric analysis of the pulmonary arteries
The medial wall thickness of the pulmonary artery
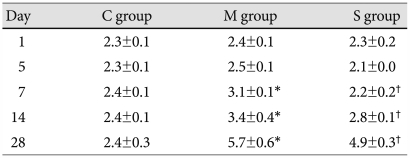
In the M group, the ratio of medial thickening to the external diameter of the pulmonary artery significantly increased from day 7, compared to control. In the simvastatin group, the ratio of medial thickening of the pulmonary artery was not significantly different from the M group (Table 4).
Table 4.
Ratio of the medial thickening of pulmonary artery in each group (%)
*p<0.05 vs. the corresponding value in the C group. C: control, M: monocrotaline, S: simvastatin
The number of muscular pulmonary arterioles
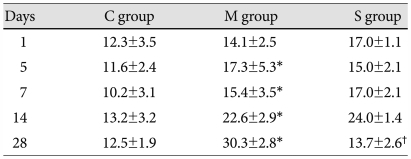
The number of muscular pulmonary arterioles significantly increased in the M group, comapared with the C group. From day 7, the number of muscular pulmonary arterioles significantly decreased in the simvastatin group, compared to the M group (Table 5).
Table 5.
Number of muscular pulmonary arterioles in each group
*p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin
Gene expressions in rat lung tissues
Gene expressions of endothelin-1
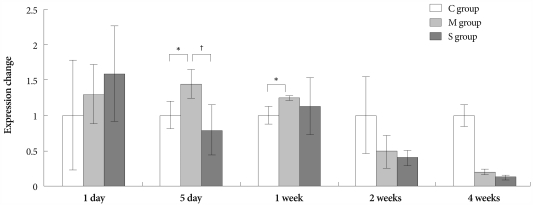
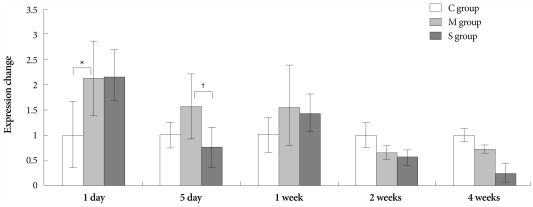
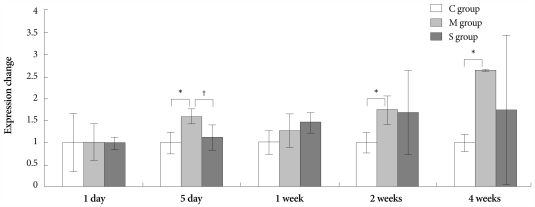
On days 5 and 7, in the M group, the expression of gene ET-1 was higher than in the control group. On day 5, in the Simvastatin group, the same gene was lower expressed than in the M group (Fig. 3).
Fig. 3.
Gene expressions of endothelin-1 in the lungs, determined at day 1, day 5, 1 week, 2 weeks and 4 weeks after simvastatin treatment. *p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin.
Gene expressions of endothelin receptor A
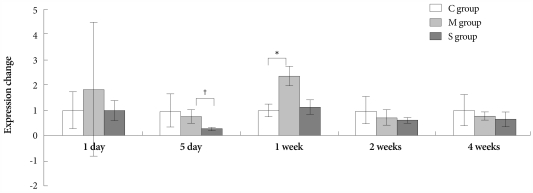
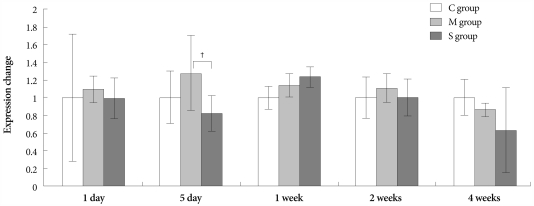
On day 1, the ERA gene was more expressed in the M group than in control group. On day 5, however, expression of the same gene significantly decreased in the simvastatin group, compared with the M group (Fig. 4).
Fig. 4.
Gene expressions of endothelin receptor A in the lungs, determined at day 1, day 5, 1 week, 2 weeks and 4 weeks after simvastatin treatment. *p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin.
