Abstract
The arterivirus lactate dehydrogenase-elevating virus (LDV) causes life-long viremia in mice. Although LDV infection generally does not cause disease, infected mice that are homozygous for the Fv1n allele are prone to develop poliomyelitis when immunosuppressed, a condition known as age-dependent poliomyelitis. The development of age-dependent poliomyelitis requires coinfection with endogenous murine leukemia virus. Even though LDV is a common contaminant of transplantable tumors, clinical signs of poliomyelitis after inadvertent exposure to LDV have not been described in recent literature. In addition, LDV-induced poliomyelitis has not been reported in SCID or ICR mice. Here we describe the occurrence of poliomyelitis in ICR-SCID mice resulting from injection of LDV-contaminated basement membrane matrix. After exposure to LDV, a subset of mice presented with clinical signs including paresis, which was associated with atrophy of the hindlimb musculature, and tachypnea; in addition, some mice died suddenly with or without premonitory signs. Mice presenting within the first 6 mo after infection had regions of spongiosis, neuronal necrosis and astrocytosis of the ventral spinal cord, and less commonly, brainstem. Axonal degeneration of ventral roots prevailed in more chronically infected mice. LDV was identified by RT-PCR in 12 of 15 mice with typical neuropathology; positive antiLDV immunolabeling was identified in all PCR-positive animals (n = 7) tested. Three of 8 mice with neuropathology but no clinical signs were LDV negative by RT-PCR. RT-PCR yielded murine leukemia virus in spinal cords of all mice tested, regardless of clinical presentation or neuropathology.
Abbreviations: GFAP, glial fibrillary acidic protein; LDV, lactate dehydrogenase-elevating virus; MuLV, murine leukemia virus
Lactate dehydrogenase-elevating virus (LDV) is an enveloped RNA virus belonging to the family Arteriviridae. Originally identified as a contaminant of transplantable tumors,54 LDV remains a common contaminant of experimental biologic materials.43,47 The virus was so named because it reliably causes an acute rise in serum LDH after infection; consequently, measurement of serum LDH after inoculation of biologic materials into mice has historically been used as the classic method for detection of LDV contamination.54,63 LDV has a highly specific tropism for a small subset of mature mouse macrophages that express F4/80 cell surface antigen, and infection results in life-long viremia.50,61,64 LDV strains are classified as neuropathogenic (strains C and v) or nonneuropathogenic (strains P and vx), and stocks of LDV contain a mixture of these strains or quaispecies.13,14,37 Infection with LDV interferes with the normal immune response, including elevation of plasma IgG,18,19,35,50,51 autoantibody production,10,50 immune complex formation,9,51,57 and enhancement or suppression of tumor development.7,28,38 Despite these alterations in immune function, most mice infected with LDV remain asymptomatic. However, mouse strains that are homozygous for the Fv1n allele, such as AKR and C58, are prone to the development of LDV-induced poliomyelitis when immunosuppressed.37,42 The Fv1 is one of multiple genes that restricts replication of endogenous murine leukemia virus (MuLV), and mice with 2 copies of the Fv1n allele are permissive to infection with N-tropic ecotropic MuLV.41 Through an unknown mechanism, this expression of MuLV renders spinal cord anterior horn neurons susceptible to cytolytic infection with LDV.16,45-47 The resultant disease, commonly known as age-dependent poliomyelitis, has been studied as a model for amyotrophic lateral sclerosis.8,58 Experiments in mice using this model have demonstrated that both CD4+ and CD8+ T cells play an essential role in the protection against paralysis in susceptible strains.4,5,40 ICR-SCID mice have significantly decreased numbers of circulating T cells, although previous studies involving LDV infection in SCID mice have not reported the development of poliomyelitis.6,26,27,32,49 Indeed, this condition has previously been reported only in C58, AKR, C3H/Fg, and PL/J mice.12,16,17,41,47,49
Despite the fact that LDV is a common contaminant of transplantable tumors, the occurrence of clinical signs of poliomyelitis after inadvertent exposure to LDV has not been described in recent literature and has not been described in ICR or SCID mice. Here we report the occurrence of poliomyelitis in ICR-SCID mice resulting from injection of LDV-contaminated basement membrane matrix.
Case Report
Clinical history.
A cohort of 50 female IcrTac:ICR-Prkdcscid (ICR-SCID; age, 6 to 7 wk) mice were injected subcutaneously in the caudal flank with 2 × 106 HeLa cells suspended in 100 µL PBS and basement membrane matrix (BD Matrigel Basement Membrane Matrix, BD Biosciences, San Jose, CA) as part of a carcinogenesis study approved by the West Haven Veterans Affairs IACUC. Approximately 6 mo after inoculation, a mouse was presented to Veterinary Services for evaluation of hindlimb paresis and tachypnea. Consultation with the laboratory staff revealed that the basement membrane matrix vials used to suspend the cells were contaminated with LDV; however, when the laboratory was informed of the contamination, the mice had already been injected, so the study proceeded as planned. At the time this case was identified, 32 mice remained in the study. Eighteen mice had been euthanized previously due to experimental endpoints. To determine whether the clinical signs observed were induced by LDV, the mice remaining in the study were submitted for necropsy at the time of experimental endpoint (tumor size or ulceration) or when clinical signs of paralysis or tachypnea developed. The endpoint criteria included tumor size greater than 10% of animal body weight or any signs of sickness or distress (weight loss greater than 20% of total body weight, skin ulceration over the tumor, tumor interference with normal gait or movement, abnormal feeding or drinking behaviors, and dehydration not responsive to fluid resuscitation).
Husbandry.
