Abstract
The aim of this study was to investigate the effects of Trypanosoma cruzi (T. cruzi) infection on myocardial morphology, single cardiomyocyte contractile function and exercise tolerance in rats. Adult Wistar rats were randomized into control (n = 14) and infected (n = 14) groups. Infected animals were inoculated with T. cruzi Y strain (300,000 trypomastigotes/50 g body weight). After 9 weeks, the animals were subjected to a treadmill running protocol. Then, the right atrium (RA) and left ventricle (LV) were removed for morphological and cell contractile evaluation. The infected animals exhibited a significant reduction in distance travelled, total time to fatigue and workload. In addition, these animals had hypertrophy, increased myocardial cellularity, and an increase in the proportion of collagen and blood vessels. RA and LV myocytes from infected animals showed marked contractile dysfunction under basal conditions and a reduced contractile response to β-adrenergic stimulation. The workload of infected animals was correlated closely with the amplitude of cell shortening of RA and LV myocytes. T. cruzi infection influenced the myocardial morphology and the mechanical properties of RA and LV single myocytes negatively and reduced exercise tolerance. Single cardiomyocyte contractile dysfunction could constitute an additional mechanism of cardiac impairment and reduced exercise tolerance in this infection.
Keywords: cellular contractility, Chagas’ cardiomyopathy, myocytes, physical capacity
Chagas' disease is an underappreciated illness caused by the intracellular protozoan parasite Trypanosoma cruzi (T. cruzi) that is an important health problem in 18 developing countries in South and Central America (Biolo et al. 2010; Rassi et al. 2010). Its main clinical manifestations are cardiac and/or digestive disturbances, with a prevalence of about 12–14 million cases worldwide, and it has been considered a major cause of cardiac infectious disease in endemic countries (WHO 2005). Chronic Chagasic cardiomyopathy is the main cause of death and occurs in approximately 30% of infected subjects (Marin-Neto et al. 2007; Rassi et al. 2010). The clinical course of Chagas’ disease shows great variability, and the mechanisms responsible for the development of this potentially lethal cardiomyopathy are not understood (Biolo et al. 2010; Rassi et al. 2010).
Cardiac denervation, interstitial mononuclear infiltrate, myocyte and vascular degenerative changes, fibrosis and hypertrophy characterize the main pathologic features of chronic Chagasic cardiomyopathy (Marin-Neto et al. 2007; Biolo et al. 2010; Rassi et al. 2010). These morphological changes coexist and are associated with abnormalities of the electrical and contractile cardiac activities characterized mainly by conduction defects, frequent and complex ventricular arrhythmias and systolic ventricular dysfunction (Marin-Neto et al. 2007; Biolo et al. 2010). In addition, the chronotropic incompetence caused by changes in the sympathetic and parasympathetic tonus induced by an immune-mediated process has been recognized as one of the mechanisms capable of interfering with the capacity of the heart to increase heart rate in response to different stimuli, including physical exercise (Colucci et al. 1989; Talvani et al. 2006; Sousa et al. 2009).
Few studies have evaluated exercise performance and the factors affecting functional capacity and exercise tolerance in patients with Chagas’ disease. Moreover, it is not known whether T. cruzi infection can also lead to changes in exercise tolerance in experimental animal models. The reduction of exercise tolerance in individuals with Chagas’ disease is multifactorial and is involved with pathological changes in several organs and tissues, such as peripheral nervous system, skeletal and cardiac muscles (Meiler et al. 1987; Montes de Oca et al. 2004). Moreover, previous studies indicated that atrial and ventricular mechanical and electrical abnormalities may have an important role in exercise intolerance in Chagas’ disease (Gallo et al. 1975; Mady et al. 2000; Lima et al. 2010). However, several aspects of the cellular and molecular basis of these changes remain to be clarified.
Recently, our group showed for the first time changes in the cellular mechanics of cardiac myocytes isolated from the atrium and ventricle of C57BL/6 mice infected with T. cruzi (Roman-Campos et al. 2009). We observed decreased myocyte contraction amplitude and a prolonged contraction and relaxation time course in the very beginning of the parasitism that remained until the chronic phase of the disease. Data from our laboratory also showed that in normal rats, exercise performance is significantly influenced by the electromechanical characteristics of cardiomyocytes (Prímola-Gomes et al. 2009). In this study, cardiomyocytes isolated from rats with high running capacity had greater calcium (Ca2+) transients, amplitude of cell contraction, maximum velocity of contraction and relaxation compared with rats of the same progeny with standard running capacity.
The aim of this study was to investigate the effects of T. cruzi infection on myocardial morphology, single cardiomyocyte contractile function and exercise tolerance in rats. We hypothesized that T. cruzi infection can lead to changes in cardiac morphology, and to single cardiomyocyte contractile dysfunction and can also influence exercise tolerance in rats.
Materials and methods
Animals and infection
Four-month-old male Wistar rats with an initial weight of 366.25 ± 31.17 g were given rodent chow and water ad libitum and maintained in animal facilities with a controlled temperature of 22 °C and 12-h light/dark inverted cycles. Animals were randomly divided into control (CG = 14) and infected (IG = 14) groups. Animals from the IG were inoculated intraperitoneally with T. cruzi Y strain (300,000 trypomastigotes/50 g body weight, about 21,00000 trypomastigotes) (Martinelli et al. 2006) contained in 700 μl of infected blood from mouse diluted in saline solution 0.9% (Brener 1962). Infection was confirmed 4 days postinoculation by the presence of trypomastigotes in peripheral blood collected from the rat's tail, and the level of parasitaemia was recorded daily after inoculation as described by Brener (1962). Mortality was investigated during the experiment. All experimental procedures were conducted in accordance with the Brazilian College of Animal Experimentation and approved by the Animal Research Ethics Commission of the Veterinary Department at the Federal University of Viçosa, Brazil (protocol 30/2009).
