Abstract
Using domestic pigs as an animal model, we here validated a reproducible and standardized myocardial infarction (MI) surgical model, to achieve the largest possible infarct extent with the lowest morbidity and mortality. To this end, we included several anesthetic and perisurgical precautions to minimize surgical complications. Mortality and morbidity rates were compared among groups of pigs that underwent permanent occlusion at different locations of either the left circumflex or left anterior descending artery. In addition, to compare the resulting MI between groups, data were collected by using cardiac biomarkers (including troponin I), electrocardiography, and echocardiography. These data were correlated to the final mean infarct size calculated by microscopic studies. Proximal occlusions lead to high mortality rates, whereas distal occlusions induced rather small MI areas. The optimal occlusion site in terms of morbidity, mortality, and lesion extent was the midpoint of the left anterior descending artery. In this group, only one pig died, and group cardiac data showed a rise in biomarker levels, marked left ventricular dysfunction on electrocardiography and echocardiography, and well-defined transmural MI in both ventricles. Infarct size quantitated through histologic studies revealed an average 15% ventricular lesion. Because interanimal variability in results from this group was negligible, we consider that the induced myocardial injury of this model is reliable.
Abbreviations: d, distal; LAD, left anterior descending artery; LCX, left circumflex artery; m, midpoint; MI, Myocardial infarction; MMB, mass assay of creatine kinase; p, proximal
Ischemic cardiomyopathies are considered the leading cause of mortality in developed countries. In this group of diseases, myocardial infarction (MI) accounts for the largest proportion of deaths.20 These facts justify the search for an ideal myocardial infarction animal model to test new treatments and optimize diagnostic tests.16
The domestic pig is considered an ideal experimental choice to study human myocardial ischemia for several reasons: 1) the heart size of pigs and its weight relative to body weight is similar to those of the human heart;13,26 2) cardiac and vascular anatomy, ventricular performance, and electrophysiology of pigs are similar to those of humans,1,5,7,10,13,16,18,19,26 and 3) unlike other laboratory animals, pigs have negligible collateral circulation, so each coronary artery supplies a specific cardiac region.1,3,5,7,10,13,16,26
Open- and closed-chest methods have been used for induction of MI in pigs. Open-chest (surgical) models have the advantage of easy access for precise control of site of occlusion and direct visual assessment of contractile function.3,17,23 More recently, to avoid the trauma associated with thoracotomy or sternotomy and its possible effects on cardiac function,10,16,23 several closed-chest techniques, mainly by means of percutaneous catheterization, have been developed. Nevertheless, these methods present important limitations: the exact location, length, and duration of arterial occlusion; rate of thrombolysis; and reflux of the injected agent cannot be controlled reliably.3,10,16,17,23 Therefore, standardization of the infarct size is not possible. In addition, most closed-chest models require substantial and expensive instrumentation to perform the occlusion, identify its location, and assess the size of the coronary artery.3,10,17,23 Furthermore, percutaneous models induce endothelial damage, and the time required to perform the procedure can vary substantially, depending on the operator's experience and tortuosity of the coronary vessels.3,10,16
In the current study, we used the simplest possible method for inducing MI in pigs: open-chest surgical ligation of a coronary branch. Our purpose was to develop a reproducible and consistent MI porcine surgical model that yielded the largest infarct area associated with the lowest morbidity and mortality. We accomplished this goal by comparing the number of deaths, extension of infarct size, and degree of ventricular dysfunction among groups of pigs that underwent surgical occlusion at different locations of either the left circumflex (LCX) or the left anterior descending (LAD) artery. To evaluate cardiac function and infarct size in each pig, we applied various state-of-the-art diagnostic methods, including cardiac biochemical markers, electrocardiography, echocardiography, and pathologic studies. In addition, we describe in detail the preemptive analgesia (intravenous opioids plus local anesthetic) and balanced anesthesia we used, because we consider these components to be absolute requirements for the wellbeing of the pigs and important factors in the final surgical outcome. Our ultimate goal is the application of this method as a control model in experimental studies to evaluate the efficacy (in reducing infarct size) of various therapies for coronary heart disease.
Materials and Methods
All experiments were conducted according to the European Union Directive number 86/609/CEE for the use of animals in research. Male mixed-breed Landrace pigs (Sus scrofa domestica; n = 17; age, 3 mo; weight, 30 to 42 kg) were used in this study. The pigs were supplied from a local commercial swine producer (Ferjosama, Aveiro, Portugal). All animals were vaccinated and dewormed and, at clinical examination before surgery, demonstrated no signs of diseases. Pigs were housed in individual cages with wooden-slat floors and had free access to food and water. They were fed dry commercial diet (soybean- and corn-based) provided by the pig supplier (Ferjosama).
Anesthetic protocol.
Pigs were acclimated for at least 24 h before use in the study. Before and after surgery, pigs were handled carefully to avoid undue excitement, and body temperature was kept constant by means of a circulating-water heating pad (Gaymar T/Pump Heating System, Eickemeyer, Tuttlingen, Germany) and cage heating.
