Abstract
Aerosol mixing resulting from turbulent flows is thought to be a major mechanism of deposition in the upper respiratory tract (URT). Because turbulence levels are a function of gas density, the use of a low-density carrier gas should reduce deposition in the URT allowing the aerosol to reach more peripheral airways of the lung. We performed aerosol bolus tests on 11 healthy subjects to investigate the effect of reduced gas density on regional aerosol deposition in the human lung. Using both air and heliox (80% helium, 20% oxygen) as carrier gas, boluses of 1 and 2 μm-diameter particles were inhaled to five volumetric lung depths (Vp) between 150 and 1200 mL during an inspiration from residual volume (RV) to 1 liter above functional residual capacity at a constant flow rate of ∼0.50 L/sec, which was immediately followed by an expiration to RV at the same flow rate. Aerosol deposition and axial dispersion were calculated from aerosol concentration and flow rate measured at the mouth. For 1 μm-diameter particles, deposition was significantly reduced by 29 ± 28% (mean ± SD, p < 0.05) when breathing heliox instead of air at shallow Vp (150 mL) and significantly increased by 11 ± 9% at deep Vp (1200 mL). For 2 μm-diameter particles, deposition was significantly higher at Vp = 500 mL by 6 ± 7% and the predicted Vp to achieve 100% deposition was significantly lower with heliox (834 ± 146 mL) compared to air (912 ± 128 mL) (p <0.05). Despite a decrease in deposition at shallow Vp, there was no change in axial dispersion, suggesting that other factors such as radial turbulent mixing result in decreased aerosol deposition. Our results suggested that heliox reduces upper airway deposition of 1 and 2 μm-diameter particles allowing more particles to penetrate and subsequently deposit in the peripheral lung.
Key words: helium, heliox, aerosol bolus inhalation
Introduction
The penetration and subsequent deposition of inhaled particles in the human lung depends upon the characteristics of both the inhaled particles and of the carrier gas, although there have been few studies focusing on the latter. Primary mechanisms of aerosol deposition include inertial impaction, gravitational sedimentation, and Brownian diffusion, as well as turbulent mixing in the upper respiratory tract (URT) and first generations of conducting airways. Differences in particle mobility between different gas mixtures may also affect particle transport and deposition rate.
A mixture of 80% helium and 20% oxygen (heliox) has a gas density about one-third that of air and a viscosity about 8% higher than air. Thus, at similar flow rates, the Reynolds number for heliox is approximately one-third that of air in any given airway. Accordingly, turbulent and transitional flow is reduced or even absent when breathing heliox. Heliox, as a carrier gas, would therefore be expected to reduce deposition in the URT and first generations of conducting airways via a reduction in turbulent mixing.
Despite the widespread medical use of heliox, relatively few investigators have looked at the effect of carrier gas density on pulmonary deposition of aerosols. Previous studies have produced conflicting results concerning the effect of carrier gas on the regional pattern of aerosol deposition in the human lung. Studies by Svartengren et al.(1) and Anderson et al.(2) were performed in nine and eight healthy human subjects, respectively, using 3.6-μm radiolabeled particles. Svartengren et al.(1) reported that there was no significant difference in deposition in the mouth and throat or in the alveolar region of the lung between air and heliox when subjects inspired at a flow rate of 0.5 L/sec. However, Anderson et al.(2) reported there was a significant decrease in deposition in the mouth and throat and a significant increase in deposition in the alveolar region of the lung when breathing heliox compared to air at inhalation flow rates of 0.4, 0.8, and 1.2 L/sec.
Previously, our lab has shown that there was a small although significant decrease in deposition in the lung as a whole when using heliox compared to air for 1- and 2-μm-diameter particles.(3) Although it could not be directly measured from those data, we speculated that deposition was decreased in the URT, and first generations of conducting airways allowing particles to penetrate more deeply and subsequently deposit in peripheral airways and alveoli. In an attempt to clarify the issue the present study used a different technique (aerosol bolus inhalation) to investigate the deposition of 1- and 2-μm-diameter particles at different volumetric depths in the lung of eleven healthy subjects using both air and heliox as the carrier gas. This technique allows a more precise investigation of deposition at different lung depths compared to other techniques such as scintigraphy. Additionally, this technique allows for determination of the axial dispersion undergone by the particles during their transport in the lung. We hypothesized that a low-density carrier gas (heliox) would reduce aerosol mixing and hence deposition in the URT, allowing particles to penetrate more deeply and subsequently deposit in the peripheral lung. To our knowledge, this is the first study to investigate the role of turbulent mixing on regional deposition patterns using the bolus method.