Measurement of exercise tolerance
Nine weeks after inoculation, all animals were evaluated for exercise tolerance using a treadmill incremental running protocol adapted from Koch and Britton (2001). Briefly, the rats were familiarized with the motor-driven treadmill (Insight Instruments®, Ribeirão Preto, Brazil) by running at a speed of 10 m/min at 5% inclination for 5 min/day for seven consecutive days. Two days after familiarization, the exercise trial was performed on three consecutive days at a constant slope of 5% with the starting speed at 10 m/min. Treadmill velocity was increased by 1 m/min every 2 min, and each rat ran until fatigue. Fatigue was defined as the point at which the animals were no longer able to keep pace with the treadmill. Travelled distance (m), time until fatigue and workload were used as indexes of exercise tolerance (Lacerda et al. 2006). Workload (W; kg) was calculated using the equation W = body mass (kg) × TTF (total time to fatigue) (min) × treadmill speed (m/min) × sine θ (treadmill inclination), where TTF is time until fatigue (Brooks et al. 1984). Because of variability in the performance data, the mean of the indices of running performance was calculated for the three trials for each rat and analysed.
Heart biometry and myocardial stereology
Forty-eight hours after the exercise test, five animals from each group were sacrificed, and the hearts were removed and weighed. The atria and ventricles were dissected, weighed separately and the right atrium (RA) and left ventricle (LV) isolated. Hypertrophy was determined by measuring RA and LV volume using the submersion method described by Scherle (1970).
The atria and ventricles were fixed for 48 h (in freshly prepared 10% w/v formaldehyde in 0.1 M phosphate buffer, pH 7.2). The fragments of the RA and LV were obtained through the Orthrip method for stereological study (Mandarim-de-Lacerda 2003). These fragments were dehydrated in ethanol, cleared in xylol and embedded in paraffin. Blocks were cut into 4-μm sections and stained by Masson's trichrome or haematoxylin–eosin (H&E) and mounted on histology slides. The slides were visualized and the images captured using a light microscope (Olympus BX-60®; Olympus, Tóquio, Japan) connected to a digital camera (Olympus QColor-3®; Olympus, Tóquio, Japan). Sixty fields from each Masson's trichromic (objective ×20) and H&E (objective ×40) stain were randomly chosen, and a total of 4.37 × 106 μm2 and 1.41 × 106 μm2 of myocardium area, respectively, were analysed. Sections stained with Masson's trichromic were used for myocardial stereological analysis. For this analysis, a test system of 72 points was used in a standard test area of 73 × 103 μm2 (Mandarim-de-Lacerda 2003). All the stereological analyses were performed according to Bezerra et al. (2008).
The stereological parameter of volume density (Vv) was estimated by point counting for cardiomyocytes [cmy], collagen [col] and intramyocardial blood vessels [ibvs] according to the formula Vv [structure] = PP [structure]/PT, where PP is the number of points that hit the structure and PT is the total number of test points. The amount of intramyocardial vascularization was defined as the ratio of Vv [ibvs]/Vv [cmy]. The mean cross-sectional area of cardiomyocytes was estimated according to the following relationship: A [cmy] = Vv [cmy]/2.QA [cmyn]; QA [cmyn] = N [cmy]/AT, where QA [cmyn] is the number of cardiomyocyte nuclei profiles in the analysed area (AT). Overestimation of the measurements was avoided by the exclusion of nuclei profiles incident on two edges of the AT.
Myocardial histopathology
For each group, 25 sections of 8 μm thickness stained with Sirius red and Fast green were used to quantify collagen and total protein in cardiac tissue using a spectrophotometric method previously described (López-De León & Rojkind 1985).
The inflammatory process was evaluated by the correlation index between the number of cells observed in the myocardium from CG and IG animals (Caldas et al. 2008). All morphological analyses were performed using the software image pro-plus 4.5® (Media Cybernetics, Silver Spring, MD, USA).
Cardiomyocytes isolation
Nine animals from each group were used in this set of experiments. At the time of sacrifice, the heart was removed rapidly and extraneous tissue dissected away. The heart was flushed immediately with modified HEPES (4-2-hydroxyethyl-1-piperazineethanesulfonic acid)-Tyrode's solution of the following composition (mM): 130 NaCl, 5.4 KCl, 1.4 MgCl2, 0.4 NaH2PO4, 0.75 CaCl2, 5 HEPES, 10 glucose, 20 taurine and 10 creatine (pH 7.4) and then blotted and weighed before being mounted onto a Langendorff perfusion apparatus for the isolation of myocytes using a collagenase–protease dispersion technique as described previously (Natali et al. 2002). Briefly, the heart was perfused for 10–15 min with a solution containing 1 mg/ml collagenase type II (Worthington Biochemical Co.; Worthington, OH, USA). The digested heart was removed from the cannula, and the RA and LV were separated and cut into small pieces. Ventricular and atrial cardiomyocyte cells were isolated mechanically (5 min at 37 °C), and single cells were separated from the non-dispersed tissue by filtration. The resulting cell suspension was centrifuged at 30 g for 45 s, resuspended in HEPES-Tyrode's and stored at 4 °C until analysis. Only calcium-tolerant, quiescent, rod-shaped cardiomyocytes showing clear cross-striations were studied. The isolated cardiac myocytes were used within 4 h after isolation.