Pigs were fasted overnight before surgery. While in their quarters, pigs were sedated with midazolam hydrochloride (0.6 mg/kg IM; Zolamid, Hospira, Birmingham, United Kingdom). After 15 to 30 min, a cannula was introduced in a superficial ear vein, and anesthesia was induced with sodium thiopental (12.5 mg/kg IV initially and then to effect; B Braun Medical, Lisbon, Portugal) and isoflurane (4% to 5% by mask; Isoflo, Abbott, Rome, Italy). After orotracheal intubation, the 7- to 7.5-mm tube was connected to the anesthetic equipment (Ohmeda, Boc Health Care, Milan, Italy), and anesthesia was maintained with a mixture of isoflurane (1.5% to 2.5% in 100% oxygen) and morphine sulphate (Labesfal, Viseu, Portugal) at constant-rate infusion of 0.2 mg/kg. After administration of atracurium (2 to 4 mg/kg IV; Faulcurium, Hospira), pigs were ventilated at a rate of 10 to 12 breaths per minute (Ohmeda, Boc Health Care). Tidal volume was adjusted to maintain the end-tidal carbon dioxide concentration in the expired air at 33 mm Hg by use of a closed rebreathing circuit with soda lime canister. Excess anesthetic gases from pop-off valve were eliminated by active gas scavenging. End-tidal CO2 and oxygen saturation were monitored continuously (Diascope NT, S and W Medico Teknik A/S, Kolding, Denmark). Pigs were placed in supine position and shaved, and electrocardiographic electrodes were attached to the chest for continuous evaluation of cardiac electrical function. Normal saline (2 to 4 mL/kg hourly) was infused through the venous cannula in the auricular vein during surgery, to maintain adequate preload stability.
In case of ventricular tachycardia, multiple bolus doses of lidocaine (2.5 to 12 mg/kg IV; Lidoject 1%, Labesfal) were administered. When resuscitation was necessary, defibrillation was performed (Cardio-Aid defibrillator, S and W Medico Teknik A/S) by applying 360 J with the paddles pressed to the anterior chest wall (above the sternum on the right side and below the sternum on the left side), along with open-chest cardiac massage, as needed. These cardioversion attempts were terminated if unsuccessful for longer than 30 min.
At the end of the surgical procedure, the pericardium and pleural spaces were drained, and pigs were allowed to recover spontaneous breathing and were extubated. They were transported back to their quarters only when arterial oxygen tension stabilized above 95%. Pigs were monitored until their body temperature normalized (with digital thermometers) and they were able to ambulate. They then were observed at least 2 or 3 times daily for locomotor activity, respiratory changes, body temperature, and food and water intake.9 Analgesia was maintained during the first 48 h after surgery by using meloxicam (0.5 mg/kg IV daily; Metacam, Boehringer Ingelheim, Ingelheim, Germany). Acetylsalicylic acid (2.5 mg/kg IV; Aspegic, Sanofi Aventis, Lisbon, Portugal) and enrofloxacin (3 mg/kg IV; Alsir 5%, Bayer, Leverkusen, Germany) were given once daily until euthanasia, to prevent platelet aggregation and postsurgical infection, respectively. Pericardial and pleural drainage was performed daily until euthanasia.
On day 4 after surgery, pigs were sedated and maintained under general anesthesia with isofluorane (as described earlier) to repeat echocardiographic and 12-lead electrocardiographic studies. At euthanasia on day 5, the animals were premedicated with midazolam hydrochloride intravenously and then anesthetized and euthanized by intravenous overdose of sodium thiopental and potassium chloride.
Surgical procedures.
Under sterile conditions, after injection of ropivacaine (approximately 5 mL; Naropeine 7.5 mg/mL, Astrazeneca, Lisbon, Portugal) at the incision site, a partial midline sternotomy was performed (the manubrium was preserved). The pericardium was sectioned, and a pericardial cradle was created to support the exposed heart. Heparin (5000 IU IV; B Braun Medical) was administered just before coronary occlusion by ligature with silk (Silkam 2/0, B Braun Medical).
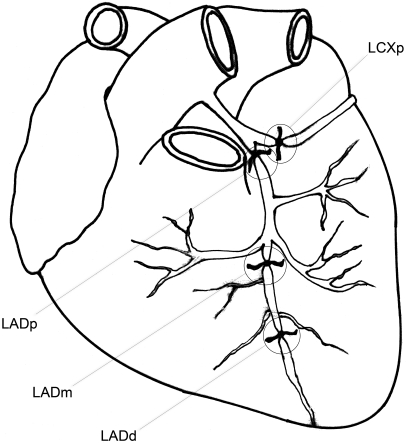
The initial series of experiments involved 3 pigs in which the proximal portion of the LCX (LCXp) was ligated. In an additional set of experiments involving 12 animals, the LAD was measured and ligated accordingly: proximally in 2 pigs (LADp), at its midpoint in 8 pigs (LADm), and distally in 2 pigs (LADd). Distal LAD occlusion (LADd) was defined as an occlusion at the distal third of the LAD. Given the size of our pigs, midpoint of the LAD (LADm; the site of occlusion) was defined as 6.5 cm from the apex (Figure 1). Two pigs were excluded from the study.
Figure 1.
Schematic coronary occlusion sites. LCXp, proximal occlusion of the left circumflex artery; LADp, proximal occlusion of the left anterior descending artery; LADm, occlusion at the midpoint of the left anterior descending artery; LADd, distal occlusion of the left anterior descending artery.
At the end of the surgery, the chest was closed, with 2 bilateral pleuropericardial tubes placed for drainage. Chest closure was performed in 3 layers: wire closure (no. 5 wire in an interrupted cruciate pattern) for the sternebrae, followed by a simple continuous suture pattern for the muscular layer and a horizontal intradermal pattern (both with Monoplus 0 [B Braun Aesculap, Tuttlingen, Germany]). Under aseptic conditions, the right or left lateral saphenous vein was dissected and cannulated with a 16-gauge, 20-cm central venous catheter (Arrow International, Reading, PA) for postsurgical collection of blood samples and administration of medication.
Diagnostic tests.
Cardiac biochemical markers.