Methods
Equipment
Aerosol bolus data were collected via the equipment shown in Figure 1. One configuration of the sliding valve SV1 allowed the subject to breathe medical air or heliox (80% helium–20% oxygen) from a reservoir bag via a two-way nonrebreathing valve. During a test breath, the configuration of SV1 transiently changed allowing the subject to inspire the aerosol bolus located between SV2 and sliding valve SV3, providing a bolus volume of ∼70 mL, followed by gas from the reservoir bag. The configuration of SV3 was manually adjusted between tests such that the bolus tube could be refilled with aerosol. The measurement of the aerosol concentration and the flow rate was provided by a photometer (model 993000, Pari)(4) and a Validyne differential pressure transducer M-45 connected via short tubes to the two ports of a pneumotachograph (Fleisch no. 1, OEM Medical), respectively. The photometer, pneumotachograph, and sliding valves were heated to body temperature to prevent water condensation during testing. A diffusion dryer (dead space ∼10 mL) was positioned between the mouthpiece and the photometer to remove water vapor from the exhaled air preventing condensation on the lenses of the photometer. The photometer was located sufficiently close to the mouthpiece such that any deposition in the experimental apparatus only minimally affected our measurements.
FIG. 1.
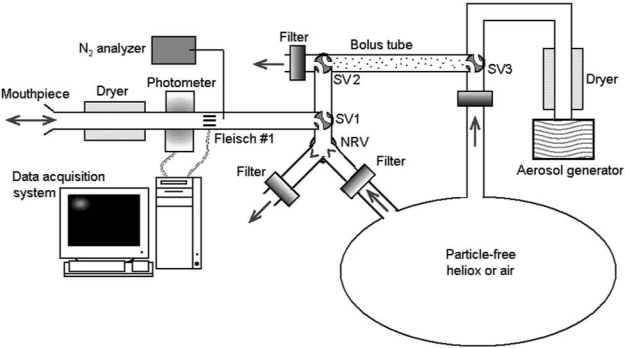
Schematic representation of experimental setup. One configuration of the sliding valve (SV) SV1 allowed subject to breathe medical air or heliox from a reservoir bag through a two-way nonrebreathing valve (NRV) equipped with filters. The other configuration of SV1 allowed subject to inspire an aerosol bolus located between SV2 and SV3. Measurements of aerosol concentration and flow rate are provided by a photometer and a pneumotachograph (Fleisch #1), respectively. A diffusion dryer is located between the photometer and the mouthpiece to remove water vapors from exhaled air.
Aerosol generation
The bolus tube (see Fig. 1) was filled with aerosol composed of monodisperse polystyrene latex particles (Duke Scientific, Fremont, CA). The particles were supplied in aqueous suspension and diluted with deionized water before being dispensed via two Acorn II nebulizers (Marquest Medical Products, Englewood, CO). Particles were nebulized with the test gas, air, or heliox. Prior to bolus tube entry, the aerosol was directed through a heated hose and a diffusion dryer to remove water droplets. The same protocol as in our previous study of total deposition in air and heliox(3) was used to ensure that the resulting aerosol consisted of dry particles of uniform size.
The sizes of the particles as specified by the manufacturer were 1.03 ± 0.022 and 2.02 ± 0.063 (SD) μm. For convenience, these are referred to as 1 and 2 μm-diameter particles. The aerosol concentrations were ∼104 particles/mL of gas for 1 μm-diameter particles and ∼5 × 103 particles/mL of gas for 2 μm-diameter particles. Previous size analysis has shown that the number of doublets in the aerosol was <3% for 1 and 2 μm-diameter particles.(5)
Data recording
A PC equipped with a 12-bit analog-to-digital card (National Instruments, Austin, TX DAQ700) was used for data acquisition. Signals from the photometer and the pneumotachograph were sampled at 100 Hz. We used the same custom software for data acquisition developed in a previous study by our group.(6)
Subjects and protocol
Eleven healthy subjects participated in the study. Their anthropometric data are listed in Table 1.
Table 1.
Anthropometric Data
| Sex | Age, (year) | Height, (cm) | Weight, (kg) | FVC, (%pred) | FEV1/FVC, (%pred) |
|---|---|---|---|---|---|
| F | 38 | 165 | 56 | 116 | 94 |
| F | 35 | 163 | 71 | 105 | 100 |
| M | 48 | 186 | 118 | 98 | 109 |
| F | 30 | 180 | 64 | 105 | 104 |
| M | 36 | 188 | 113 | 102 | 97 |
| M | 39 | 182 | 90 | 128 | 83 |
| M | 23 | 180 | 85 | 108 | 83 |
| M | 22 | 185 | 101 | 95 | 103 |
| M | 22 | 185 | 80 | 88 | 109 |
| M | 23 | 167 | 95 | 80 | 97 |
| M | 21 | 185 | 85 | 102 | 91 |
F, female; M, male; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 sec; %pred, % predicted.