Mice were housed in static autoclaved cages with corncob bedding on a 12:12-h photocycle and were offered free access to rodent chow (no. 2018, Harlan Teklad, Madison, WI) and autoclaved water. All mice were free of epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, ectromelia virus, mouse hepatitis virus, pneumonia virus of mice, mouse parvovirus, Sendai virus, mouse encephalomyelitis virus, mouse minute virus, Mycoplasma spp., and endo- and ectoparasites based upon sentinel data.
Necropsy.
After transport to the necropsy suite, mice were euthanized by carbon dioxide exposure and blood was collected by cardiac puncture. Serum was submitted for measurement of serum lactate dehydrogenase (LDH) concentration (Antech Diagnostics, Lake Success, NY). The remaining blood and samples of spinal cord, brain, spleen, liver, and kidney were frozen and stored at −80 °C for RT-PCR analysis. Remaining tissues were fixed in 10% formalin or Bouin fixative for histopathologic analysis.
Histopathology and immunohistochemistry.
After fixation, tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. All organs systems, including the brain, brainstem, multiple segments of spinal cord, and sciatic nerve, were examined in each mouse. Qualitative abnormalities in the CNS (such spongiosis, neuronal necrosis, astrocytosis, astrocytic hyperplasia, and axonal degeneration) were recorded and their distribution noted. Immunohistochemical detection of glial fibrillary acidic protein (GFAP) was performed by using rabbit polyclonal antihuman GFAP primary antibody (1:100; BioCare, Concord, CA). Antigen retrieval was not performed. Secondary antibody and horseradish peroxidase application was combined by using the Rat-and-Mouse Double-Stain Polymer Detection Kit (BioCare) as indicated by the manufacturer. Staining was visualized by using diaminobenzidine (BioCare). To detect LDV, a mouse antiLDV monoclonal antibody (a kind gift from Dr Peter Plagemann; University of Minnesota) was applied to spinal cord sections of 8 mice (of which 2 were paretic and all had spinal neuropathology) at a concentration of 1:100 after antigen retrieval (microwaving of tissues in 10 mM sodium citrate pH 6). Subsequent steps were similar to those for GFAP immunohistochemistry. In both reactions, negative controls omitting the primary antibody were used. Counterstaining with hematoxylin was used only on the negative control slide. Immunoreactivity was scored as negative (similar intensity to negative control) or positive (greater staining intensity than negative control).
PCR analysis.
Tissue samples collected at necropsy were analyzed by RT-PCR for LDV or MuLV RNA. In addition, the 2 cell lines used in the study and 1 of 2 vials of the basement membrane matrix (the other vial was unavailable for testing) were analyzed by PCR and RT-PCR for common biologic contaminants: lymphocytic choriomeningitis virus, minute virus of mice, mouse parvovirus, mouse hepatitis virus, Mycoplasma, reovirus, Sendai, Theiler murine encephalomyelitis virus, pneumonia virus of mice, epzootic diarrhea of infant mice virus, mouse norovirus, and LDV. Spleen, liver, kidney, brain, spinal cord, and tumor were homogenized in Dulbecco media. RNA was isolated from 50 µL of tissue homogenates (10% w/v) or whole blood by using RNeasy kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. LDV RT-PCR was performed by using 2 µL RNA, Brilliant SYBR Green qRT-PCR kit (Agilent Technologies, Cedar Creek, TX), and primers specific for LDV open reading frame 5/6 (LDV12943: 5′ CCT TCA TTT TCT TCT GCT GT 3′ and LDV13478: 5′ GGG GCT AGG ATG TAA CTT CT 3′). Reaction conditions were 30 min at 50 °C, 10 min at 95 °C, and 40 cycles of 94 °C for 30 s, 57 °C for 1 min, and 72 °C for 1 min. A 1130-bp fragment of the LDV genome spanning from the 3′ end of the GP3 gene through the N gene amplified from spinal cords from 7 mice was sequenced by the WM Keck Foundation Biotechnology Resource Laboratory at Yale University. Prior to MuLV RT-PCR, RNA samples were treated with RNase-free DNase (Qiagen) to remove any integrated MuLV DNA. Samples were considered to be actively infected with MuLV if RT-PCR, but not PCR, yielded a product. MuLV primers used were MLV1579 (5′ AAC GTC CCG ATT GGG ATT ACA CCA 3′) and MLV 2089 (5′ TGT CCA CTA ACT ACG GTG GCC AA 3′). PCR primers were obtained from the WM Keck Foundation Biotechnology Resource Laboratory at Yale University. All assays included positive and negative controls.
Statistical analysis.
Because LDH values were not normally distributed, we performed a nonparametric Wilcoxon rank sum test to determine whether the median values of LDH differed between mice that were PCR-negative and PCR-positive for LDV. Nonnormally distributed values ranges are presented as the interquartile range (25th to 75th percentile of the data). To test whether the presence of LDV was associated with neurologic lesions, we performed a χ2 test of association and used a Fisher exact test to correct for the small sample size. All statistical tests were 2-tailed, and a P value of less than 0.05 was used to denote statistical significance. Analyses were performed by using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Clinical and gross findings.
Of the 32 mice remaining in the study, a total of 23 mice (age, 7 to 15 mo) were submitted for pathologic evaluation (Table 1). Mice found dead (n = 9) were not analyzed. Mice were submitted due to hindlimb paresis or paralysis with or without forelimb involvement (n = 7), severe tachypnea (n = 2), and experimental endpoint (n = 14). Gross findings included atrophy of the limb musculature in all mice demonstrating paresis or paralysis and severe enlargement of the thymus consistent with thymic lymphoma in both mice that presented with severe dyspnea. Additional gross findings included multicentric organ enlargement consistent with lymphoma (n = 4) and lack of tumor development at inoculation site (n = 8).