A blood sample was collected from the jugular vein of each anesthetized pig for baseline analysis of cardiac biomarkers.
In order to capture the true up-slopes and peak values of cardiac biomarkers (and thus more accurately estimate the infarct size), serial blood samples (approximately 3mL each) were collected every 2 h during the first 10 h after surgery, at 24 h after surgery, and then once daily until euthanasia.6 Blood samples were centrifuged at 3100 × g for 5 min within 30 min of sampling, and the serum was stored in 2 microfuge tubes at −4 °C. The analysis included assessment of troponin I, myoglobin, total creatine kinase, isoenzyme MB of creatine kinase (measured by mass and activity assays), and lactate dehydrogenase. Although several biomarkers were used, analysis focused on the combination of 3: myoglobin (helpful in early detection of MI), cardiac troponin I (a marker that takes longer to rise but is highly specific) and MMB, as mass assay of creatine kinase (considered as effective as cardiac troponins in estimating infarct size).15,25 Calculations of areas under the curve (AUC) are presented to complement data provided by peak values. Myoglobin, cardiac troponin I, and MMB were determined by enzyme immunoassays (Dimension, Dade Behring, Newark, DE) which were performed according to the manufacturer's instructions.
Electrocardiography and echocardiography.
At baseline, with the pigs under general anesthesia and in right lateral recumbency, 12-lead electrocardiography and 2D and M-mode echocardiography were performed. By using a multichannel electrocardiography analyzer (Cardisuny, Fukuda ME, Tokyo, Japan), precordial leads were included to precisely determine the ventricular wall segment affected. Transthoracic echocardiography (Vivid 3 Pro digital ultrasonic scanner, GE Medical Systems, Ontario, Canada) allowed assessment of myocardial echogenicity, cardiac chamber size, wall thicknesses and amplitudes of motion, and valve morphology and motion. Wall and cavity dimensions allowed for calculation of indices of cardiac motion (shortening fraction and ejection fraction). For these measurements, short-axis views were obtained at the level of the chordae tendineae, by using the right parasternal window. To avoid intraobserver variability, 3 different M-mode images were obtained for each pig, and the mean of these 3 measurements was calculated.
To detect the maximal value of ST deviation, 12-lead electrocardiography was repeated 1 h after coronary occlusion (with pigs still under anesthesia), by using the same technical approach.22
On day 4 after surgery, pigs again were sedated and anesthetized for reevaluation of cardiac performance and electrical activity. Echocardiography and 12-lead electrocardiography as performed at baseline were repeated by using the same technical approach. To estimate left ventricular performance, the recommendations from ASE Committee for echocardiographic measurements of left ventricular dimensions, volumes, and wall thicknesses were followed.11 To avoid operator bias, both baseline and postocclusion results from electrocardiography and echocardiography were analyzed by independent observers who were blinded to groups and the focus of the investigation.
Histopathologic studies.
After euthanasia of pigs on day 5 after surgery, hearts were excised immediately, cleaned, and washed in saline solution for macroscopic and histologic evaluation. In all cases, the lengths to the occlusion site in the proximal and distal coronary artery segments were measured. By cannulating the proximal segment of the coronary artery, saline solution (NaCl 0.9%) was injected to confirm complete occlusion.
Hearts were stored in 10% buffered formaldehyde (Panreac Quimica SAU, Barcelona, Spain) for 24 h. Each heart then was filled and embedded in agar–agar suspension (Merck KGaA, Darmstadt, Germany) at 4%, which was allowed to harden by incubating for 4 h at 4 °C. The hearts were sliced circumferentially parallel to the atrioventricular sulcus from the apex to the base. Each slice was 10 mm thick. The basal surface of each slice was photographed with a digital camera (FE360, Olympus, Tokyo, Japan).
From the basal surfaces of the cardiac transverse slices, circumferential transmural 2- to 3-mm thick samples were cut (including septum and both ventricles). These tissue samples were processed routinely for histologic analysis and stained with hematoxylin and eosin and Masson trichrome. Masson trichrome provided a clear contrast of colors between viable myocardium (bright red to pink), rim of infarct with inflammatory tissue (gray), and infarction (purple to blue).14 We used Image J software (NIH, Bethesda, MD) to measure these 3 regions in each trichrome-stained sample by tracing the corresponding tissue boundaries. Infarct size of both ventricles was expressed as the percentage of affected myocardial area (necrosis + inflammatory tissue) in all myocardial areas analyzed, with infarct area % = infarct area × 100 / total myocardial area.
As for electrocardiographic and echocardiographic studies, an independent blinded investigator calculated mean infarct size through histologic studies.
Statistics.
Statistical analysis was performed using the PASW package, version 18.00 (SPSS, Chicago, IL). Data are presented as mean ± SE (where appropriate) and were considered to differ significantly if the P value was less than 0.05.
Results
The results presented are from 15 pigs; 2 pigs (both from the LADm group) were excluded from the study. One pig developed malignant hyperthermia during surgery (before coronary occlusion) and died. Sternotomy revealed that the other pig had pleural effusion and adherences from underlying disease; we therefore rejected this animal from analysis. Macroscopically, the effusion was serous and transparent, and culture demonstrated that it was aseptic. At echocardiography and necropsy, this pig showed an abnormal dimension of the right ventricle and thickness of its walls.
In the LCXp group, only 1 pig of 3 survived. This pig had very high peak values and AUC of cardiac biomarkers (Table 1). In addition, echocardiography reflected severe ventricular dysfunction 4 d after surgery (Table 2): ejection fraction fell from 60.9% to 36.8% (a 40% decrease) and the thickness of the posterior wall fell from 12.7 to 5.6 mm (a 56% decrease); the septal wall was nearly unaffected. Macroscopic analysis revealed a transmural infarct located at the inferiolateral myocardial region, affecting mostly the posterior wall. Calculated infarct size was 17.91%.