Aerosol boli were administered to several specific volumetric lung depths during inhalation of air or heliox. The volumetric lung depth or penetration volume (Vp) was defined as the volume of air inhaled from the mode of the aerosol bolus to the end of the inhalation. The test breaths consisted of an inspiration from residual volume (RV) to 1 liter above functional residual capacity (FRC) at a flow rate of ∼0.50 L/sec, immediately followed by an expiration to RV at the same flow rate of 0.50 L/sec. A flowmeter provided visual feedback to the subject. During the inspiration, an aerosol bolus was injected at different target penetration volumes: 150, 300, 500, 800, and 1200 mL. Penetration volumes were measured from the mouth such that the dead space of the experimental apparatus was not included in the measured penetration volumes. For tests performed with heliox, subjects were asked to perform several vital capacity maneuvers inhaling from the reservoir filled with particle-free heliox and remained breathing heliox between aerosol bolus tests in order to ensure the lungs were equilibrated with heliox. The wash-in was monitored by a nitrogen analyzer measuring the concentration of nitrogen in the exhaled gases and the protocol began when the expired nitrogen concentration was less than 10%. Tests were performed with two different particle sizes (1 and 2 μm) during two separate sessions. The protocol was repeated three times for each combination of penetration volume, carrier gas, and particle size. Prior to each experiment, flow calibration was performed with the test gases (air or heliox) using a 3-L calibration syringe providing accurate flow measurements for both gases. The protocol was approved by the Committee on Investigations Involving Human Subjects at the University of California, San Diego, and each subject signed a statement of informed consent prior to experimentation.
Data analysis
For each bolus test, both aerosol deposition (DE) and dispersion (H) were calculated. Deposition was determined using the following equation
 |
(1) |
where Nin and Nex are the number of particles in the inspired and expired bolus, respectively, calculated by the integration of the aerosol concentration multiplied by the instantaneous flow rate. The integration was done only for concentrations exceeding 5% of maximal expired aerosol concentration to reduce error due to baseline noise in the signal.(7)
Aerosol dispersion is defined as the change in half-width between the inspired and expired bolus and was calculated using the following equation
 |
(2) |
where Hin and Hex were the half-width between the inspired and expired bolus, respectively.(8) The half-width was defined as the volume over which particle concentration was higher than half the maximum concentration of the bolus.(5,7)
Data were grouped on the basis of four categorical variables: carrier gas (air or heliox), particle size (1 or 2 μm), subject number (1–11), and penetration volume (150, 300, 500, 800, and 1200). A oneway ANOVA for two correlated samples was performed to test for differences between the chosen categorical variables. Post-ANOVA pair-wise comparisons using Tukey HSD test was performed for tests showing significant F ratios. Significant differences were accepted at the p < 0.05 level.
Previous studies(9) have shown that, for Vp > 100 mL, both deposition and dispersion increase linearly with increasing penetration volume in air. The same held true for tests performed in heliox as illustrated in Figure 2 (open symbols). Actual Vp values measured during testing varied from the five target penetration volumes specified in the computer software. In order to compare deposition at target penetration volumes, data points for a given subject, particle size, and carrier gas were obtained for each target Vp via linear regression analysis (closed symbols on Fig. 2).
FIG. 2.
Aerosol deposition (DE, A) and dispersion (H, B) in one subject. Data are for 1 μm-diameter particles with heliox as the carrier gas. Raw data are shown with open symbols (○). Closed symbols (●) were obtained for each target Vp via linear regression analysis (solid line). See text for details.
For the 2-μm analysis, recorded expired bolus tracings at deeper penetration volumes had unacceptably high noise-to-signal ratios because of high deposition, and hence low exhaled aerosol concentrations. Data with DE < 80% were therefore eliminated from the analysis.
Results
Data were obtained in all 11 subjects for tests performed with 1-μm-diameter particles. For the 2-μm-diameter particle analysis, one subject did not provide a sufficient number of data points and was eliminated from the analysis. Deposition and dispersion increased with increasing penetration volume for both particle sizes and for each carrier gas.