Gene expressions of inducible nitric oxide synthase
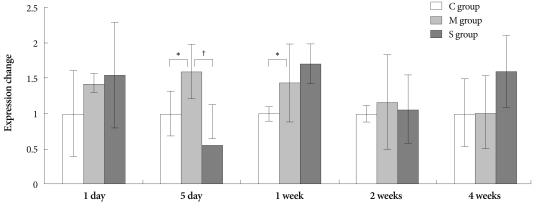
On day 7, expression of NOS2 significantly increased in the M group compared to control group. On day 5, NOS2 was less expressed in the simvastatin group than in the M group (Fig. 5).
Fig. 5.
Gene expressions of inducible nitric oxide synthase in the lungs, determined at day 1, day 5, 1 week, 2 weeks and 4 weeks after simvastatin treatment. *p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin.
Gene expressions of endothelial nitric oxide synthase
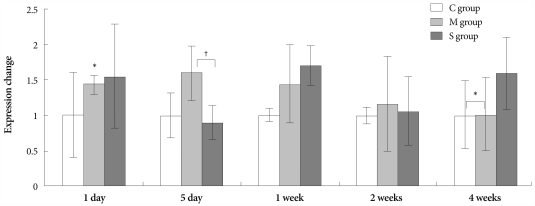
On days 5 and 7, expression of NOS3 significantly increased in the M group compared to control group. On day 5, NOS3 was less expressed in the simvastatin group than in the M group (Fig. 6).
Fig. 6.
Gene expressions of endothelial nitric oxide synthase mRNA in the lungs, determined at day 1, day 5, 1 week, 2 weeks and 4 weeks after simvastatin treatment. *p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin.
Gene expressions of matrix metalloproteinases
On days 1 and 28, expression of MMP2 significantly increased in the M group compared to control group. On day 5, MMP2 was less expressed in the simvastatin group than in the M group (Fig. 7).
Fig. 7.
Gene expressions of matrix metalloproteinase 2 in the lungs, determined at day 1, day 5, 1 week, 2 weeks and 4 weeks after simvastatin treatment. *p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin.
Gene expressions of tissue inhibitor of matrix metalloproteinases
On days 5, 14 and 28, expression of TIMP significantly increased in the M group compared to control group. On day 5, TIMP was less expressed in the simvastatin group than in the M group (Fig. 8).
Fig. 8.
Gene expressions of tissue inhibitor of matrix metalloproteinases in the lungs, determined at day 1, day 5, 1 week, 2 weeks and 4 weeks after simvastatin treatment. *p<0.05 vs. the corresponding value in the C group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin.
Gene expressions of caspase 3
On day 5, expression of caspase 3 significantly decreased in the simvastatin group, compared to the M group (Fig. 9).
Fig. 9.
Gene expressions of caspase 3 in the lungs, determined at day 1, day 5, 1 week, 2 weeks and 4 weeks after simvastatin treatment. †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotaline, S: simvastatin.
Liver enzymes and serum lipid profiles
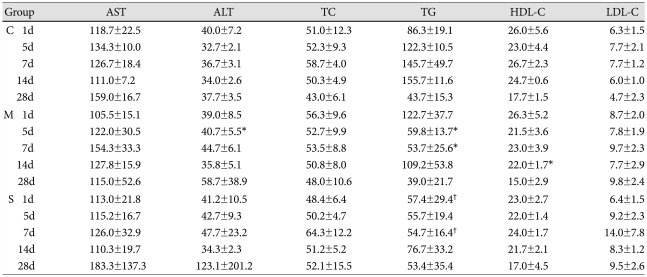
On day 5, ALT significantly increased in the M group compared to control group. In contrast, on days 5 and 7, triglyceride significantly decreased in the M group compared to control group.
On days 1 and 7, triglyceride also significantly decreased in the simvastatin group compared to the M group. On day 14, HDL-C significantly decreased in the M group compared to control group. LDL-C, AST and total cholesterol showed no significant changes after either M or simvastatin treatment (Table 6).
Table 6.