Table 1.
Peak values and areas under the curve (AUC; mean ± SE) of troponin I, myoglobin, and mass assay of creatine kinase of surviving pigs with coronary occlusions
| Troponin I |
Myoglobin |
Creatine kinase |
|||||
| Occlusion site | n | Peak (ng/mL) | AUC | Peak (ng/mL) | AUC | Peak (ng/mL) | AUC |
| LCXp | 1 | 109 | 7.964 | 2.341 | 81.342 | 165 | 10.620 |
| LADd | 2 | 41 ± 17 | 2.637 ± 227 | 696 ± 301 | 18.079 ± 2973 | 16 ± 3 | 587 ± 150 |
| LADm | 6 | 43 ± 10 | 2.647 ± 310 | 668 ± 115 | 10.374 ± 1.186 | 15 ± 4 | 360 ± 102 |
Results from LADp are not shown, because only baseline values were obtained.
Table 2.
Variation (mean ± SE) of ejection fraction, interventricular septal thickness at end-systole, left ventricular diameter at end-systole, and posterior wall thickness at end-systole of surviving pigs with coronary occlusions
| Ejection fraction (%) |
Interventricular septal thickness at end-systole (mm) |
Left ventricular diameter at end-systole (mm) |
Posterior wall thickness at end-systole (mm) |
||||||
| Occlusion site | n | 0 h | 96 h | 0 h | 96 h | 0 h | 96 h | 0 h | 96 h |
| LCXp | 1 | 60.9 | 36.8 | 12.4 | 13.7 | 28.1 | 40.3 | 12.7 | 5.6 |
| LADd | 2 | 69.9 ± 2.7 | 65.2 ± 0.6 | 16.2 ± 0.8 | 14.5 ± 1.5 | 20.8 ± 1.4 | 32.4 ± 3.2 | 13.9 ± 1.4 | 13.9 ± 2.8 |
| LADm | 7 | 65.5 ± 4.1 | 53.4 ± 3.9 | 14.9 ± 1.1 | 10.3 ± 1.2 | 24.6 ± 2.1 | 33.8 ± 2.4 | 13.7 ± 1.2 | 16.0 ± 1.2 |
Results from LADp are not shown, because only baseline values were obtained.
Neither of the 2 pigs in the LADp group survived. Therefore, no cardiac data could be obtained from diagnostic tests.
In the LADd group (n = 2), both pigs survived. Some discrepancies were observed in peak values and AUC of the various analyzed cardiac biomarkers (Table 1). At 4 d after ligation, the ejection fraction fell from 69.9% to 65.2% (a 7% decrease); changes in the thickness of the septal and posterior walls were negligible. Infarct sizes were 9.95% and 10.09%.
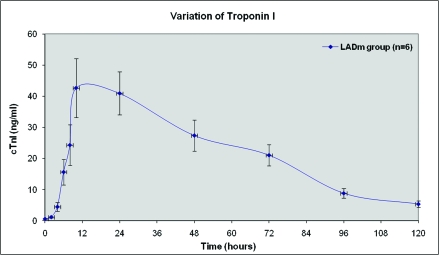
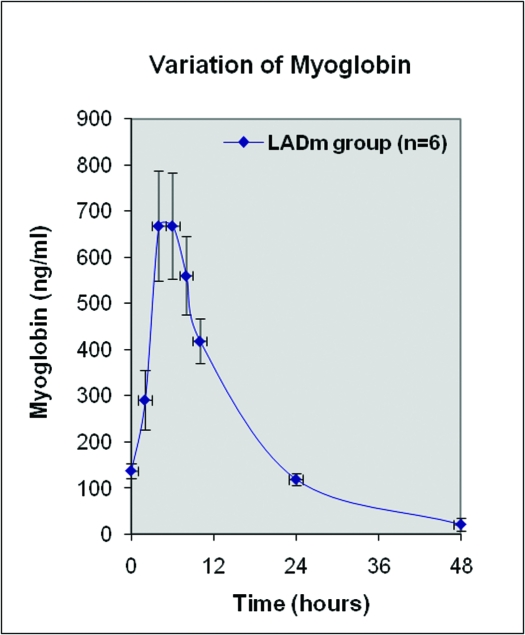
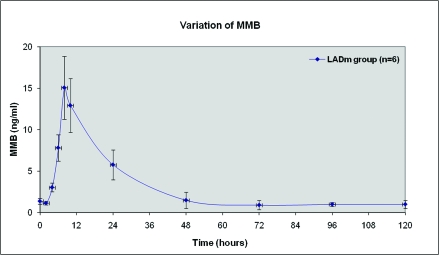
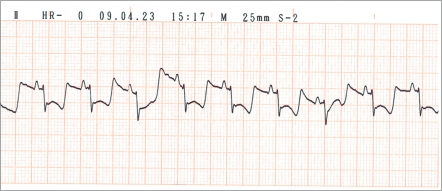
At baseline, all 8 pigs in the LADm group had normal values of biochemical markers, ECG tracings, and echocardiographic data. In this group, 7 of the 8 pigs survived. As shown in Figures 2 through 4, levels of cardiac biomarkers rose during the first 10 h after occlusion, followed by a gradual decrease throughout the remaining 4 d. The curves of cardiac biomarkers were somewhat distinct in the 6 pigs analyzed (2 animals were excluded from analysis). In all 8 pigs of the LADm group, electrocardiographic recordings obtained 1 h after coronary occlusion confirmed MI as represented by ST-segment deviations (elevations or depressions; Figure 5). These ST changes resolved completely by day 4 after surgery. Inversions of T waves occurred also. On average, heart rate at baseline was 85.1 ± 3.0 bpm, at 1 h after occlusion was 86.7 ± 10.3 bpm, and on day 4 was 105.0 ± 19.0 bpm.