Aerosol deposition
The effect of heliox on aerosol deposition is shown in Table 2 and Figure 3 for tests performed with 2-μm-diameter particles. Deposition (mean ± SD) is shown as a function of volume penetration for both air (black circles) and heliox (gray triangles) for Vp = 150, 300, and 500 mL. A high noise-to-signal ratio associated with high deposition prevents accurate measurement of high deposition values. Because the penetration volume at which 100% deposition was reached ranged from 691–1056 mL with air as the carrier gas, and 608–980 mL with heliox as the carrier gas, an average deposition at Vp = 800 and 1200 was not used due to the high signal-noise-ratio associated with high deposition. The penetration volume at which full deposition was predicted to occur was used as an additional data point. This value was extrapolated from the linear regression equations describing the deposition patterns for a given particle size and carrier gas for each subject. In Figure 3, this value averaged over the 10 subjects (mean ± SD) is plotted at DE = 100 with both air and heliox as the carrier gas. For 2-μm-diameter particles, deposition was significantly higher by 6 ± 7%, with heliox as the carrier gas at Vp = 500 ml (p < 0.05). The penetration volume at which full deposition was reached was significantly lower with heliox (834 ± 145 mL) than with air (912 ± 127 mL, p < 0.05).
Table 2.
Deposition (DE) and Dispersion (H) data (Mean ± SD) for 2 μm-Diameter Particles
| |
DE, % |
H, mL |
||
|---|---|---|---|---|
| Vp, mL | Air | Heliox | Air | Heliox |
| 150 | 20.0 ± 7.6 | 15.8 ± 4.7 | 203 ± 27 | 206 ± 32 |
| 300 | 36.2 ± 7.2 | 35.2 ± 4.9 | 287 ± 25 | 274 ± 20 |
| 500 | 57.8 ± 8.9 | 61.0 ± 10.2 | 399 ± 36 | 365 ± 24 |
Vp, penetration volume.
FIG. 3.
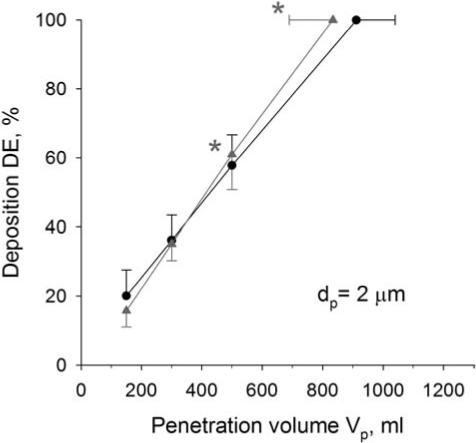
Deposition of 2 μm-diameter particles in air (●) and heliox (▲) as a function of penetration volume. Data with DE < 100% are means ± SD averaged over 10 subjects as a function of Vp. Data with DE = 100% (mean ± SD) are predictions extrapolated by linear regression from measured values for each subject. *Significantly different compared to air data. For clarity purposes, only one-sided error bars are shown on the figure.
Deposition data obtained with 1 μm-diameter particles are presented in Table 3 and in Figure 4, in which the relative change in deposition between heliox and air is plotted as a function of penetration volume. The relative change was calculated as follows for each subject at each penetration volume
 |
(3) |
Table 3.
Deposition (DE) and Dispersion (H) Data (Mean ± SD) for 1 μm-Diameter Particles
| |
DE, % |
H, mL |
||
|---|---|---|---|---|
| Vp, mL | Air | Heliox | Air | Heliox |
| 150 | 14.7 ± 4.6 | 10.5 ± 5.3 | 212 ± 39 | 209 ± 22 |
| 300 | 22.9 ± 4.7 | 20.4 ± 5.2 | 287 ± 38 | 279 ± 21 |
| 500 | 33.8 ± 5.2 | 33.7 ± 5.8 | 388 ± 40 | 372 ± 27 |
| 800 | 50.1 ± 6.5 | 53.5 ± 7.9 | 539 ± 51 | 513 ± 43 |
| 1200 | 71.8 ± 8.7 | 80.0 ± 11.7 | 741 ± 71 | 699 ± 69 |
Vp, penetration volume.
FIG. 4.
Relative increase in deposition of 1 μm-diameter particles when breathing heliox instead of air. Data are plotted as a function of penetration volume with the symbols representing individual data and the bars the averaged data (mean ± SD, N = 11). A negative relative increase corresponds to a decrease in deposition in heliox compared to air. *Significantly different compared to air data.
Both individual data (symbols) and averaged data (bars) are shown on Figure 4 (mean ± SD, N = 11). Data showed significantly less deposition with heliox as carrier gas compared to air at Vp = 150 mL and Vp = 300 mL (p < 0.05) and significantly more deposition with heliox as the carrier gas compared to air at Vp = 1200 mL (p < 0.05).