Table 1.
Summary of clinicopathologic findings in 23 mice submitted for pathologic evaluation after injection with basement membrane matrix contaminated with LDV
| Clinicopathologic findings |
|||
| Paresis | LDV PCR | Neuropathology | No. of mice |
| + | + | + | 6 |
| +a | — | — | 1 |
| — | — | + | 3 |
| — | + | + | 6 |
| — | + | — | 3 |
| — | — | — | 4 |
Paresis developed secondary to meningeal lymphoma
Lactate dehydrogenase.
LDH concentration was measured in serum samples from 20 mice. Concentrations were highly variable, with a median of 2649 U/L (interquartile range, 0 to 5824 U/L) in mice PCR-negative for LDV and 7425 U/L (interquartile range, 2881 to 9990 U/L) in mice PCR-positive for LDV. LDH values showed a trend (2.8 times higher concentrations; P = 0.09) toward higher values in mice that were PCR-positive for LDV.
Histopathology.
Of the 23 mice submitted for pathologic examination, 15 demonstrated histologic lesions within the brainstem and spinal cord (Table 1). Of the 15 mice with neurologic lesions, 8 were submitted for necropsy approximately 6 mo after inoculation. Lesions in these mice were characterized by variably sized, asymmetric, and well-defined regions of spongiosis affecting ventral and lateral horns of the spinal cord, associated funiculi, and to a lesser extent, the brainstem (Figure 1). Spongiosis resulted from both intraneuronal vacuolation and vacuolation of neuropil and was accompanied by neuronal necrosis and loss, with intense astrocytic hyperplasia and hypertrophy. Lateral and ventral funiculi were affected by axonal degeneration, spongiosis, and a similar astrocytic response. Axonal degeneration was also evident in ventral roots and the sciatic nerve. No leukocytic response was present at this stage. The remaining 7 mice were presented over the next 8 mo and displayed much more subtle histologic lesions. Those with clinical evidence of paresis (n = 4) all had axonal degeneration of ventral nerve roots accompanied by modest predominantly mononuclear infiltration (Figure 2). Astrocytosis and spongiosis were mild to undetectable. Seven of the 23 mice presented with paresis—of these, all but one demonstrated histopathology as described above. The remaining paretic mouse had meningeal lymphoma and lacked spinal cord lesions. An additional 7 mice had lymphoma. Tumors arising from HeLa cells were localized to the subcutis as circumscribed masses. There was no evidence of an infiltrative growth pattern or metastasis in any mouse.
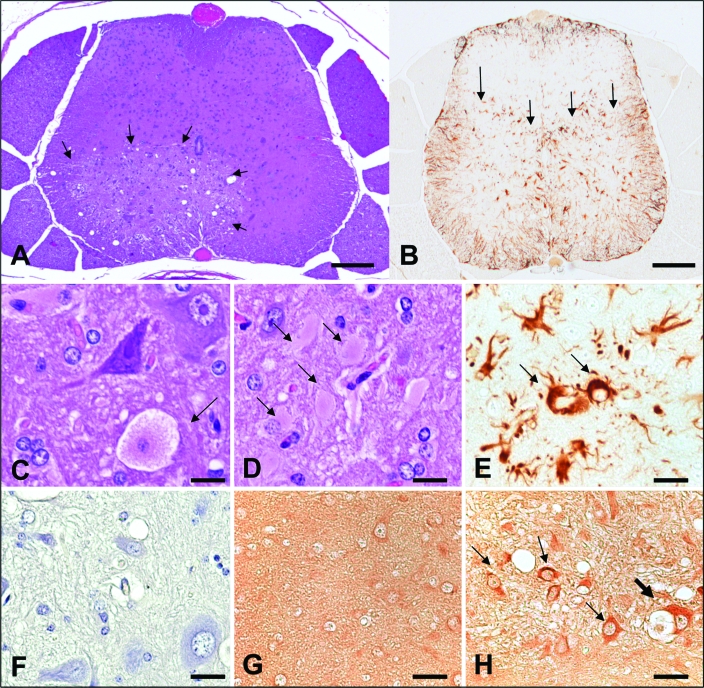
Figure 1.
Histopathology of mice presenting with paresis (mice presenting in the first 6 mo) (A) Cross-section of caudal lumbar spinal cord. A focal well-circumscribed region of malacia is present in the ventral horn (arrows). Hematoxylin and eosin stain; bar, 20 μm. (B) Cross-section of midlumbar spinal cord. Immunoreactive astrocytes are limited to regions corresponding to malacia in the ventral horns (arrows). AntiGFAP immunohistochemistry; bar, 20 μm. (C) Ventral horn, spinal cord. A dying neuron with swollen granular cytoplasm and a pale nucleus is indicated (arrow). Hematoxylin and eosin stain; bar, 5 μm. (D) Ventral horn, spinal cord. Numerous hypertrophic astrocytes with dense eosinophilic cytoplasm are indicated (arrows). Hematoxylin and eosin stain; bar, 5 μm. (E) Ventral horn, spinal cord. Strongly immunoreactive hypertrophic astrocytes with thick processes and abundant cytoplasm are indicated (arrows). AntiGFAP immunohistochemistry; bar, 5 μm. (F) Ventral horn, spinal cord. Omission of the primary antibody confirms lack of nonspecific immunoreactivity from the secondary antibody. Negative control, antiLDV immunohistochemistry; bar, 5 μm. (G) Dorsal horn, spinal cord. Dorsal horn regions lacking evidence of pathology also fail to demonstrate antiLDV immunoreactivity. AntiLDV immunohistochemistry; bar, 5 μm. (H) Ventral horn, spinal cord. AntiLDV immunoreactivity is noted within vacuolated cytoplasm of a degenerating neuron (thick arrow) as well as within astrocytes (small arrows). AntiLDV immunohistochemistry; bar, 5 μm.