Liver enzymes and lipid profiles in each group (mg/dL)
*p<0.05 vs. the corresponding value in the control group, †p<0.05 vs. the corresponding value in the M group. C: control, M: monocrotalin, S: simvastatin, AST: aspartate aminotransterase, ALT: alanine aminotransferase, TC: total cholesterol, TG: triglyceride, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol
Discussion
We confirmed that pulmonary hypertension was induced by M, as evidenced by pathologic finding and gene expressions. The evidence includes; first, a progressive increase in the ratio of RV to LV+S was significantly noted in both our previous study14-16) and in the present study. Second, the RVP progressively increased in the M group, compared with the control group. Third, in the M group, predominant changes in the pulmonary vasculature included the development of medial thickening in the pulmonary arterioles and increased number of intra-acinar muscular arteries. Fourth, the expressions of ET-1, ERA, NOS2, NOS3, MMP and TIMP genes were significantly increased in the M group. These pathologic findings were consistent with other results demonstrating a significant rise in pulmonary blood pressure and apparent RVH after M injection in the rat model.17),18)
During the development of M-induced pulmonary hypertension in rats, administration of simvastatin exerted inhibitory effects on RVH and on a number of muscular pulmonary arterioles. On day 28, RVH significantly decreased in the simvastatin group compared to the M group. Starting with the first week, the number of muscular pulmonary arterioles was significantly reduced in the simvastatin group, compared to M group. However, the ratio of medial thickening of the pulmonary artery has not changed significantly in the simvastatin group. On day 5, expressions of ET-1, ERA, NOS2, NOS3, MMP and TIMP genes significantly decreased in the simvastatin group.
Statins, such as 3-hydroxyl-3-methylglutaryl (HMG)-CoA reductase inhibitors, have been developed as lipid-lowering drugs. In addition, recent experiments and clinical trials have demonstrated that statins also exert vasculoprotective effects, independent of their cholesterol-lowering effects.19) Thus, recent studies have suggested that statins may have potential use in the treatment of pulmonary hypertension.8),9)
However, there is still controversy on the effect of simvastatin on pulmonary hypertension. Simvastatin attenuates neointimal thickening of the smooth muscle.10) Simvastatin is effective in inducing apoptosis in hyperproliperative pulmonary vascular lesions and could be considered as a potential drug for treatment of human severe pulmonary hypertension. Nishimura et al.10) demonstrated impressive hemodynamic responses to simvastatin therapy, and also suggested that simvastatin promotes apoptosis of the smooth muscle cells in pulmonary arterioles. That data10) demonstrated the down-regulation of the inflammatory genes such as fos, jun, and TNF-a and the up-regulation of the cell cycle inhibitor p27Kip1, the NOS3 gene, and the bone morphogenetic protein receptor type 1. In contrast, our experiments demonstrated a significant down-regulation of NOS3 expression in simvastatin treated animals, suggesting that any increase in NO by simvastatin would relate to increased bioavailability, rather than enzyme expression.
However, McMurtry et al.11) reported no effect of simvastatin on pulmonary hypertension, i.e., simvastatin could not reverse the M-induced pulmonary arterial hypertension. In that study, simvastatin improved echocardiographic parameters of pulmonary hypertension, including the pulmonary artery acceleration time and the RV thickness after 1-2 weeks, but the effect was not sustained, and ultimately, rats treated with simvastatin developed severe pulmonary arterial hypertension and expired. Our study revealed a weak inhibitory effect of simvastatin on RVH and pulmonary hypertension. In our model, treatment with simvastatin initially appeared to improve lung pathologic findings, but did not reverse the M-induced pulmonary hypertension. In general the simvastatin effect of attenuation of RVH and pulmonary hypertension seems to be weaker than that of bosentan.14)
NO is best known for its marked vasodilator activity. Exogenous NO is useful in the clinical management of acute pulmonary hypertensive crisis. However, NO is also a reactive radical capable of tissue injury, either directly or after reacting with other radicals. For example, an extremely fast reaction of NO with superoxide yields the highly cytotoxic peroxynitrite.20) There are three subtypes of NOS {i.e., NOS2, NOS3 and neuronal NOS (nNOS)}. The NO can contribute to oxidative stress and, in association with tissue injury, NOS2 is often up-regulated. Transient NOS2 induction in the pulmonary vascular wall at the beginning of chronic hypoxia participates in the pathogenesis of pulmonary hypertension. In our study, on day 7, NOS2 significantly increased in the M group compared to control and significantly decreased in the simvastatin group, compared to the M group, on day 5. In chronic hypoxia, many authors have reported elevated NOS mRNA and protein in both the lungs and the pulmonary vessels.21) However, most of those studies either focused on NOS3 or did not discriminate between various NOS isoforms. In a study comparing NOS3, nNOS and NOS2 activity in mice, NOS3 showed the most important role in regulating pulmonary circulation.22) Similarly, the endothelial NO production has been shown to play a key role in vascular reactivity and in the modulation of smooth muscle cell proliferation, as well as inflammation.21) Therefore, at least, in part, the protective effects of statins on the endothelium may contribute to recover pulmonary hypertension. In our study, NOS3 has not increased significantly in the simvastatin group.