Aerosol dispersion
Aerosol bolus dispersion increased continuously with increasing penetration volume for each particle size both in air and heliox (Tables 2 and 3). Figure 5 displays the relative change in dispersion of 1 μm-diameter particles in heliox compared to air as a function of penetration volume with the symbols representing individual data and the bars the averaged data (mean ± SD, N = 11). The averaged values were small, however statistically significant for Vp = 800 and 1200 mL. Relative changes in dispersion were computed in a similar manner than for relative changes in deposition [see Equation (3)].
FIG. 5.
Relative increase in dispersion of 1 μm-diameter particles when breathing heliox instead of air. Data are plotted as a function of penetration volume with the symbols representing individual data and the bars the averaged data (mean ± SD, N = 11). A negative relative increase corresponds to a decrease in dispersion in heliox compared to air. *Significantly different compared to air data.
Comparison with previous aerosol bolus studies
Data presented in this study were collected using the same technique as in previous studies by our group in which 3 of the 11 subjects also participated.(5,7,10) Data from the present study that were obtained in these three subjects agreed well with that obtained previously.(7) In both studies, data were obtained with air at a flow rate of ∼0.5 L/sec and with 1 μm-diameter particles. There was no significant difference between the two datasets.
Discussion
Deposition
The objective of this study was to determine the effect of the carrier gas on aerosol deposition patterns within the human lung. A previous study by our group showed that there was a statistically significant decrease in deposition when using heliox compared to air for 1 and 2 μm-diameter particles (p <0.05).(3) Regional deposition could not be directly measured from those data but we speculated that when breathing heliox instead of air, deposition was reduced in the URT and increased in the respiratory bronchioles and alveoli. In the present study, regional deposition was measured using the aerosol bolus technique.
The penetration and subsequent deposition of inhaled aerosols in the human lung depends on both the physical properties of the particles (including size, shape, and density) and on the flow regime of the carrier gas. Flow regime is affected by the physical properties of the gas (including viscosity and mean free path), and also by the breathing pattern and by the geometry of the respiratory tract.
Our data show that heliox increases particle deposition in the peripheral lung. For 2 μm-diameter particles (Fig. 3), while small in absolute values, deposition was significantly higher at deeper penetration volumes (Vp = 500 mL) with heliox compared to air and full deposition was reached at a shallower penetration volume with heliox than with air. Even though deposition was not significantly lower at Vp = 150 mL (p = 0.14), seven out of 10 subjects showed a trend for lower deposition at shallow penetration volumes. For 1 μm-diameter particles (Fig. 4), DE was significantly lower (p < 0.05) at shallow penetration volumes (Vp = 150 and 300 mL) and significantly higher at deep penetration volumes (Vp = 1200 mL) with heliox. These results show increased particle deposition in the respiratory bronchioles and beyond with heliox as carrier gas compared to air. Because the flow rate and inspired volumes were tightly controlled and were the same for experiments in both air and heliox, a deeper penetration of the aerosol while breathing heliox cannot account for the differences in peripheral deposition. Rather, the increase in peripheral lung deposition must be attributed to a larger number of particles reaching and depositing in the lung periphery because of less deposition at shallow penetration volumes. It should also be noted that deposition measured this way is the cumulative deposition at all depths up to a given penetration volume. Thus, the increase in peripheral deposition in heliox is larger than the data might at first suggest, as decreased deposition at shallow penetration volumes will decrease cumulative deposition in measurements made at higher penetration volumes as the bolus traverses the proximal airways.
Deposition of 1–2 μm-diameter particles in the URT and first generations of conducting airways occurs by two main mechanisms: inertial impaction and turbulent mixing. Deposition by inertial impaction is a velocity-dependent mechanism that occurs predominantly at the nose, pharynx, and bifurcations of large airways. Turbulent gas flow, associated with high Reynolds numbers (Re), generates random and dispersed particle motion that increases deposition in the URT and larger conducting airways. Deposition may also be enhanced near the initial bifurcations of the lung by secondary vortical flows also favored by a high Re.(11)
A study by Matida et al.(12) suggests that turbulent mixing is as important as inertial impaction for particle deposition. Using computational modeling where both factors could be studied separately, Matida et al.(12) predicted aerosol deposition of 2.5 to 5 μm-diameter particles in an idealized mouth model for adults. When both the averaged values of mean velocities and the fluctuating component of turbulent flow were modeled, predicted deposition agreed remarkably well for all particle sizes with experimental data obtained in a similar physical model. When only averaged values of mean velocities were calculated and when, as a consequence, only the inertial effects were accounted for in particle transport, Matida et al.(12) predicted deposition that was ∼40% of that measured in the physical model. These predictions suggest that deposition due to turbulent mixing is of about the same magnitude as that due to inertial impaction.