Measurements of cell contractility
Cellular contractile function was evaluated as described by Natali et al. (2002). Isolated cells were placed in a chamber with a glass coverslip base mounted on the stage of an inverted phase-contrast video microscope (Eclipse-TS100®; Nikon, Tóquio, Japan). The chamber was perfused with Tyrode's solution (in mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 10 HEPES and 10 glucose (pH 7.4) at room temperature (approximately 28 °C). Myocytes were stimulated via platinum bath electrodes with voltage pulses of a duration of 5 ms and an intensity of 20 V at the stimulation frequency of 3 Hz. Cells were visualized on a PC monitor with a NTSC camera (Myo-Cam CCD100V®; Ionoptix, Milton, MA, USA) in partial scanning mode. This image was used to measure cell shortening (index of contractile function) in response to electrical stimulation using a video motion edge detection system (Ionoptix). The cell image was sampled at 240 Hz, and cell shortening was calculated from the output of the edge detector using an IonWizard A/D converter (Ionoptix). Eight to 16 consecutive contractions were averaged, and cell shortening (expressed as a percentage of resting cell length), time to peak shortening and time to half relaxation were calculated (Roman-Campos et al. 2009).
β-Adrenergic stimulation
The contractile response of isolated cardiomyocytes to β-adrenergic stimulation was assessed using the non-selective agonist isoproterenol (ISO, 1, 2 and 3 mM) at a stimulation rate of 1 Hz. After recording the baseline cell shortening, ISO was infused in the experimental chamber through an automatic pipette. The cells were electrically stimulated after 5 min of infusion when cell shortening was recorded (Prahash et al. 2000). This procedure was repeated for each ISO concentration in different myocytes. Cell contractile function was analysed, and the variation (Δ) from the baseline to the larger stimulus (ISO, 3 mM) was used as an index of β-adrenergic sensitivity.
Statistics
Data are presented as mean and standard error of the mean (mean ± SEM). The normal distribution of the data was verified using the Kolmogorov–Smirnov test. Parameters of exercise tolerance, biometric and cell contractile function data were compared using the Student's t-test. Stereological data and karyometric parameters were compared using the Mann–Whitney U test. The relationship between cell contractile function and exercise workload was assessed by linear regression. A probability of P < 0.05 was considered statistically significant.
Results
Parasitaemia and mortality
The presence of parasites in the bloodstream of IG animals began on the fourth day, disappearing completely on the eighth day. The peak parasitaemia occurred on the sixth day after inoculation. The same analysis was performed to CG animals and demonstrates the absence of circulating parasites (Figure 1). No animals died during the experiment in both groups.
Figure 1.
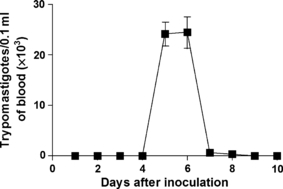
Parasitemia curve in Wistar rats inoculated with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weight). Data of 14 animals are expressed as mean ± SEM.
Exercise tolerance
Infection with T. cruzi impaired the exercise tolerance of IG animals resulting in significantly reduced distance travelled, total time to fatigue and workload compared to CG animals (Figure 2).
Figure 2.
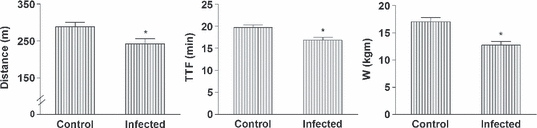
Exercise tolerance of control and infected rats. Infected animals were inoculated intraperitoneally with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weight). TTF, total time to fatigue; W, workload. Data of 14 animals from each group were collected 9 weeks after inoculation and are expressed as mean ± SEM. *Denotes statistical difference from the Control (P < 0.001).
Heart biometry and myocardial stereology
There was no significant difference in body weight between the CG and the IG (Table 1). Infected group animals presented a higher heart and ventricular weight compared to CG animals, whereas the AT weight did not differ between the groups. Left ventricle volume was significantly higher in IG animals as compared to CG animals.
Table 1.
Biometric parameters of control and infected rats
| Control | Infected | |
|---|---|---|
| Body mass (g) | 509.76 ± 16.48 | 497.90 ± 17.31 |
| Heart mass (g) | 2.01 ± 0.06 | 2.17 ± 0.41* |
| AT mass (g) | 0.59 ± 0.05 | 0.59 ± 0.08 |
| VE mass (g) | 1.42 ± 0.05 | 1.58 ± 0.04* |
| RA volume (mm3) | 140.83 ± 3.79 | 143.91 ± 4.52 |
| LV volume (mm3) | 447.15 ± 9.21 | 496.08 ± 7.95* |
AT, atrium; VE, ventricle; RA, right atrium; LV, left ventricle.
Data of five animals from each group were collected 9 weeks after inoculation and are expressed as mean ± SEM. Animals of the infected group were inoculated intraperitoneally with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weight).
Denotes statistical difference from Control (P < 0.001).