Figure 2.
Average variation of troponin I from baseline values to the fifth day, in LADm occlusions. Two pigs are not included: pig 8 did not survive MI and pig 7 was submitted to numerous cardioversion maneuvers and showed extraordinary increase of troponin I.
Figure 3.
Average variation of myoglobin from baseline to day 5 after LADm occlusion. Two pigs are not included: pig 8 did not survive MI, and pig 7 underwent numerous cardioversion maneuvers and showed extraordinary increase of myoglobin.
Figure 4.
Average variation of MMB (mass assay of creatine kinase) from baseline to day 5 after LADm occlusion. Two pigs are not included: pig 8 did not survive MI, and pig 7 underwent numerous cardioversion maneuvers and showed extraordinary increase of myoglobin.
Figure 5.
Derivation II on ECG recording of pig 6 at 1 h after LADm occlusion, showing marked ST-segment depression.
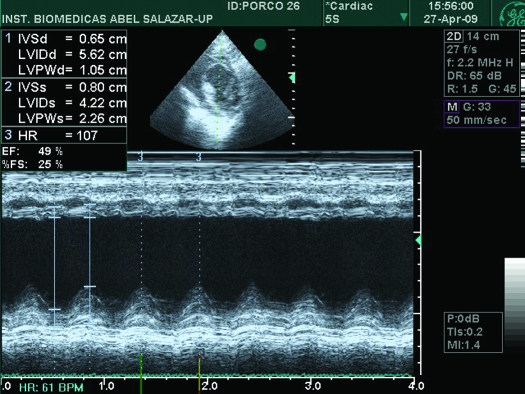
Echocardiography in all 7 LADm pigs that survived until analysis revealed a decrease in systolic function from baseline to 4 d after occlusion, with remarkable wall-motion abnormalities. Akinesis and dyskinesis were predominant at the septal wall (Figure 6). In addition, LADm pigs showed an 18% reduction (P < 0.01) in ejection fraction compared with baseline (from 65.5% to 53.4%), which was accompanied by enlargement (P < 0.01) of the left ventricular diameter from 24.6 to 33.8 mm (a 37% increase); Table 2). The thickness of the septal wall fell (P < 0.01) from 14.9 to 10.3 mm (a 31% decrease), and the thickness of the posterior wall tended (P = 0.09) to increase.
Figure 6.
M-mode echocardiography of pig 6 on day 4 after LADm occlusion. IVSd, thickness of interventricular septum at end-diastole; LVIDd, left ventricular internal diameter at end-diastole; LVPWd, left ventricular posterior wall at end-diastole; IVSs, thickness of interventricular septum at end-systole; LVIDs, left ventricular internal diameter at end-systole; LVPWs, left ventricular posterior wall at end-systole; HR, heart rate; EF, ejection fraction; %FS, percentage of fractional shortening.
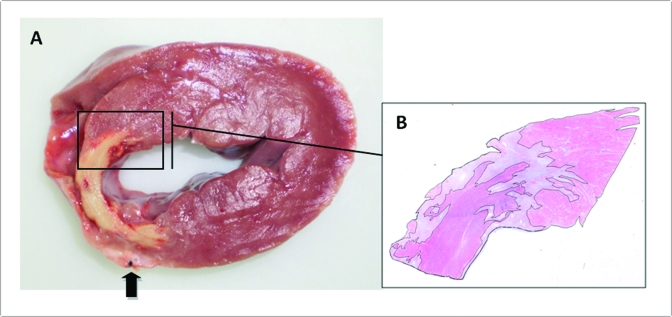
Histopathologic studies after necropsy confirmed complete coronary vessel occlusion in all LADm pigs. All hearts showed some degree of pericarditis, with layers of fibrin covering the epicardium, especially over ischemic areas. Macroscopic analysis of transverse cardiac sections revealed transmural MI located at the anteroseptal myocardial region. The infarcted area was lighter in color than was normal tissue, shrunken, thin, and firm (Figure 7 A). No abnormal changes were detected in any other organ evaluated (lung, liver, and kidney). Histologic total mean infarct size was 14.9% ± 0.8%, with coagulative necrosis (6.8% ± 0.8%) in approximately equal proportion to granulation tissue (8.1% ± 0.6%). All infarcts were transmural in both ventricles, but the left ventricle was always more severely damaged than was the right (11.9% ± 1.0% compared with 3.0% ± 0.3%).
Figure 7.
(A) Transverse cardiac slice at the level of the papillary muscles of pig 6 after LADm. A transmural infarct is visible at the left part of interventricular septum and anterior wall of the left ventricle. Dark arrow shows the location of the occluded LAD. (B) Histologic section stained with Masson trichrome, with clear distinction of necrotic tissue (purple), surrounding granulation tissue (gray), and viable myocardium (bright pink). Magnification, 1.0× (A); 3.0× (B).
Discussion
In our search for the ideal surgical model of MI, we were confronted with 2 main issues: achievement of a standard insult of MI along with minimal morbidity and mortality. Surgical models are associated with high rates of complications, resulting in high mortality.3,10,12 We believe mortality rates depend mostly on the occlusion site, duration of occlusion, and the anesthetic strategies and precautions.