Gravitational sedimentation becomes the mechanism of deposition in the narrower airways of the low-speed regions of the peripheral lung. The sedimentation velocity of the aerosols depends upon the mean free path of the gas molecules and the gas viscosity. Sedimentation velocity can be calculated by the following equation (Stoke's law)
 |
(4) |
where ρp and dp are the density and diameter of the particles, respectively, μ is the gas viscosity, g is gravitational acceleration and Cc is the Cunningham correction factor. This correction factor accounts for the decreased air resistance due to slippage when the size of the particle approaches the mean-free path of the gas molecules. Sedimentation velocities were calculated at a temperature of 37 Celsius and a pressure of 760 mmHg. Sedimentation velocity was slightly lower in air (35.8 μm/sec) than in heliox (37.2 μm/sec) for 1 μm-diam particles but slightly higher in air (132.4 μm/sec) than in heliox (130.1 μm/sec) for 2 μmdiameter particles. Given the similar sedimentation velocities in the different gases, it is unlikely that the increased peripheral deposition we observed resulted from changes in intrinsic mobility of the particles between air and heliox. Our results rather suggest that flow characteristics are more important than sedimentation velocity for determining peripheral deposition.
The flow regime within an airway is characterized by the Reynolds number (Re = 2 rvρ/η, where r is the airway radius, v is mean gas velocity in the airway, ρ and η are gas density and viscosity, respectively). Turbulent flow is favored over laminar flow when Re exceeds a critical value. Turbulence is probable when Re > 2000 in a straight, smooth tube.(13) However, within the complex geometry of the lung, turbulence occurs at lower Re's. Dekker(14) has observed turbulence beginning to appear in tracheal casts for Reynolds numbers as low as ∼500. In this study, flow rate was ∼450 mL/sec, which corresponds to a Reynolds number of ∼1700 in air implying the likely presence of turbulence in our study. Flow in heliox is characterized by a Re approximately three times lower than air for the same conditions (Re ≈ 560). Turbulent airflow in the trachea and secondary transitional flows in the first generations of conducting airways, which are both present at normal breathing rates, are therefore greatly reduced or even absent when breathing heliox instead of air. In the low speed regions of the peripheral lung, laminar flow is likely for both air and heliox. Thus, the flow regime of the two gases differs only as air moves through the URT and upper conducting airways.
Dispersion
The measurement of aerosol bolus dispersion is a means of probing convective mixing at different depths within the lung. There were no significant differences in dispersion between heliox and air at shallow penetration volumes (Fig. 5). Yet, deposition at shallow penetration volumes was significantly lower when breathing heliox instead of air. It should be noted that aerosol dispersion as measured by the bolus technique only provides information on axial mixing and not on radial mixing processes. While radial mixing induced by turbulent flows tend to reduce axial streaming by blunting the flow profiles, other factors such as ventilation inhomogeneities and flow asynchrony are thought to be dominant mechanisms affecting aerosol dispersion.(15) Therefore, while there was no change in axial mixing, there might be significant reductions in radial mixing processes within the URT and larger conducting airways when breathing heliox instead of air. Such a difference would not be reflected in our measurement of dispersion.
Although small, there was a significant reduction in dispersion at large penetration volumes (800 and 1200 mL) when breathing heliox instead of air. A previous study by our group(5) linked a reduction in dispersion observed in microgravity compared to normal gravity to a reduction in gravitational convective ventilatory inhomogeneities. The smaller dispersion observed when breathing heliox instead of air suggest a decrease in ventilation inhomogeneities when breathing a reduceddensity gas. Christopherson and Hlastala(16) studied pulmonary gas exchange during altered density gas breathing. They determined both blood and ventilation distributions by the multiple inert gas elimination technique and characterized the distributions by its mean VA/Q ratio and log standard deviation (log SD). In their study, the ventilation distribution showed a significant decrease in the log SD when breathing heliox instead of air, indicating an improvement of ventilation distribution during heliox breathing heliox instead of air, indicating an improvement of ventilation distribution during heliox breathing. These observations(16) are consistent with the reduced dispersion we measured at large penetration volumes.
This reduction in dispersion at large penetration volumes is likely to have a minimal impact on deposition. Indeed, Anderson et al.(17) have shown a significant increase in aerosol bolus dispersion in smokers compared to nonsmokers for penetration volumes larger than 400 mL, while there was no difference in deposition between the two groups. They attributed the increase in aerosol dispersion to an increase in ventilation heterogeneity. These observations suggest that the improvement in ventilation brought by heliox breathing has a minimal effect on the deposition measurements. Indeed, the change in ventilation distribution between heliox and air in healthy subjects is likely to be less that the change in ventilation distribution in smokers compared to nonsmokers.