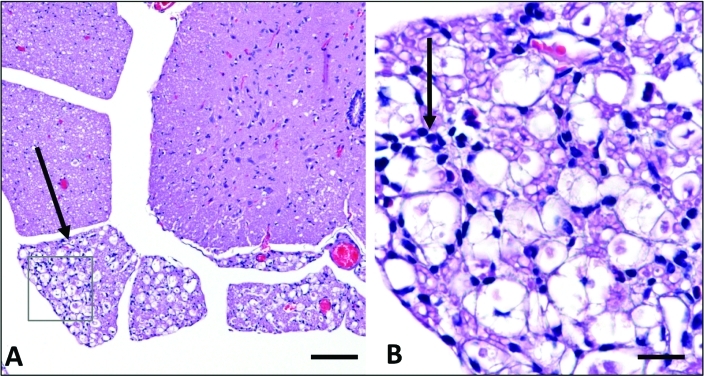
Figure 2.
Histopathology of mice presenting with paresis (mice presenting after 6 mo) (A) Ventral horn, spinal cord. Marked regional axonal degeneration of ventral roots is evident (arrow). Hematoxylin and eosin stain; bar, 50 μm. (B) Ventral root, spinal cord: boxed area from A. Small numbers of mononuclear cells admixed with occasional neutrophils infiltrate degenerate ventral roots (arrow). Hematoxylin and eosin stain; bar, 50 μm.
Immunohistochemistry.
GFAP immunohistochemistry was performed on representative sections of spinal cord of the 8 mice submitted during the first 6 mo. GFAP immunoreactivity was limited to ventral horn or brainstem regions displaying astrogliosis and astrocytic hypertrophy on sections stained with hematoxylin and eosin (Figure 1 A through F). The spinal cords of the same 8 mice were immunolabeled for LDV. Of these, 6 demonstrated cytoplasmic immunolabeling of neurons and astrocytes. Immunolabeling was evident in affected ventral or intermediate horn regions, but not dorsal horns, thus corresponding to the pattern of astrocytosis noted on GFAP immunolabeling (Figure 1 G through H). All 6 mice with LDV immunolabeling were also positive for LDV by RT-PCR. The 2 mice that were negative for LDV immunostaining were negative for LDV by RT-PCR but had neuropathologic lesions.
PCR analysis of tissues, cells, and basement membrane matrix.
RT-PCR analysis for LDV was performed on multiple tissues including spleen, liver, kidney, brain, spinal cord, and blood. Testing of the first 9 mice revealed 100% correlation between multiple tissues of the same mice, and thereafter RT-PCR analysis was performed on spinal cord samples only. All negative results were confirmed by testing multiple tissues. Fifteen of the 23 mice evaluated tested positive for LDV by RT-PCR. All 6 paretic mice with typical neuropathologic lesions were LDV-positive. Of the 15 mice with typical neuropathology described earlier, 12 were LDV-positive and 3 were LDV-negative. Of the 8 animals with no neuropathology, 5 were LDV-negative, and 3 were LDV-positive (Table 1). The Fisher exact χ2 test of association showed that the odds of neurologic lesions in LDV-positive mice were 6.7 times higher than those that are LDV-negative, but this finding was not statistically significant (P = 0.07). A 1130-bp fragment of the LDV genome spanning from the 3′ end of the GP3 gene through the N gene was amplified from spinal cords from 7 mice. The fragment was sequenced and was found to have the highest homology with the nonneuropathogenic LDV-P virus61 (91% at the nucleotide level and 96% at the amino acid level).
DNase-treated MuLV RNA was amplified by RT-PCR from the spinal cords of all mice, confirming the presence of actively replicating retrovirus within spinal tissue. The amplified product was sequenced and was found to have 100% homology with MuLV isolates AKV and SL3-3. PCR and RT-PCR analysis of the 2 cell lines inoculated into the mice were negative for all pathogens tested. A representative vial of basement membrane matrix used in the mouse study was positive for LDV by RT-PCR and was negative for all other pathogens by RT-PCR and PCR. The other vial of basement membrane matrix was unavailable for testing.