Two of the 3 pigs in the LCXp group died from ventricular arrhythmias refractory to cardioversion: one during surgery and the other at 21 h after surgery. The remaining pig in this group survived likely due to an individual variation: its LAD was more developed than was typical and thus supplied a wider myocardial region. However, this pig showed distress throughout postsurgical period. In fact, the diagnostic tests confirmed and justified its clinical instability: extremely elevated peaks and AUC of analyzed cardiac biomarkers (Table 1), substantial ST-segment elevation, severe decreases in the motion and thickness of the posterior wall (compensated by increase of the septal wall), along with remarkable enlargement of left ventricular lumen and reduction in ejection fraction (Table 2). The calculated infarct size for this pig was 17.91%, the highest value in our study (Table 3).
Table 3.
Ventricular fibrillation (VF) rate, mortality rate, and calculated mean infarct size of pigs with coronary occlusions at different locations.
| Occlusion site | n | VF rate (%) | Mortality rate (%) | Mean infarct size (%) |
| LCXp | 3 | 67 | 67 | 17.9 |
| LADp | 2 | 100 | 100 | — |
| LADd | 2 | 0 | 0 | 10.0 ± 0.1 |
| LADm | 8 | 25 | 12.5 | 14.9 ± 0.8 |
Due to early death in LADp, no histologic lesions were observed.
Due to severe left ventricular dysfunction and intractable ventricular arrhythmias, both pigs of the LADp group died shortly after coronary ligation, thereby preventing subsequent diagnostic tests. We therefore abandoned LADp experiments to proceed with more distal occlusions.
Only 1 of the 8 LADm pigs died before final evaluation. In the pig that died, the LAD supplied a larger cardiac region than was typical. This pig developed several arrhythmias after coronary occlusion, all of which were reversed successfully. Nevertheless, the pig died 2 h after surgery. One other pig in the LADm group presented with ventricular fibrillation and survived only after several cardioversion maneuvers, including defibrillation. The mortality rate and diagnostic data from the LADm group typically fell between the extremes of those pigs with proximal occlusion (LCXp and LADp) and those with distal occlusion (LADd; Tables 1 through 3).
Most changes in cardiac values in LADd pigs were not significant. Contrary to our expectation, the peak values and AUC of myoglobin and MMB of LADd pigs were higher than the ones from the LADm group. This difference is explained by the fact that, due to technical difficulties with the placement of a central venous catheter, blood samples had to be collected from the jugular vein (inducing muscular damage) in 1 LADd pig.15,25 Although mortality was 0% in the LADd group, infarct sizes were considered to be too small (9.95% and 10.09%) to be useful, and these experiments were abandoned.
As we confirmed for proximal occlusions (LCXp and LADp), if the infarcted myocardial region is too large, pigs develop ventricular fibrillation and die.16,23 Therefore, during the acute phase of MI, these models do not provide enough information from diagnostic tests to correlate with, for instance, experimental groups in which new treatments for coronary heart disease are tried. In contrast, if the infarcted area is too small (as in LADd), diagnostic tests will detect only mild changes which may not be significantly different from those obtained from treatment groups. Pigs that underwent LADm surgery seem to offer the optimal balance between low mortality (12.5%) and sufficient infarct size (14.9%). Echocardiographic changes correlated well with histopathologic results, with a reduction of 18% in ejection fraction. The increase in heart rate corroborated left ventricular dysfunction and insufficiency. Ventricular fibrillation occurred in 2 pigs (25%) of the LADm group and resulted only in one death. Intragroup variability in most cardiac data was small, so reproducibility of the swine LADm model is feasible.
Our results are in accordance to other studies performed in domestic swine (Table 4). Analysis of Table 4 reveals several important points. First, closed-chest porcine models are associated with high mortality rates,16 similar to or even higher than those of open-chest models. Second, independent of method, mortality rates of LCX occlusions (even in its distal part) are higher than those for LADm occlusions. Finally, mortality rates (as regards infarct size) in LADp occlusions are higher than those of LADm occlusions.
Table 4.
Ventricular fibrillation (VF) rate, mortality rate and calculated mean infarct size in previous porcine models
| Occlusion site | Method used | VF rate (%) | Mortality rate (%) | Mean infarct size (%) | Reference | |
| Open-chest models | ||||||
| LADp | Surgical ligation | — | >70 | — | 26 | |
| LADm | Surgical ligation | 34.6 | 11.5 | ≈15.8 | 1 | |
| LADm | Surgical ligation | 33 | 19.4 | — | 2 | |
| Closed-chest models | ||||||
| LADp | Balloon inflation | 90 | 66.7 | 18.3 | 23 | |
| LADm | Balloon inflation | 18.4 | 16.3 | 13 | 23 | |
| LADm | Embolization coils | — | 13.6 | 14.8 | 3 | |
| LADm | Balloon inflation | 59.1 | 31.8 | 21.5 | 10 | |
| LCXd | Balloon inflation | 76.2 | 52.4 | — | 17 | |
| LCXd | Tungsten spirals | 50 | 27.8 | — | 16 | |
Along with site and duration of occlusion, we consider that anesthetic strategies and management precautions play a key role in minimizing mortality after surgical induction of MI in pigs. In addition to a human cardiothoracic surgeon, our team included 3 veterinarians to meet all specific requirements for the wellbeing of the pigs. For example, in contrast to the typical human surgery, sternotomy in our pigs was incomplete and did not involve the manubrium. This precaution was taken to avoid undue postsurgical pain in pigs because of their quadrupedal stance.