Infected group animals exhibited a higher LV cardiomyocyte cross-sectional area and volumetric density of blood vessels (Vv [ibvs]) and collagen (Vv [col]) compared to CG animals. According to the spectrophotometric analysis, the amount of collagen in the LV of the IG animals was also significantly higher compared to CG animals. In addition, IG animals showed a higher index of myocardial vascularization in both RA and LV as compared to CG, demonstrated by the increased Vv [ibvs]-to-Vv [cmy] ratio (Table 2).
Table 2.
Quantitative parameters of the myocardium from control and infected rats
| Right atrium | Left ventricle | |||
|---|---|---|---|---|
| Control | Infected | Control | Infected | |
| A [cmy] (μm2) | 101.94 ± 14.09 | 105.24 ± 16.69 | 376.11 ± 39.98 | 414.85 ± 42.74* |
| Vv [cmy] (%) | 72.04 ± 1.87 | 72.18 ± 2.64 | 72.82 ± 3.02 | 67.11 ± 2.96* |
| Vv [ibvs] (%) | 11.11 ± 0.92 | 13.29 ± 1.43* | 15.83 ± 1.15 | 18.91 ± 1.58* |
| Vv [ibvs]/Vv [cmy] | 15.66 ± 2.17 | 18.85 ± 2.68† | 22.31 ± 3.52 | 29.82 ± 4.59* |
| Vv [col] (%) | 16.81 ± 2.09 | 14.68 ± 2.96 | 11.25 ± 1.40 | 14.03 ± 1.56* |
| Collagen (μg/mg protein) | 19.02 ± 3.85 | 18.52 ± 3.27 | 20.33 ± 2.83 | 27.51 ± 3.39* |
A, cross-sectional area of cardiomyocytes; Vv, volumetric density; cmy, cardiomyocytes; ibvs, intramyocardial blood vessels; col, collagen.
Data of five animals from each group were collected 9 weeks after inoculation and are expressed as mean ± SEM. Infected animals were inoculated intraperitoneally with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weight).
Denotes statistical difference from Control (P < 0.001) for the same segment.
Denotes statistical difference from Control (P < 0.01) for the same segment.
Myocardial histopathology
The intensities of the fibrosis and the interstitial inflammatory infiltrate in the LV of IG and CG animals were significantly different (Figure 3). The histopathology of the myocardium showed an occurrence of inflammatory infiltrate with a predominance of mononuclear cells and the presence of mast cells in IG animals, which characterizes the chronic inflammatory processes (Figure 3c). Intracellular amastigote forms of T. cruzi were identified after the infection persisted for 9 weeks (Figure 3f). The LV of IG animals presented a higher collagen content (Figure 3h), and the myocardial cellularity was significantly more intense in IG animals (3422 ± 732.60 cells in 1.4 × 106 μm2) compared to CG animals (2217 ± 520.19 cells in 1.4 × 106 μm2). The RA of IG animals showed no significant differences either in collagen content or in myocardial cellularity compared to CG animals (data not shown).
Figure 3.
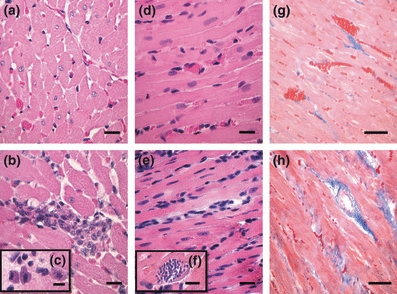
Representative photomicrographs of the left ventricle from control (a, d and g) and infected (b, c, e, f and h) rats. The infected animals were inoculated intraperitoneally with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weight). Nine weeks after inoculation, five animals from each group were euthanized and heart fragments were collected for morphological analysis. (a) A myocardial cross-section showing a well-organized structure (H&E staining, bar = 30 μm). In panel b cardiac myocytes with increased diameters and focal inflammatory infiltrate are observed (H&E staining, bar = 30 μm). Mast cells were also observed in the infected myocardium (c, bar = 12 μm). Differences in myocardial cellularity between the control (d) and infected (e) groups are also shown (H&E staining, bar = 30 μm). Intracellular amastigotes of T. cruzi can be seen in panel f (H&E staining, bar = 10 μm). (g) A longitudinal section of myocardium showing blood vessels and thin collagen bundles between muscle fibers (Masson trichrome staining, bar = 20 μm). In panel h thick bundles of collagen with pericellular and perivascular distribution are shown (Masson trichrome staining, bar = 20 μm).
Cell contractility and β-adrenergic stimulation
Right atrium myocytes from the IG had a significant reduction in the amplitude of shortening and an increase in time to half relaxation compared to the CG (Figure 4, upper panel). The time to peak shortening did not differ between the groups. Left ventricular myocytes from IG animals exhibited a significant reduction in amplitude of shortening and an increase in the time to peak shortening and the time to half relaxation as compared to CG animals (Figure 4, lower panel).
Figure 4.