Comparison with previous studies
Previous scintigraphy studies have investigated the effect of carrier gas on the pattern of aerosol deposition in healthy individuals. Svartengren et al.(1) administered 3.6 μm-diameter radio-tagged particles suspended in air or a helium–oxygen mixture via deep inhalations at a flow rate of 0.5 L/sec. They reported a trend for lower deposition in the mouth and throat (p < 0.05) and a trend for higher peripheral deposition with heliox compared to air (p < 0.05). Anderson et al.(2) performed a similar scintigraphic study with 3.6 μm-diameter radio-labeled particles in healthy human subjects. Anderson's study was slightly different in that it included a 1- to 2-sec breathhold between inhalation and exhalation. Anderson's study reported a significant decrease in deposition within the mouth and throat and a significant increase in alveolar deposition when breathing heliox compared to air. Our data supports these results.
Simulation studies predict a lower aerosol deposition in the upper respiratory tract and first generations of conducting airways in heliox than in air. Kleinstreuer et al.(18) performed computer simulations of laminar-transitional-turbulent air/particle flows in realistic human oral airways using a low Reynolds number turbulence model. Their simulations predict that particles follow the basic relationship between air flow and particle motion very well at low inspiratory flow rates, but particle motion seems to be random and dispersed due to turbulence associated with high inspiratory flow rates. They predicted that turbulent dispersion would enhance deposition of smaller particles throughout the trachea. Another computer simulation study by Comer et al.(11) focused on deposition patterns in the first two bifurcations of the lung. They predicted that particle motions near the initial bifurcations of the human lung are dependent upon secondary vortical flows favored by high Reynolds numbers, and they predicted these secondary flows enhance deposition. Both studies suggest deposition should be lower in the URT and first generations of conducting airways with heliox compared to air because the lower Reynolds number of flow in heliox. Our data agree with these predictions, as we observed a trend for a lower deposition with heliox at the lowest measured penetration volume (Vp = 150), corresponding to the approximate depth of the conducting airways.
We would expect that heliox would cause an even greater increase in peripheral deposition if tests were performed on subjects suffering from narrowed airways based on increased Reynolds number in these circumstances. In persons with narrowed airways, the radius of the tube is smaller and the velocity of the gas is higher for a given flow rate. These two values combine to produce a higher Reynolds number. Because of increased baseline turbulence the density-dependent conversion of turbulent flow to laminar flow associated with heliox would have a more pronounced effect in such persons. Anderson et al.(19) showed that the increase in alveolar deposition with heliox compared to air was larger in asthmatic subjects than in healthy subjects. Svartengren et al.(1) showed that a trend for decreased mouth and throat DE and increased alveolar DE became statistically significant after pharmacologically induced airway constriction. Piva et al.(20) administered radiolabeled aerosols to children with lower airway obstructions using heliox or oxygen. They showed that subjects who used heliox deposited the aerosols at a higher rate, and that this effect was most prominent in children with the highest degree of obstruction and that no significant differences existed if patients had only moderate airway obstruction.
In summary, our data show that heliox increases aerosol deposition in the periphery of the lung and reduces aerosol deposition in the upper respiratory tract. Heliox reduces turbulent flow in the trachea and reduces secondary transitional flows in the conducting airways, thereby reducing deposition by turbulent mixing. Reduced deposition in the upper airways allows more particles to penetrate, and subsequently, deposit via sedimentation in the periphery of the lung. Our data also suggest that the reduction in turbulent mixing mainly affects radial mixing, as no change in axial dispersion was observed at shallow lung depth between air and heliox despite a reduction in deposition. Finally, our data suggest that heliox improves ventilation distribution, as evidenced by a significant decrease in axial dispersion at deeper penetration volumes with heliox.
Acknowledgments
This work was supported by NIH Grant 1 RO1 ES11184 and NIH Grant 5 T35 HL07491-25, and by NSBRI/NASA Grant TD00701.
Disclosure Statement
No conflicts of interest exist.