Discussion
This case report presents an outbreak of hindlimb paresis in a cohort of immunodeficient mice exposed to basement membrane matrix harboring LDV. Because LDV can induce age-dependent poliomyelitis in immunosuppressed C58 and AKR mice,37 this agent became an immediate suspect as the cause of the syndrome. Age-dependent poliomyelitis is characterized by hindlimb paresis and neuronal necrosis, gliosis, and myelopathy affecting the ventral gray matter and funiculi.37,60 Two additional components are essential for development of LDV-induced poliomyelitis: the presence of multiple proviral copies of N-tropic endogenous murine leukemia viruses (MuLV) and homozygosity of the permissive allele for N-tropic viral replication (Fv1n/n).1,15,39 Therefore, LDV-associated poliomyelitis is a complex disease requiring immunosuppression (due to age or by genetic or chemical means), the presence of 2 viruses (LDV and MuLV), and the presence of a replication permissive allele of Fv1n/n. In the case reported here, all 3 of these requirements were met: the mice were genetically immunosuppressed; we demonstrated the presence of LDV in the majority of mice; we were able to identify MuLV by RT-PCR from spinal tissue of all animals, implying the presence of active MuLV replication; and ICR mice, based on their origination from Swiss mice,29 are homozygous for the Fv1n allele.30
The LDV-negative mice described in this case report showed elevated and varied levels of serum lactate dehydrogenase. In normal mice, lactate dehydrogenase levels are reported to be in the general range of 360 to 550 U/L,11,21,22 whereas the LDV-negative mice we report here had a median serum LDH level of 2649 U/L . LDH levels can be elevated falsely by hemolysis; however, in the serum samples we analyzed, hemolysis was minimal or absent. In mice infected with LDV, LDH becomes acutely elevated secondary to destruction of a subset of macrophages involved in clearance of LDH from the blood.59 However, because LDH is a leakage enzyme, serum levels can be elevated secondary to cell destruction in a variety of tissues, including skeletal tissue. Tumors can cause cellular lysis in adjacent tissues, including skeletal muscle, as the tumors progress in size; all mice in this study were inoculated with tumor cells, and 7 mice had lymphoma. Therefore, in these particular mice, LDH levels would not have been a reliable indicator of which mice were infected with LDV. However, as we report in the results, LDH concentrations were 2.8 times higher in mice that were PCR-positive compared with negative for LDV, although this difference was not statistically significant (P = 0.07).
The standard tumor xenograft models in this carcinogenesis study use human tumor cell lines with rapid growth and therefore reach endpoint criteria quickly. The mice included in our case report were from a cohort of xenografts with genetically altered tumors displaying much slower growth curves. The additional latency in these aging mice allowed the presentation of the characteristic neurologic symptoms of age-dependent poliomyelitis, which would normally not be seen in rapid tumor models. However, some inconsistencies in our findings need to be addressed.
First, although LDV was retrieved from all paretic mice (with the exception of one with meningeal lymphoma), tissues from 8 mice did not amplify LDV by RT-PCR, including 3 that had evidence of typical neuropathology. One potential explanation for this finding is that exposure to LDV was not controlled in every mouse. Although a representative vial of the infected lot numbers were verified to be contaminated with LDV, one of the vials was unavailable for testing, and the primary emphasis of this carcinogenesis study was to standardize the number of tumor cells implanted and not the viral load. Therefore, we considered the possibility that mice that were LDV-negative by RT-PCR were not equally exposed to LDV, and the viral load may not have been high enough to result in an active infection. However, this possibility is unlikely, because mice become highly viremic regardless of the infectious dose.41 We also considered the possibility of false-negative RT-PCR results; however, this outcome is unlikely also, as supported by an earlier study.63 In the cited study, even though PCR was not as sensitive as the mouse bioassay in detecting very low levels of infection 3 d after inoculation, it was 100% successful in detecting infections in mice 1 and 3 wk after inoculation. Another potential cause for negative RT-PCR findings in virus-inoculated mice is viral neutralization; however, in this case, neutralization is not expected to occur, because SCID mice have no active B cells, and ICR-SCID mice in particular do not exhibit ‘leakiness’ (that is, the development of small numbers of antibody-producing B cells) as they age, as can occur with SCID mice on a C.B-17 background .62 Similarly, viral clearance is unlikely, given that LDV infection results in lifelong viremia with persistence in multiple organs, including the spleen and liver.2,44,50,51 In addition, to be certain, we tested multiple tissues on all LDV-negative mice, including the spleen. Moreover, viral levels appear to be even higher in SCID mice compared with immunocompetent mice;6,27 however, the effect of synchronous tumor development on the immune biology of LDV in SCID mice is unknown. Taken together, we are confident that that the LDV-negative mice truly were negative.
Second, apart from mild predominantly mononuclear radiculoneuritis in mice evaluated toward the end of the study, neuropathology was conspicuous by its lack of inflammation. LDV can induce a variable lymphohistiocytic inflammatory response that can vary from intense perivascular cuffing or leptomeningeal infiltration to a noninflammatory spongiform myelopathy of the ventral spinal cord.60 The genotype of these mice (SCID) may explain the general lack of inflammation in our cohort; however, an alternative hypothesis is that active MuLV was the primary agent driving neuropathology and that LDV coinfection increased the pathology caused by MuLV.36,55 This hypothesis is supported by the presence of lymphoma in 8 of 25 mice, a finding consistent with MuLV pathogenesis, although SCID mice are known to develop lymphoma in later life.47 Aside from induction of lymphoma, MuLV infection can result in other pathology such as CNS disease.48,53,56 The most widely studied example of MuLV-induced neuropathology is the syndrome seen in feral Lake Casitas mice.47 The Cas-Br-E strain of MuLV originates from these wild mice that exhibit spontaneous hindlimb paresis or lymphoma or both.23,24 Neuropathology is limited to the latero-ventral spinal cord and brainstem, and is characterized by neuronal necrosis, intense astrogliosis, and spongiform change of both gray matter and ventrolateral funiculi.3 Described lesions in this syndrome closely resemble those seen in our cohort. Transmission of the disease in the wild is vertical. It can be reproduced experimentally by inoculation of newborn mice with cloned Cas-Br-E MuLV derived from wild mice23,25,34 or by transgenic expression of either Cas-Br-E MuLV or its env gene.33 Genetic control of the naturally occurring disease in wild mice is achieved by segregation of a defective endogenous ecotropic virus restriction gene, Akvr1R/Fv4R, which is found in wild and strain G mice only.20,25,31 In addition to this classic example of MuLV-induced neuropathology, other strains of MuLV have been shown to induce neurodegenerative disease in mice.48,53,56 Although we detected active MuLV infection in all mice, not all of the mice developed neurologic lesions. This outcome is not surprising, given that experimental infection with some MuLV strains has resulted in a low incidence of disease or development of disease over a long period of time after inoculation.52,53 Another possibility is that the mice in the current report that did not develop neurologic disease would eventually have done so had they not been euthanized due to experimental endpoints.