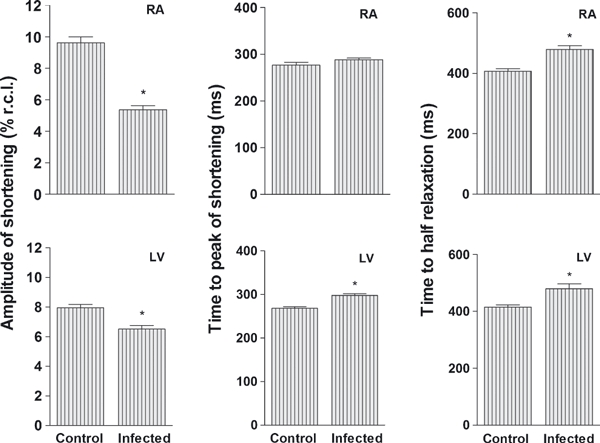
Cell shortening in myocytes isolated from the right atrium and left ventricle from control and infected rats. For each cardiac segment were analyzed 81 ± 18 cardiomyocytes. Infected animals were inoculated intraperitoneally with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weigh). Data of nine animals from each group were collected 9 weeks after inoculation and are expressed as mean ± SEM. Amplitude of shortening is expressed as a % of resting cell length (% r.c.l.). *Denotes statistical difference from the Control in the same segment (P < 0.001).
The RA and LV myocytes response to ISO is shown in Figure 5. Myocytes from IG animals exhibited an impaired cell contractile response to β-adrenergic stimulation compared to CG animals, with significant differences observed mainly with 2 and 3 mmol concentrations of ISO. Myocytes from the IG presented significantly less variation of shortening than those from the CG in both cardiac segments for amplitude (RA, 5.44 ± 2.13 vs. 8.87 ± 2.05% respectively; LV, 6.36 ± 1.72 vs. 10.32 ± 2.17% respectively), time to peak shortening (RA, −144.65 ± 18.26 vs.−206.56 ± 23.19 ms respectively; LV, −153.93 ± 11,53 vs.−183.46 ± 14.07 ms respectively) and time to half relaxation (RA, −132.51 ± 23.89 vs.−200.05 ± 19.37 ms respectively; LV, −98.45 ± 29.15 vs.−220.65 ± 24.71 ms respectively).
Figure 5.
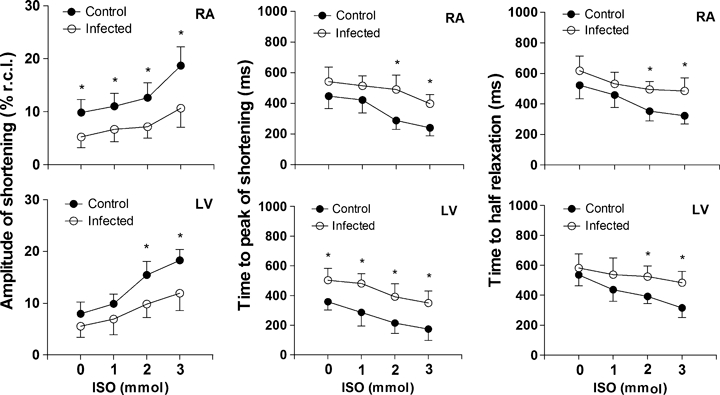
Cardiomyocyte response to β-adrenergic stimulation. Cell shortening, time to peak and time to half relaxation of shortening in myocytes from the right atrium (upper panel) and left ventricle (lower panel) of control (closed circles) and infected (open circles) rats plotted vs. concentration (0–3 mmol) of isoproterenol. For each cardiac segment were analyzed 74 ± 8 cardiomyocytes. The infected animals were inoculated intraperitoneally with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weight). Data of nine animals from each group were collected 9 weeks after inoculation and are expressed as mean ± SEM for the numbers of myocytes indicated from nine animals per group. Shortening is expressed as a % of resting cell length (% r.c.l.). *Denotes statistical difference from the Control in the same segment (P < 0.001).
The linear regression analysis showed a moderate and significant correlation between the amplitude of cell shortening in basal and ISO-stimulated conditions and the workload of IG and CG animals in the exercise tolerance protocol (Figure 6).
Figure 6.
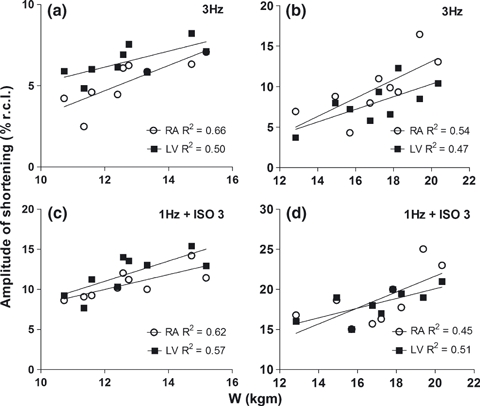
Correlation between cell shortening and workload. Myocytes from right atrium (open circle) and left ventricle (LV, closed square) stimulated at 3 Hz without isoproterenol in infected (a) and control rats (b). Right atrium and LV myocytes stimulated at 1 Hz in the presence of 3 mmol of isoproterenol in infected (c) and control group (d). The mean of cell shortening per animal was plotted against the workload of each animal per group. Data used in the correlation were collected from nine animals in each group 9 weeks after inoculation. Shortening expressed as % of resting cell length (% r.c.l.). W, workload. The infected animals were inoculated intraperitoneally with Trypanosoma cruzi Y strain (300,000 trypomastigotes/50 g body weight). All correlations presented statistical significance (P < 0.05).
Discussion
Our results confirmed our hypothesis that T. cruzi infection is able to impair myocardium morphology and single cardiomyocyte contractile function and influence negatively the exercise tolerance in the murine model investigated.