References
- 1.Svartengren M. Anderson M. Philipson K. Camner P. Human lung deposition of particles suspended in air or in helium/oxygen mixtures. Exp. Lung Res. 1989;15:575–585. doi: 10.3109/01902148909069619. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M. Svartengren M. Philipson K. Camner P. Deposition in man of particles inhaled in air or helium-oxygen at different flow rates. J Aerosol Med. 1990;3:209–216. [Google Scholar]
- 3.Darquenne C. Prisk GK. Aerosol deposition in the human respiratoy tract breathing air and 80:20 heliox. J Aerosol Med. 2004;17:278–285. doi: 10.1089/jam.2004.17.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westenberger S. Gebhart J. Jaser S. Knoch M. Köstler R. A novel device for the generation and recording of aerosol micro-pulses in lung diagnostic. J Aerosol Sci. 1992;23:S449–S452. [Google Scholar]
- 5.Darquenne C. West JB. Prisk GK. Dispersion of 0.5–2 μm aerosol in micro- and hypergravity as a probe of convective inhomogeneity in the human lung. J Appl Physiol. 1999;86:1402–1409. doi: 10.1152/jappl.1999.86.4.1402. [DOI] [PubMed] [Google Scholar]
- 6.Darquenne C. Prisk GK. Effect of small flow reversals on aerosol mixing in the alveolar region of the human lung. J Appl Physiol. 2004;97:2083–2089. doi: 10.1152/japplphysiol.00588.2004. [DOI] [PubMed] [Google Scholar]
- 7.Darquenne C. West JB. Prisk GK. Deposition and dispersion of 1 μm aerosol boluses in the human lung: effect of micro- and hypergravity. J Appl Physiol. 1998;85:1252–1259. doi: 10.1152/jappl.1998.85.4.1252. [DOI] [PubMed] [Google Scholar]
- 8.Heyder J. Blanchard JD. Feldman HA. Brain JD. Convective mixing in human respiratory tract: estimates with aerosol boli. J Appl Physiol. 1988;64:1273–1278. doi: 10.1152/jappl.1988.64.3.1273. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard JD. Aerosol bolus dispersion and aerosol-derived airway morphometry: assessment of lung pathology and response to therapy, part 1. J Aerosol Med. 1996;9:183–205. doi: 10.1089/jam.1996.9.183. [DOI] [PubMed] [Google Scholar]
- 10.Mills CN. Darquenne C. Prisk GK. Mode shift of an inhaled aerosol bolus is correlated with flow sequencing in the human lung. J Appl Physiol. 2002;92:1232–1238. doi: 10.1152/japplphysiol.00655.2001. [DOI] [PubMed] [Google Scholar]
- 11.Comer JK. Kleinstreuer C. Kim CS. Flow structures and particle deposition patterns in double-bifurcation airway models. Part 2. Aerosol transport and deposition. J Fluid Mech. 2001;435:55–80. [Google Scholar]
- 12.Matida EA. Finlay WH. Breuer M. Lange CF. Improving prediction of aerosol deposition in an idealized mouth using large-eddy simulation. J Aerosol Med. 2006;19:290–300. doi: 10.1089/jam.2006.19.290. [DOI] [PubMed] [Google Scholar]
- 13.West JB. Respiratory Physiology—The Essentials. Lippincott Williams & Wilkins; Baltimore, MD: 2000. [Google Scholar]
- 14.Dekker E. Transition between laminar and turbulent flow in human trachea. J Appl Physiol. 1961;16:1060–1064. doi: 10.1152/jappl.1961.16.6.1060. [DOI] [PubMed] [Google Scholar]
- 15.Darquenne C. Prisk GK. Aerosols in the study of convective acinar mixing. Respir Physiol Neurobiol. 2005;148:207–216. doi: 10.1016/j.resp.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christopherson SK. Hlastala MP. Pulmonary gas exchange during altered density gas breathing. J Appl Physiol. 1982;52:221–225. doi: 10.1152/jappl.1982.52.1.221. [DOI] [PubMed] [Google Scholar]
- 17.Anderson PJ. Hardy KG. Gann LP. Cole R. Hiller FC. Detection of small airway dysfunction in asymptomatic smokers using aerosol bolus behavior. Am J Respir Crit Care Med. 1994;150:995–1001. doi: 10.1164/ajrccm.150.4.7921475. [DOI] [PubMed] [Google Scholar]
- 18.Kleinstreuer C. Zhang Z. Laminar-to-turbulent fluid-particle flows in a human airway model. Int J Multiphase Flow. 2003;29:271–289. [Google Scholar]
- 19.Anderson M. Svartengren M. Bylin G. Philipson K. Camner P. Deposition in asthmatics of particles inhaled in air or in helium–oxygen. Am Rev Resp Dis. 1993;147:524–528. doi: 10.1164/ajrccm/147.3.524. [DOI] [PubMed] [Google Scholar]
- 20.Piva JP. Menna Barreto SS. Zelmanovitz F. Amantea S. Cox P. Heliox versus oxygen for nebulized aerosol therapy in children with lower airway obstruction. Pediatr Crit Care Med. 2002;3:6–10. doi: 10.1097/00130478-200201000-00002. [DOI] [PubMed] [Google Scholar]