Li et al.23) investigated the hypothesis that statins regulate vascular remodeling by modulation of MMP secretion in primary cultured pulmonary artery smooth muscle cells. Thus, angiotension II induced a concentration-dependent MMP2 secretion. Pre-exposure of cells to simvastatin blocked the angiotension II-stimulated MMP2 release. Simvastatin suppressed ET-1-induced MMP2 release through the Rho/ROCK pathway. In contrast, there was no effect of angiotensin II on the expression of TIMP genes. These results indicated a novel statins-regulation of Rho/ROCK signaling against pulmonary vascular remodeling, and suggested that statins could prove usefulness in targeting this pathway in pulmonary hypertension and other disease conditions.24)
In rats, during treatment of severe pulmonary hypertension with a HMG-CoA reductase inhibitor, simvastatin caused apoptosis of the lumen-obliteration cells, which was associated with significant reduction of pulmonary artery pressure and of RVH.13) In normal rat lungs, blood vessels are negative for caspase 3 staining. Simvastatin treatment causes activation of caspase-3 and significantly decreases the number of pulmonary vascular lesions. Treatment with simvastatin induced apoptosis of endothelial cells in obliterated vessels. Simvastatin reduced pulmonary hypertension through apoptosis-induced reopening of the occluded vessels, as shown by caspase-3 immunohistochemistry.13) It is now becoming clear that statins can trigger apoptosis using both caspase-dependent and caspase-independent Rho kinase-dependent pathways.25) The ability of HMG-CoA reductase inhibitors to induce apoptosis was demonstrated in both rat pulmonary vein endothelial cells26) and human hepatocytes.27) In our study, the simvastatin effect on caspase-3 mRNA was not apparent, suggesting that more study on high dose simvastatin treatment is needed.
Simvastatin has both anti-proliferative and pro-apoptotic effects on vascular smooth muscle cells and enhances the endothelial production of NO. Simvastatin stabilizes the endothelial barrier function and promotes anti-inflammatory effect, which leads to atherogenesis.
The dose of simvastatin, used both experimentally and clinically, was inconsistent among the various other investigators. Girgis et al.24) used 20 mg/kg/day of simvastatin and found that simvastatin attenuated and induced regression of chronic hypoxic pulmonary hypertension. Wilkins et al.28) gave the patients 80 mg/day of simvastatin for 6 months, and found that, when added to conventional therapy in patients with pulmonary arterial hypertension, simvastatin produced a small and transient early reduction in both the RV mass and the N terminal pro B type natriuretic peptide (NT-pro-BNP) level. Liu et al.29) used 2 mg/kg/d of simvastatin for 35 days in rats, and resulted in prevention of pulmonary hypertension. However, in McMurtry's rat study,11) the same dose of 2 mg/kg/d of simvastatin did not reverse the M-induced pulmonary arterial hypertension.
In conclusion, treatment of simvastatin in M-induced hypertensive rats blunted the development of M-induced pulmonary hypertension and resulted in a weak attenuation of both RVH and vascular remodeling. Our results in M-induced pulmonary hypertension suggest that all seven genes increased, and all of those increases may play a role in RVH and remodeling. The sensitive markers of decreased expression of ERA and NOS2 genes appeared earlier than other pathologic findings. Also, the decreased gene expression of MMP2 could be a marker of pulmonary hypertension treatment. Further studies are in progress to determine the effects of simvastatin on other gene expressions in the M-induced pulmonary hypertension rat model.
Larger sample sizes are needed to measure hemodynamics and follow-ups should extend for more than 4 weeks. The follow up duration is a critical factor to evaluate the effectiveness of the simvastatin treatment on M-induced pulmonary hypertension. Finally, further studies are necessary to explore the intricate mechanisms of simvastatin treatment in M-induced pulmonary hypertension.