Infected animals had LV hypertrophy that was evidenced by the presence of cellular hypertrophy and an increased amount of collagen in the myocardium. The abnormal pattern of accumulation and organization of collagen during the progression of the disease has been previously described in Chagas’ disease-induced pathological cardiac hypertrophy (Higuchi et al. 1999; Marin-Neto et al. 2007; Rassi et al. 2010). This new organization of collagen fibres may decrease the myocardial mechanical efficiency to the extent that part of the force used for pumping blood is diverted to correct the geometric distortion determined by the abnormal organization of collagen and muscle bundles (Mady et al. 1999). Moreover, there is evidence that the progressive accumulation of collagen reduces the myocardium compliance and the efficiency of the regulatory mechanism of cellular and muscular contraction force based on the length–tension relationship (Kitzman et al. 1991; Higuchi et al. 1999).
The volumetric density of the blood vessels and the blood vessel-to-cardiomyocyte volumetric density ratio did not indicate a reduction in the myocardial vascularization of infected animals. However, these findings do not exclude the possibility of vascular dysfunction of vasomotor origin and an inadequate balance in the blood flow distribution. For example, our stereological data indicated the occurrence of microvascular dilatation that may have resulted from altered blood flow induced by diffuse fibrosis and vascular derangement. The presence of inflammatory infiltrate and mast cells 9 weeks after infection with T. cruzi favour this hypothesis because the continuous production of cytokines and oxidant components by these cells in a chronic inflammatory process may be conducive to vascular dysfunction. Indeed, mechanisms such as endothelial dysfunction, persistence of T. cruzi antigens and release of nitric oxide associated with the chronic inflammatory process have been implicated in vascular dilatation and dysfunction of Chagas’ disease (Higuchi et al. 1999; Marin-Neto et al. 2007). The presence of vascular damage is not unusual in T. cruzi infection, and the reduction in myocardial vascularization has been considered as an important component involved in the deterioration of cardiac function (Higuchi et al. 1999; Marin-Neto et al. 2007) and exercise tolerance (Meiler et al. 1987). Previous studies have shown that myocardial hypoperfusion significantly limits the exercise tolerance because of the occurrence of abnormal heart rhythm with the onset of arrhythmias and cardiac pump dysfunction (Verani et al. 1981; Meiler et al. 1987).
Our data showed contractile dysfunction of RA and LV myocytes (i.e. reduced cell shortening amplitude and increased time to peak shortening and time to half relaxation) in T. cruzi -infected rats. Mechanisms such as downregulation of ion channels that modulate Ca2+ flux and cell contraction and relaxation have been implicated in the pathogenesis of cardiomyocyte mechanical dysfunction observed in heart disease of different aetiologies (Wisloff et al. 2002; Kemi & Wisloff 2010). In myocardial infarction, diabetic cardiomyopathy and autoimmune myocarditis, the reduced expression and/or inhibition of the sodium and calcium exchanger of the sarcolemma (NCX), the ryanodine channel (RyR2), phospholamban (PLB) and the calcium ATPase of the sarcoplasmic reticulum (SERCA-2) have been reported to be important in chronotropic, inotropic and lusitropic cardiomyocyte dysfunction (Wisloff et al. 2002; Afanasyeva et al. 2004; Kemi & Wisloff 2010). However, whether these molecular changes are promoted by T. cruzi infection warrants further investigation.
Previous studies have demonstrated a positive relationship between improved cardiomyocyte mechanical properties and parameters of exercise performance, such as higher maximal oxygen consumption (Wisloff et al. 2002; Kemi & Wisloff 2010) and intrinsic aerobic exercise capacity in healthy rats (Prímola-Gomes et al. 2009). There is evidence that in animals without disease (Kemi et al. 2004; Kemi & Wisloff 2010) and in animal models of cardiovascular disease, the improvement in mechanical properties of cardiomyocytes because of chronic physical exercise programmes occurs in association with an increased density and sensitivity of Ca2+ ion channels of the sarcolemma and sarcoplasmic reticulum. An important finding is that these contractile and molecular adaptations of cardiomyocytes in response to physical exercise are accompanied by simultaneous improvement in physical performance (Wisloff et al. 2002; Kemi & Wisloff 2010). It is believed that the results of high physical fitness are because of better provision and use of oxygen in exercised tissues and that part of this adaptation is because of the high capacity of cells and the myocardium to produce greater cardiac output (Kemi & Wisloff 2010). In this context, it is not unrealistic to assume that conditions that impair cellular contractility and, consequently, myocardial function have the potential to reduce physical capacity and exercise tolerance. The reduction in physical capacity in individuals with Chagas’ disease has been linked to disturbances in cardiac mechanics and haemodynamics (Gallo et al. 1975; Lima et al. 2010). Indeed, in the present study, the amplitude of cell shortening in RA and LV myocytes correlated closely with workload in control and infected animals. This correlation was observed in the absence and in the presence of β-adrenergic stimulation. Thus, this finding indicates that the level of cell shortening was an important component impaired by T. cruzi infection that contributed to the reduction in exercise tolerance. However, it is well recognized that the determination of exercise tolerance is multifactorial. Thus, as T. cruzi is able to parasitize and damage structures such as peripheral nerves and skeletal muscles, equally important elements in determining the exercise tolerance (Meiler et al. 1987; Montes de Oca et al. 2004), we cannot attribute the results exclusively to cardiac and cellular changes. In this context, the weak correlation between single cardiomyocyte contractile parameters and workload indicates that other organs and tissues should be investigated to improve the knowledge about the pathophysiological mechanism related to exercise intolerance in Chagas’ disease.