In the current study, anesthesia was maintained without complication, except for one pig that developed malignant hyperthermia. To avoid complications, infections were prevented with administration of antibiotics during and after surgery, and pain was minimized by local anesthesia at the sternotomy incision site and systemic analgesia given intra- and postsurgically. The anesthetic and analgesic regimen we selected was aimed at preventing cardiovascular depression and delayed recovery from anesthesia.21,24 Consistent with our goal of developing a model for human MI, this anesthetic protocol mimicked one of the most common regimens used in human medicine.4 Antiarrhythmic agents, atropine, other tranquilizers, and opioids were not used in the current study in order to simplify and standardize the model (reducing variability of individual reactions to each drug) and to prevent masking or influencing mortality and morbidity rates caused by coronary occlusion. For example, fentanyl patches were not used due to variability in absorption.24 Therefore, we used only the anesthetic and analgesic agents necessary to prevent pain and distress. The protocol we used seemed adequate because all animals were able to stand, walk, and eat within the first 24 h after surgery. This behavior did not change after withdrawal of meloxicam.
As demonstrated previously,13 avoidance of stress in surgical models for MI is necessary to reduce the incidence of ventricular arrhythmias. In the present model, overall stress was carefully managed. To this end, animal restraint and IM injections were avoided by use of central venous catheters, pigs were allowed to roam freely for 30 min daily, and care was taken to avoid hypothermic conditions. To avoid the need for animal restraint, the ends of the catheters and chest tubes were easily accessible by being left external to the bandages. However, because of their placement in a rear leg (a saphenous vein was used for the central venous catheter) and at the pig's back (chest tubes), these devices were inaccessible to the pigs themselves. In addition, bandages did not require changing during postsurgery days, because the cage was always kept dry and clean. Due to the pigs’ sternal recumbency, no straw, paper, or cardboard was used for bedding because these materials might have hampered the healing of the surgical wound.
Some authors mention that exposure to room temperatures can lead to epicardial cooling and desiccation, with eventual influence on infarct size, local metabolism, and inflammatory responses.12,17 We believe that this effect was not an issue during our study because the whole surgical procedure (from sternotomy to chest closure) took less than 1 h, fluid replacement was provided by continuous intravenous infusion of saline solution, and antiinflammatory drugs were given intra- and postsurgically.
Another complication associated with open-chest models is pneumothorax, leading to complex local and hemodynamic effects and influencing the imaging characteristics of animals, mainly in chronic models.10,12,23 We prevented these effects in our study by placing pleuropericardial tubes, which were drained (through intermittent syringe drainage) right after chest closure and then at each blood sample collection.
Our initial experimental design involved a total of 32 pigs, with coronary occlusions at LCXp (n = 8) and then at the LAD—its proximal part (n = 8), its midpoint (n = 8), and its distal part (n = 8). However, because the results were satisfactory only with the LADm group, only 17 pigs were used. Indeed, our study is limited by the small number of animals used for LCXp, LADp, and LADd occlusions. We considered that increasing the number of pigs in the LCXp and LADp groups would only raise the number of deaths, and ethical issues would arise. In the LADd group, although mortality rate was 0%, the low-value results and small infarct size were suboptimal, so we opted to discontinue this model to avoid euthanasia of excess numbers of pigs.
Surgical coronary occlusion has been reported in several publications. As a topic still under discussion, one group has recently performed LAD occlusions in miniature pigs.8 However, cardiac biomarkers were not evaluated, and the heart size of these miniature pigs is not comparable to the adult human heart. To our knowledge, the comparison of MI produced by occlusion in different locations in the coronary arteries and the use of noninvasive diagnostic tests for its characterization in domestic pigs has not yet been documented.
As stated previously, “A model has validity only in relation to the experimental question being asked.”7 Most recent studies use minimally invasive percutaneous methods in attempts to produce a more physiologic model of MI. Our intention in the present study differed. Our open-chest model is advantageous as a control model for comparison with experiments on testing or optimizing treatment strategies for patients with coronary heart disease. Our swine model is easily reproducible and consistent, with precise location of the site of occlusion, low mortality and surgical complication rates, and minimization of pain, suffering, and distress to the animals involved.
The results from the current study demonstrate that the occlusion at the middle portion of LAD is the most favorable option for creation of MI, associating a low mortality rate with a sufficiently large infarcted area, with clear left ventricular dysfunction on electrocardiography and echocardiography and cellular damage as evidenced by assessment of cardiac biomarkers. The estimation of infarct size was considered to be reliable, because intragroup variation was negligible in all diagnostic tests. The diagnostic tools used are simple, readily available, noninvasive, and applicable in routine clinical practice, providing important data on cardiac morphology and function. Cardiac biochemical markers, including one of the most recent and specific (troponin I), were assessed quantitatively.
Acknowledgments
We express our gratitude to all members of the Departments of Anatomy and Veterinary Clinics of ICBAS-UP, especially to Ana Pinto and Madalena Santos and the Department of Clinical Chemistry of Centro Hospitalar do Porto–HGSA. We also thank Renato Margato and João Rodrigues for their technical assistance in ECG and echocardiographic interpretation. This investigation was funded by grants from FCT to UMIB.