Failure to amplify LDV from 3 mice with neuropathology and lack of neuropathology in 3 LDV-positive mice suggests that LDV infection alone does not fully explain the observed syndrome. We propose the following model of the findings in this cohort of mice. Given that MuLV was present in all mice and that those with neuropathology exhibited changes consistent with reports of Cas-Br-E MuLV pathology, active MuLV infection was likely necessary for expression of neuropathology in these mice. In addition, the mice were immunodeficient (SCID) and exposed to LDV, thus fulfilling known requirements for LDV-associated poliomyelitis. Furthermore, LDV was amplified from all mice with paresis and from most of those with neuropathology. Therefore, the combined effects of immunodeficiency, active MuLV infection, and LDV exposure likely contributed to development of this neurologic syndrome. However, failure to amplify LDV from some mice with neurologic lesions and the presence of lesions more consistent with those described for MuLV suggests that MuLV may be a required cause of neuronal damage, with a variable contribution by LDV.
Acknowledgments
We thank Dr Peter Plagemann for his kind gift of antiLDV monoclonal antibody and Dr Jody Willis for provision of veterinary care. Funding was provided in part by the American Geriatrics Society/Hartford Foundation Project Jahnigen Scholar Grant and the Yale Claude D Pepper Older Americans Independence Center (P30AG21342). Presented at the 59th National Meeting of the American Association for Laboratory Animal Science, Indianapolis, IN, on 11 November 2008.
References
- 1.Anderson GW, Palmer GA, Rowland RR, Even C, Plagemann PG. 1995. Infection of central nervous system cells by ecotropic murine leukemia virus in C58 and AKR mice and in-utero–infected CE/J mice predisposes mice to paralytic infection by lactate dehydrogenase-elevating virus. J Virol 69:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson GW, Rowland RR, Palmer GA, Even C, Plagemann PG. 1995. Lactate dehydrogenase-elevating virus replication persists in liver, spleen, lymph node, and testis tissues and results in accumulation of viral RNA in germinal centers, concomitant with polyclonal activation of B cells. J Virol 69:5177–5185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews JM, Gardner MB. 1974. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol 33:285–307 [DOI] [PubMed] [Google Scholar]
- 4.Bentley DM, Morris RE. 1982. T cell subsets required for protection against age-dependent polioencephalomyelitis of C58 mice. J Immunol 128:530–534 [PubMed] [Google Scholar]
- 5.Bentley DM, Watson SR, Morris RE. 1983. Age-related loss of Lyt1,2 cells in C58 mice results in susceptibility to lactic dehydrogenase virus-induced polioencephalomyelitis. Infect Immun 41:1389–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley DS, Broen JJ, Cafruny WA. 1991. Infection of SCID mice with lactate dehydrogenase-elevating virus stimulates B-cell activation. Viral Immunol 4:59–70 [DOI] [PubMed] [Google Scholar]
- 7.Brinton-Darnell M, Brand I. 1977. Delayed foreign-body tumorigenesis in mice infected with lactate dehydrogenase-elevating virus: brief communication. J Natl Cancer Inst 59:1027–1029 [DOI] [PubMed] [Google Scholar]
- 8.Bromberg MB. 1999. Pathogenesis of amyotrophic lateral sclerosis: a critical review. Curr Opin Neurol 12:581–588 [DOI] [PubMed] [Google Scholar]
- 9.Cafruny WA, Chan SP, Harty JT, Yousefi S, Kowalchyk K, McDonald D, Foreman B, Budweg G, Plagemann PG. 1986. Antibody response of mice to lactate dehydrogenase-elevating virus during infection and immunization with inactivated virus. Virus Res 5:357–375 [DOI] [PubMed] [Google Scholar]
- 10.Cafruny WA, Hovinen DE. 1988. Infection of mice with lactate dehydrogenase-elevating virus leads to stimulation of autoantibodies. J Gen Virol 69:723–729 [DOI] [PubMed] [Google Scholar]
- 11.Caisey JD, King DJ. 1980. Clinical chemical values for some common laboratory animals. Clin Chem 26:1877–1879 [PubMed] [Google Scholar]
- 12.Chen Z, Li K, Plagemann PG. 2000. Neuropathogenicity and sensitivity to antibody neutralization of lactate dehydrogenase-elevating virus are determined by polylactosaminoglycan chains on the primary envelope glycoprotein. Virology 266:88–98 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Li K, Rowland RR, Anderson GW, Plagemann PG. 1998. Lactate dehydrogenase-elevating virus variants: cosegregation of neuropathogenicity and impaired capability for high viremic persistent infection. J Neurovirol 4:560–568 [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Rowland RR, Anderson GW, Palmer GA, Plagemann PG. 1997. Coexistence in lactate dehydrogenase-elevating virus pools of variants that differ in neuropathogenicity and ability to establish a persistent infection. J Virol 71:2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contag CH, Plagemann PG. 1988. Susceptibility of C58 mice to paralytic disease induced by lactate dehydrogenase-elevating virus correlates with increased expression of endogenous retrovirus in motor neurons. Microb Pathog 5:287–296 [DOI] [PubMed] [Google Scholar]