We also observed that in association with reduced basal myocyte contractility, T. cruzi -infected animals shared reduced sensitivity to β-adrenergic stimulation. The inotropic and lusitropic responses to ISO were dose dependent in both IG and CG animals; however, all of the contractile parameters examined showed lower amplitude of variation in animals from the IG. In clinical and experimental studies on Chagas’ disease, physical and pharmacological cardiac tests have shown reduced ability of the myocardium to respond to stimuli of progressive intensity, suggesting a lower cellular functional reserve (Gallo et al. 1975; Talvani et al. 2006; Sousa et al. 2009; Lima et al. 2010). In this disease, changes in electrical and mechanical cardiac function have been more pronounced in conditions of cardiac stress, as occurs during exercise, and the reduction in cardiac responsiveness to β-adrenergic stimulation has been considered to be an important factor involved in reducing exercise tolerance (Gallo et al. 1975; Colucci et al. 1989). Data exist to support the role of the immune system in pathological remodelling of cardiomyocyte contractility (Sterin-Borda et al. 1999; Chakraborti et al. 2000; Afanasyeva et al. 2004), including Chagas’ disease (Roman-Campos et al. 2009). It has been demonstrated that in humans and experimental animals with Chagas’ disease, anti-β-adrenoreceptor antibodies produced during infection by T. cruzi can inhibit the signalling pathway triggered by these receptors (Sterin-Borda et al. 1999; Chakraborti et al. 2000). Under normal conditions, β-adrenergic pathways lead to the phosphorylation and inhibition of PLB, which reduces its activity on SERCA-2 and improves inotropic, lusitropic and chronotropic activity of cardiomyocytes (Afanasyeva et al. 2004). However, direct allosteric inhibition of β-adrenoreceptors by autoantibodies or desensitization mediated by upregulation of β-adrenergic receptor kinase may impair cardiomyocyte contractile function because this receptor is the main signalling pathway that regulates cellular mechanics through adjustments in Ca2+ kinetics. Furthermore, inhibition of β signalling reduces the phosphorylation and activation of RyR2 and Ca2+ entry into the cell via the L-type current mediated by the Ca2+-induced Ca2+-release mechanism (Afanasyeva et al. 2004).
In summary, we showed that experimental T. cruzi infection negatively influenced myocardial morphology, the mechanical properties of single RA and LV myocytes and exercise tolerance in rats. The results of the cell mechanics associated with β-adrenergic stimulation support the hypothesis that single cardiomyocyte contractile dysfunction could constitute an additional mechanism of cardiac impairment and reduced exercise tolerance in animals infected with T. cruzi. The experimental model presented here can be useful for future studies investigating, in addition to the cardiac muscle, the participation of other tissues in exercise intolerance. However, little is known about the influence of the parasite in the signalling pathways through which it acts to modulate the single cardiomyocyte mechanics: thus, further studies are needed in this area.
Acknowledgments
Research supported by FAPEMIG (PRONEX). Rômulo D. Novaes was a recipient of the MS scholarship from FAPEMIG. Antonio J. Natali is a CNPq fellow.
Contributions
All listed authors meet ICMJE authorship criteria and nobody who qualifies for authorship has been excluded. Authors contributed to research design, acquisition, analysis and interpretation of data; drafting the paper or revising it critically; and approval of the submitted and final versions.
References
- Afanasyeva M, Georgakopoulos D, Rose NR. Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmun. Rev. 2004;3:476–486. doi: 10.1016/j.autrev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Bezerra DGA, Andrade LML, Cruz FOP, Mandarim-de-lacerda CA. Atorvastatin attenuates cardiomyocyte loss in adult rats from protein-restricted dams. J. Card. Fail. 2008;14:151–160. doi: 10.1016/j.cardfail.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Biolo A, Ribeiro AL, Clausell N. Chagas cardiomyopathy-where do we stand after a hundred years? Prog. Cardiovasc. Dis. 2010;52:300–316. doi: 10.1016/j.pcad.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- Brooks GA, Donovan CM, White TP. Estimation of anaerobic energy production and efficiency in rats during exercise. J. Appl. Physiol. 1984;56:520–525. doi: 10.1152/jappl.1984.56.2.520. [DOI] [PubMed] [Google Scholar]
- Caldas IS, Talvani A, Caldas S, et al. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol. Res. 2008;103:413–421. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Chakraborti T, Shaw G. Beta-adrenergic mechanisms in cardiac diseases: a perspective. Cell. Signal. 2000;12:499–513. doi: 10.1016/s0898-6568(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure: role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- Gallo L, Jr, Neto JA, Manco JC, Rassi A, Amorim DS. Abnormal heart rate responses during exercise in patients with Chagas’ disease. Cardiology. 1975;60:147–162. doi: 10.1159/000169713. [DOI] [PubMed] [Google Scholar]