Acknowledgments
This work was supported by the Korean Society of Cardiology (2009) and National Research Foundation of Korea (NRF) grant founded by the Korean government (MEST) (KRF-2009-0064784).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 3.Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 1992;263:H647–H659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- 4.Keck EW. Pulmonary hypertension and pulmonary vascular disease in congenital heart defects. Z Kardiol. 1989;78(Suppl 7):65–73. [PubMed] [Google Scholar]
- 5.Yamaki S, Endo M, Takahashi T. Different grades of medial hypertrophy and intimal changes in small pulmonary arteries among various types of congenital heart disease with pulmonary hypertension. Tohoku J Exp Med. 1997;182:83–91. doi: 10.1620/tjem.182.83. [DOI] [PubMed] [Google Scholar]
- 6.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 7.Todd L, Mullen M, Olley PM, Rabinovitch M. Pulmonary toxicity of monocrotaline differs at critical periods of lung development. Pediatr Res. 1985;19:731–737. doi: 10.1203/00006450-198507000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Girgis RE, Li D, Zhan X, et al. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol. 2003;285:H938–H945. doi: 10.1152/ajpheart.01097.2002. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura T, Faul JL, Berry GJ, et al. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med. 2002;166:1403–1408. doi: 10.1164/rccm.200203-268OC. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura T, Vaszar LT, Faul JL, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 11.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamycin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007;293:L933–L940. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- 12.Kim MY, Kim YK, Jung YW, Kim WT, Kwon TH, Lee DS. The protective effect of simvastatin on monocrotaline-induced pulmonary hypertension in rats. Korean Circ J. 2008;38:313–319. [Google Scholar]
- 13.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–L676. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- 14.Lim KA, Shim JY, Cho SH, Kim KW, Han JJ, Hong YM. Effect of endothelin receptor blokade on monocrotaline-induced pulmonary hypertension in rats. Korean J Pediatr. 2009;52:689–695. [Google Scholar]
- 15.Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor A on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010;40:459–464. doi: 10.4070/kcj.2010.40.9.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo HS, Kim KC, Hong YM. Gene expression of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011;41:83–90. doi: 10.4070/kcj.2011.41.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyauchi T, Yorikane R, Sakai S, et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotalin-induced pulmonary hypertension. Circ Res. 1993;73:887–897. doi: 10.1161/01.res.73.5.887. [DOI] [PubMed] [Google Scholar]
- 18.Itoh T, Nagaya N, Fujii T, et al. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;169:34–38. doi: 10.1164/rccm.200303-346OC. [DOI] [PubMed] [Google Scholar]
- 19.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JS, Koppenol WH. Nitric oxide, superoxide and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 21.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- 22.Miller AA, Hislop AA, Vallance PJ, Haworth SG. Deletion of the eNOS gene has a greater impact on the pulmonary circulation of male than female mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L299–L306. doi: 10.1152/ajplung.00022.2005. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Li Z, Sun X. Statins suppress MMP2 secretion via inactivation of RhoA/ROCK pathway in pulmonary vascular smooth muscle cells. Eur J Pharmacol. 2008;591:219–223. doi: 10.1016/j.ejphar.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 24.Girgis RE, Mozammel S, Champion HC, et al. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1105–L1110. doi: 10.1152/ajplung.00411.2006. [DOI] [PubMed] [Google Scholar]
- 25.Kim YC, Song SB, Lee MH, et al. Simvastatin induces caspase-independent apoptosis in LPS-activated RAW264.7 macrophage cells. Biochem Biophys Res Commun. 2006;339:1007–1014. doi: 10.1016/j.bbrc.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 26.Kaneta S, Satoh K, Kano S, Kanda M, Ichihara K. All hydrophobic HMG-CoA reductase inhibitors induce apoptotic death in rat pulmonary vein endothelial cells. Atherosclerosis. 2003;170:237–243. doi: 10.1016/s0021-9150(03)00301-0. [DOI] [PubMed] [Google Scholar]
- 27.Kubota T, Fujisaki K, Itoh Y, Yano T, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG-CoA reductase inhibitors. Biochem Pharmacol. 2004;67:2175–2186. doi: 10.1016/j.bcp.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 28.Wilkins MR, Ali O, Bradlow W, et al. Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med. 2010;181:1106–1113. doi: 10.1164/rccm.2009111-699OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B, Wang XQ, Yu L, Zhou TF, Wang XM, Liu HM. Simvastatin restores down-regulated GATA-6 expression in pulmonary hypertensive rats. Exp Lung Res. 2009;35:411–426. doi: 10.1080/01902140902736819. [DOI] [PMC free article] [PubMed] [Google Scholar]