References
- 1.Brooks H, Al-Sadir J, Schwartz J, Rich B, Harper P, Resnekov L. 1975. Biventricular dynamics during quantitated anteroseptal infarction in the porcine heart. Am J Cardiol 36:765–775 [DOI] [PubMed] [Google Scholar]
- 2.Cinca J, Bardají A, Carreño A, Mont L, Bosch R, Soldevilla A, Tapias A, Soler-Soler J. 1995. ST segment elevation at the surface of a healed transmural myocardial infarction in pigs. Circulation 91:1552–1559 [DOI] [PubMed] [Google Scholar]
- 3.Dib N, Diethrich EB, Campbell A, Gahremanpour A, McGarry M, Opie SR. 2006. A percutaneous swine model of myocardial infarction. J Pharmacol Toxicol Methods 53:256–263 [DOI] [PubMed] [Google Scholar]
- 4.DiNardo JA, Zvara DA. 2008. Anesthesia for myocardial revascularization, p 90–129 : Anesthesia for cardiac surgery, 3rd ed Boston (MA): Blackwell Publishing [Google Scholar]
- 5.Galiñanes M, García-Dorado D, Elízaga J, Solares J, Riesgo M, Fdez-Avilés F, Gómez Nebreda MJ. 1987. Transient occlusion of the left anterior descending coronary artery in pigs. Eur Surg Res 19:246–253 [DOI] [PubMed] [Google Scholar]
- 6.Gibbons RJ, Valeti US, Araoz PA, Jaffe AS. 2004. The quantification of infarct size. J Am Coll Cardiol 44:1533–1542 [DOI] [PubMed] [Google Scholar]
- 7.Fozzard HA. 1975. Validity of myocardial infarction models. Circulation 52(6 Suppl): III131–III146 [PubMed] [Google Scholar]
- 8.Huang Z, Ge J, Sun A, Wang Y, Zhang S, Cui J, Zhang S, Qian J, Zou Y. 2010. Ligating LAD with its whole length rather than diagonal branches as coordinates is more advisable in establishing stable myocardial infarction model of swine. Exp Anim 59:431–439 [DOI] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research, Committee on Recognition and Alleviation of Pain in Laboratory Animals 2009. Recognition and alleviation of pain in laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 10.Krombach GA, Kinzel S, Mahnken AH, Günther RW, Buecker A. 2005. Minimally invasive close-chest method for creating reperfused or occlusive myocardial infarction in swine. Invest Radiol 40:14–18 [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. 2005. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Group, developed in conjunction with the European Association of Echocardiography. J Am Soc Echocardiogr 18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 12.Mitsos S, Katsanos K, Dougeni E, Koletsis EN, Dougenis D. 2009. A critical appraisal of open- and closed-chest models of experimental myocardial ischemia. Lab Anim (NY) 38:167–177 [DOI] [PubMed] [Google Scholar]
- 13.Näslund U, Häggmark S, Johansson G, Marklund SL, Reiz S. 1992. A closed-chest myocardial occlusion–reperfusion model in the pig: techniques, morbidity, and mortality. Eur Heart J 13:1282–1289 [DOI] [PubMed] [Google Scholar]
- 14.Ouyang J, Guzman M, Desoto-Lapaix F, Pincus MR, Wieczorek R. 2009. Utility of desmin and a Masson trichrome method to detect early acute myocardial infarction in autopsy tissues. Int J Clin Exp Pathol 3:98–105 [PMC free article] [PubMed] [Google Scholar]
- 15.Panteghini M. 2002. Acute coronary syndrome—biochemical strategies in the troponin era. Chest 122:1428–1435 [DOI] [PubMed] [Google Scholar]
- 16.Peukert D, Laule M, Kaufels N, Schnorr J, Taupitz M, Hamm B, Dewey M. 2009. A minimally invasive method for induction of myocardial infarction in an animal model using tungsten spirals. Int J Cardiovasc Imaging 25:529–535 [DOI] [PubMed] [Google Scholar]
- 17.Reffelmann T, Sensebat O, Birnbaum Y, Stroemer E, Hanrath P, Uretsky BF, Schwarz ER. 2004. A novel minimal-invasive model of chronic myocardial infarction in swine. Coron Artery Dis 15:7–12 [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues M, Silva AC, Águas AP, Grande NR. 2005. The coronary circulation of the pig heart: comparison with the human heart. European Journal of Anatomy 9:67–87 [Google Scholar]
- 19.Sahni D, Kaur GD, Jit H, Jit I. 2008. Anatomy and distribution of coronary arteries in pig in comparison with man. Indian J Med Res 127:564–570 [PubMed] [Google Scholar]
- 20.Schoen FJ, Mitchell RN. 2010. The heart. p 529–590 : Kumar V, Abbas AK, Fausto N, Aster AC. Robbins and Cotran pathologic basis of disease, 8th ed Philadelphia (PA): Saunders Elsevier [Google Scholar]
- 21.Smith AC, Swindle MM. 2008. Anesthesia and analgesia in swine p 413–440, : Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals, 2nd ed New York (NY): Academic Press [Google Scholar]
- 22.Smith GT, Geary G, Ruf W, Roelofs TH, McNamara JJ. 1979. Epicardial mapping and electrocardiographic models of myocardial injury. Circulation 60:930–938 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Lyons JK, Yeung AC, Ikeno F. 2008. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area / area at risk. Catheter Cardiovasc Interv 71:100–107 [DOI] [PubMed] [Google Scholar]
- 24.Swindle MM. 2007. Swine in the laboratory: surgery, anesthesia, imaging and experimental techniques, 2nd ed Boca Raton (FL): CRC Press [Google Scholar]
- 25.Thygesen K, Alpert JS, White HD. 2007. Universal definition of myocardial infarction. J Am Coll Cardiol 50:2173–2195 [DOI] [PubMed] [Google Scholar]
- 26.White FC, Roth DM, Bloor CM. 1986. The pig as a model for myocardial ischemia and exercise. Lab Anim Sci 36:351–356 [PubMed] [Google Scholar]