- Higuchi ML, Fukasawa S, Brito T, Parzianello LC, Bellotti G, Ramires JAF. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas’ disease: a three dimensional confocal microscopy study. Heart. 1999;82:279–285. doi: 10.1136/hrt.82.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi OJ, Wisloff U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol. 2010;199:425–439. doi: 10.1111/j.1748-1716.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Haram PM, Wisloff U, Ellingsen O. Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation. 2004;109:2897–2904. doi: 10.1161/01.CIR.0000129308.04757.72. [DOI] [PubMed] [Google Scholar]
- Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J. Am. Coll. Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Lacerda AR, Marubayashi U, Balthazar CH, Coimbra CC. Evidence that brain nitric oxide inhibition increases metabolic cost of exercise, reducing running performance in rats. Neurosci. Lett. 2006;393:260–263. doi: 10.1016/j.neulet.2005.09.076. [DOI] [PubMed] [Google Scholar]
- Lima MMO, Pereira MC, Rocha MOC, Beloti FR, Alencar MCN, Ribeiro ALP. Left ventricular diastolic function and exercise capacity in patients with Chagas cardiomyopathy. Echocardiography. 2010;27:519–524. doi: 10.1111/j.1540-8175.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- López-De León A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. Histochem. Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- Mady C, Ianni BM, Arteaga E, et al. Relation between interstitial myocardial collagen and the degree of clinical impairment in Chagas’ disease. Am. J. Cardiol. 1999;84:354–356. doi: 10.1016/s0002-9149(99)00295-7. [DOI] [PubMed] [Google Scholar]
- Mady C, Ianni BM, Arteaga E, Salemi VMC, Frimm CC. Maximal functional capacity in patients with chagas’ cardiomyopathy without congestive heart failure. J. Card. Fail. 2000;3:220–224. doi: 10.1054/jcaf.2000.8828. [DOI] [PubMed] [Google Scholar]
- Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An. Acad. Bras. Ciênc. 2003;75:469–486. doi: 10.1590/s0001-37652003000400006. [DOI] [PubMed] [Google Scholar]
- Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas’ heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- Martinelli PM, Camargos ERS, Azevedo AA, Chiari E, Morel G, Machado CRS. Cardiac NGF and GDNF expression during Trypanosoma cruzi infection in rats. Auton. Neurosci. 2006;130:32–40. doi: 10.1016/j.autneu.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Meiler SEL, Ashton JJ, Moeschberger ML, Unverferth DV, Leier CV. An analysis of the determinants of exercise performance in congestive heart failure. Am. Heart J. 1987;113:1207–1217. doi: 10.1016/0002-8703(87)90935-5. [DOI] [PubMed] [Google Scholar]
- Montes de Oca M, Torres SH, Loyo JG, et al. Exercise performance and skeletal muscles in patients with advanced Chagas disease. Chest. 2004;125:1306–1314. doi: 10.1378/chest.125.4.1306. [DOI] [PubMed] [Google Scholar]
- Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J. Physiol. 2002;541:863–875. doi: 10.1113/jphysiol.2001.013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahash AJC, Gupta S, Anand IS. Myocyte response to β-adrenergic stimulation is preserved in the noninfarcted myocardium of globally dysfunctional rat hearts after myocardial infarction. Circulation. 2000;102:1840–1846. doi: 10.1161/01.cir.102.15.1840. [DOI] [PubMed] [Google Scholar]
- Prímola-Gomes TN, Campos LA, Lauton-Santos S, et al. Exercise capacity is related to calcium transients in ventricular cardiomyocytes. J. Appl. Physiol. 2009;107:593–598. doi: 10.1152/japplphysiol.91218.2008. [DOI] [PubMed] [Google Scholar]
- Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Roman-Campos D, Duarte HLL, Sales PA, Jr, et al. Changes in cellular contractility and cytokines profile during Trypanosoma cruzi infection in mice. Basic Res. Cardiol. 2009;104:238–246. doi: 10.1007/s00395-009-0776-x. [DOI] [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–63. [PubMed] [Google Scholar]
- Sousa LAP, Rocha MOC, Britto RR, Lombardi F, Ribeiro AL. Chagas disease alters the relationship between heart rate variability and daily physical activity. Int. J. Cardiol. 2009;135:257–259. doi: 10.1016/j.ijcard.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Sterin-Borda L, Gorelik G, Postan M, Gonzalez Cappa S, Borda E. Alterations in cardiac beta-adrenergic receptors in chagasic mice and their association circulating beta-adrenoceptor-related autoantibodies. Cardiovasc. Res. 1999;41:116–125. doi: 10.1016/s0008-6363(98)00225-9. [DOI] [PubMed] [Google Scholar]
- Talvani A, Rocha MOC, Ribeiro AL, Borda E, Sterin-Borda L, Teixeira MM. Levels of anti-M2 and anti-β1 autoantibodies do not correlate with the degree of heart dysfunction in Chagas’ heart disease. Microbes Infect. 2006;8:2459–2464. doi: 10.1016/j.micinf.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Verani MS, Hartung GH, Hoepfel-Harris J, Welton DE, Pratt CM, Miller RR. Effects of exercise training on left ventricular performance and myocardial perfusion in patients with coronary artery disease. Am. J. Cardiol. 1981;47:797–803. doi: 10.1016/0002-9149(81)90176-4. [DOI] [PubMed] [Google Scholar]
- WHO, World Health Organization. Tropical Disease Research: Progress 2003–2004 seventeenth programme report of the UNICEF/UNDP/World Bank/WHO. 2005. pp. 31–33. Special Programme for Research & Training in Tropical Diseases (TRD). Programme report, n. 17. Geneva pp.
- Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc. Res. 2002;54:162–174. doi: 10.1016/s0008-6363(01)00565-x. [DOI] [PubMed] [Google Scholar]