- 16.Contag CH, Plagemann PG. 1989. Age-dependent poliomyelitis of mice: expression of endogenous retrovirus correlates with cytocidal replication of lactate dehydrogenase-elevating virus in motor neurons. J Virol 63:4362–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutelier JP, Brinton MA. Lactate dehydrogenase-elevating virus. : Fox J, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A. The mouse in biomedical research. San Diego (CA): Elsevier [Google Scholar]
- 18.Coutelier JP, Coulie PG, Wauters P, Heremans H, van der Logt JT. 1990. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol 64:5383–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutelier JP, Van Snick J. 1985. Isotypically restricted activation of B lymphocytes by lactic dehydrogenase virus. Eur J Immunol 15:250–255 [DOI] [PubMed] [Google Scholar]
- 20.Dandekar S, Rossitto P, Pickett S, Mockli G, Bradshaw H, Cardiff R, Gardner M. 1987. Molecular characterization of the Akvr1 restriction gene: a defective endogenous retrovirus-borne gene identical to Fv4r. J Virol 61:308–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillberger JE, Monroy P, Altman NH. 1987. The effect of 3 bleeding techniques on lactic dehydrogenase levels in mice: implications for lactic dehydrogenase virus bioassay. Lab Anim Sci 37:356–359 [PubMed] [Google Scholar]
- 22.Frith CH, Suber RL, Umholtz R. 1980. Hematologic and clinical chemistry findings in control BALB/c and C57BL/6 mice. Lab Anim Sci 30:835–840 [PubMed] [Google Scholar]
- 23.Gardner MB. 1985. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis 7:99–110 [DOI] [PubMed] [Google Scholar]
- 24.Gardner MB, Henderson BE, Officer JE, Rongey RW, Parker JC, Oliver C, Estes JD, Huebner RJ. 1973. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst 51:1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner MB, Wiley CA. 1994. Murine retroviral spongiform polioencephalopathy. Ann N Y Acad Sci 724:385–395 [DOI] [PubMed] [Google Scholar]
- 26.Hayashi T, Mori I, Noguchi Y, Itoh T, Saitoh M. 1992. Immunofluorescent antibody response to lactic dehydrogenase virus in different strains of mice. J Comp Pathol 107:179–183 [DOI] [PubMed] [Google Scholar]
- 27.Hayashi T, Ozaki M, Mori I, Saitoh M, Itoh T, Yamamoto H. 1992. Enhanced clearance of lactic dehydrogenase 5 in severe combined immunodeficiency (SCID) mice: effect of lactic dehydrogenase virus on enzyme clearance. Int J Exp Pathol 73:173–181 [PMC free article] [PubMed] [Google Scholar]
- 28.Howard RJ, Notkins AL, Mergenhagen SE. 1969. Inhibition of cellular immune reactions in mice infected with lactic dehydrogenase virus. Nature 221:873–874 [DOI] [PubMed] [Google Scholar]
- 29.The Jackson Laboratory [Internet] 2010. ICR/HaJ. JAX mice database. [Cited 15 June 2010]. Available at: http://jaxmice.jax.org/strain/009122.html
- 30.The Jackson Laboratory [Internet] 2011. Fv1 gene detail. JAX mice database. [Cited 28 February 2011]. Available at: http://www.informatics.jax.org/searches/accession_report.cgi?id=MGI:95595
- 31.The Jackson Laboratory [Internet] 2011. Fv4 gene detail. JAX mice database. [Cited 28 February 2011]. Available at: http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=markerDetail&key=8918
- 32.The Jackson Laboratory [Internet] 2011. Prkdcscid spontaneous allele detail. JAX Mice Database. [Cited 1 March 2011]. Available at: http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=alleleDetail&key=1285
- 33.Kay DG, Gravel C, Pothier F, Laperrière A, Robitaille Y, Jolicoeur P. 1993. Neurological disease induced in transgenic mice expressing the env gene of the Cas-Br-E murine retrovirus. Proc Natl Acad Sci USA 90:4538–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kay DG, Gravel C, Robitaille Y, Jolicoeur P. 1991. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci USA 88:1281–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Hu B, Harty J, Plagemann PG. 1990. Polyclonal B cell activation of IgG2a and IgG2b production by infection of mice with lactate dehydrogenase-elevating virus is partly dependent on CD4+ lymphocytes. Viral Immunol 3:273–288 [DOI] [PubMed] [Google Scholar]
- 36.Marques R, Antunes I, Eksmond U, Stoye J, Hasenkrug K, Kassiotis G. 2008. B lymphocyte activation by coinfection prevents immune control of Friend virus infection. J Immunol 181:3432–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez D, Brinton MA, Tachovsky TG, Phelps AH. 1980. Identification of lactate dehydrogenase-elevating virus as the etiological agent of genetically restricted, age-dependent polioencephalomyelitis of mice. Infect Immun 27:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaelides MC, Schlesinger S. 1974. Effect of acute or chronic infection with lactic dehydrogenase virus (LDV) on the susceptibility of mice to plasmacytoma MOPC315. J Immunol 112:1560–1564 [PubMed] [Google Scholar]
- 39.Monteyne P, Coulie PG, Coutelier JP. 2000. Analysis of the Fv1 alleles involved in the susceptibility of mice to lactate dehydrogenase-elevating virus-induced polioencephalomyelitis. J Neurovirol 6:89–93 [DOI] [PubMed] [Google Scholar]
- 40.Monteyne P, Meite M, Coutelier JP. 1997. Involvement of CD4+ cells in the protection of C58 mouse against polioencephalomyelitis induced by lactate dehydrogenase-elevating virus. J Neurovirol 3:380–384 [DOI] [PubMed] [Google Scholar]
- 41.Murphy WH, Nawrocki JF, Pease LR. 1983. Age-dependent paralytic viral infection in C58 mice: possible implications in human neurologic disease. Prog Brain Res 59:291–303 [DOI] [PubMed] [Google Scholar]
- 42.Nawrocki JF, Pease LR, Murphy WH. 1980. Etiologic role of lactic dehydrogenase virus infection in an age-dependent neuroparalytic disease in C58 mice. Virology 103:259–264 [DOI] [PubMed] [Google Scholar]
- 43.Nicklas W, Kraft V, Meyer B. 1993. Contamination of transplantable tumors, cell lines, and monoclonal antibodies with rodent viruses. Lab Anim Sci 43:296–300 [PubMed] [Google Scholar]
- 44.Notkins AL, Mahar S, Scheele C, Goffman J. 1966. Infectious virus–antibody complex in the blood of chronically infected mice. J Exp Med 124:81–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pease LR, Abrams GD, Murphy WH. 1982. Fv1 restriction of age-dependent paralytic lactic dehydrogenase virus infection. Virology 117:29–37 [DOI] [PubMed] [Google Scholar]
- 46.Pease LR, Murphy WH. 1980. Coinfection by lactic dehydrogenase virus and C-type retrovirus elicits neurological disease. Nature 286:398–400 [DOI] [PubMed] [Google Scholar]
- 47.Percy DH, Barthold SW. 2007. Pathology of laboratory rodents and rabbits. Ames (IA): Blackwell Publishing [Google Scholar]
- 48.Peterson KE, Du M. 2009. Innate immunity in the pathogenesis of polytropic retrovirus infection in the central nervous system. Immunol Res 43:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plagemann PG. 2001. Complexity of the single linear neutralization epitope of the mouse arterivirus lactate dehydrogenase-elevating virus. Virology 290:11–20 [DOI] [PubMed] [Google Scholar]
- 50.Plagemann PG, Moennig V. 1992. Lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus: a new group of positive-strand RNA viruses. Adv Virus Res 41:99–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plagemann PG, Rowland RR, Even C, Faaberg KS. 1995. Lactate dehydrogenase-elevating virus: an ideal persistent virus? Springer Semin Immunopathol 17:167–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Portis JL, Czub S, Garon CF, McAtee FJ. 1990. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag–pol sequences from nondefective Friend murine leukemia virus. J Virol 64:1648–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Portis JL, Czub S, Robertson S, McAtee F, Chesebro B. 1995. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J Virol 69:8070–8075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riley V, Wroblewski F. 1960. Serial lactic dehydrogenase activity in plasma of mice with growing or regressing tumors. Science 132:151–152 [DOI] [PubMed] [Google Scholar]
- 55.Robertson SJ, Ammann CG, Messer RJ, Carmody AB, Myers L, Dittmer U, Nair S, Gerlach N, Evans LH, Cafruny WA, Hasenkrug KJ. 2008. Suppression of acute anti-friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J Virol 82:408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robertson SJ, Hasenkrug KJ, Chesebro B, Portis JL. 1997. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J Virol 71:5287–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rowland RR, Even C, Anderson GW, Chen Z, Hu B, Plagemann PG. 1994. Neonatal infection of mice with lactate dehydrogenase-elevating virus results in suppression of humoral antiviral immune response but does not alter the course of viraemia or the polyclonal activation of B cells and immune complex formation. J Gen Virol 75:1071–1081 [DOI] [PubMed] [Google Scholar]
- 58.Sillevis Smitt PA, de Jong JM. 1989. Animal models of amyotrophic lateral sclerosis and the spinal muscular atrophies. J Neurol Sci 91:231–258 [DOI] [PubMed] [Google Scholar]
- 59.Smit MJ, Duursma AM, Koudstaal J, Hardonk MJ, Bouma JM. 1990. Infection of mice with lactate dehydrogenase-elevating virus destroys the subpopulation of Kupffer cells involved in receptor-mediated endocytosis of lactate dehydrogenase and other enzymes. Hepatology 12:1192–1199 [DOI] [PubMed] [Google Scholar]
- 60.Stroop WG, Brinton MA. 1983. Mouse strain-specific central nervous system lesions associated with lactate dehydrogenase-elevating virus infection. Lab Invest 49:334–345 [PubMed] [Google Scholar]
- 61.Stueckemann JA, Ritzi DM, Holth M, Smith MS, Swart WJ, Cafruny WA, Plagemann GW. 1982. Replication of lactate dehydrogenase-elevating virus in macrophages: 1. Evidence for cytocidal replication. J Gen Virol 59:245–262 [DOI] [PubMed] [Google Scholar]
- 62.Taconic [Internet] 2011. ‘Leakiness’ in C.B-17 SCID versus ICR SCID. Taconic library. [Cited 1 March 2011]. Available at: http://www.taconic.com/wmspage.cfm?parm1=326
- 63.Wagner AM, Loganbill JK, Besselsen DG. 2004. Detection of lactate dehydrogenase-elevating virus by use of a fluorogenic nuclease reverse transcriptase-polymerase chain reaction assay. Comp Med 54:288–292 [PubMed] [Google Scholar]
- 64.Zitterkopf NL, Haven TR, Huela M, Bradley DS, Cafruny WA. 2002. Transplacental lactate dehydrogenase-elevating virus (LDV) transmission: immune inhibition of umbilical cord infection, and correlation of fetal virus susceptibility with development of F4/80 antigen expression. Placenta 23:438–446 [DOI] [PubMed] [Google Scholar]