Abstract
An animal study was conducted to evaluate the antilithogenic effect of a combination of dietary fenugreek seeds and onion. Lithogenic conditions were induced in mice by feeding them a high (0.5%) cholesterol diet (HCD) for 10 weeks. Fenugreek (12%) and onion (2%) were included individually and in combination in this HCD. Fenugreek, onion and their combination reduced the incidence of cholesterol gallstones by 75%, 27% and 76%, respectively, with attendant reduction in total cholesterol content by 38–42%, 50–72% and 61–80% in serum, liver and bile respectively. Consequently, the cholesterol/phospholipid ratio was reduced significantly in serum, liver and bile. The cholesterol saturation index of bile was reduced from 4.14 to 1.38 by the combination of fenugreek and onion and to 2.33 by onion alone. The phospholipid and bile acid contents of the bile were also increased. Changes in the hepatic enzyme activities (3-hydroxy-3-methylglutaryl Coenzyme A reductase, cholesterol-7α-hydroxylase and cholesterol-27-hydroxylase) induced by HCD were countered by fenugreek, onion and their combination. Hepatic lipid peroxides were reduced by 19–22% and 39–45% with fenugreek, onion and their combination included in the diet along with the HCD. Increased accumulation of fat in the liver and inflammation of the gallbladder membrane produced by HCD were reduced by fenugreek, onion and their combination. The antilithogenic influence was highest with fenugreek alone, and the presence of onion along with it did not further increase this effect. There was also no additive effect of the two spices in the recovery of antioxidant molecules or in the antioxidant enzyme activities.
Keywords: antilithogenic effect, cholesterol gallstones, dietary fenugreek seeds, dietary onion, hypocholesterolemic effect
Cholesterol gallstone (CGS) pathogenesis is a primary disorder arising out of altered hepatic and biliary cholesterol homeostasis. Gallstones are formed in the gallbladder because of precipitation of cholesterol, bilirubin and calcium salts in bile. The majority of gallstones are contributed by cholesterol, and a small number of gallstones are primarily composed of calcium salts of bilirubin and phosphate. Pathologic conditions that generally precede the occurrence of CGS are lithogenic bile, gallbladder stasis and short nucleation time. Lithogenicity of bile is determined by relative concentration of three main components viz., bile acids (BA), phospholipids and cholesterol. Generally, lithogenic bile occurs with alteration of cholesterol homeostasis, leading to increased cholesterol secretion and subsequent supersaturation of cholesterol in bile (Marzolo et al. 1990; Apstein & Carey 1996). Gallbladder stasis increases the opportunity for concentration of supersaturated bile in the gallbladder to form gallstones. It is well known that cholesterol supersaturation in bile is the first step in the onset of CGSs. The onset of CGS is associated with various physicochemical disturbances in bile, which include excess secretion of cholesterol and reduced secretion of BAs by the liver into bile and increased absorption of water from the gallbladder thus creating a hydrophobic environment. These series of events lead to supersaturation of bile with cholesterol, a prerequisite for CGS formation (Von Erpecum & Von Berge-Henegouwen 1989).
Several animal studies have evaluated the role of dietary components in preventing CGS. It has been shown that dietary proteins, carbohydrates, fibre and fat play a role in the induction of CGSs (Thornton et al. 1983; Ebihara & Kiriyama 1985; Mott et al. 1992). A few instances of dietary factors playing a role in the regression or amelioration of already existing CGS are also reported. Spices such as garlic, onion, fenugreek, red pepper and turmeric have been documented to be effective as hypocholesterolemic agents under conditions of experimentally induced hypercholesterolemia and hyperlipidemia (Srinivasan et al. 2004). It has been shown that curcumin of turmeric, capsaicin of red pepper, onion and garlic reduced the incidence of CGSs in mice when included in the diet (Hussain & Chandrasekhara 1992; Vidyashankar et al. 2009). Dietary fenugreek seeds were also found to exert antilithogenic effect by reducing the incidence of CGS formation (Reddy & Srinivasan 2009a). Dietary spice bioactive compounds (Hussain & Chandrasekhara 1994), Allium spices (Vidyashankar et al. 2010) and fenugreek seeds (Reddy & Srinivasan 2009b) have also been evidenced to regress preformed CGSs in experimental mice when included in the diet after CGS induction.
Spices are an integral part of Indian diet, and they are included in the diet to enhance the sensory quality of food. As Allium spices (onion and garlic) and fenugreek have already been reported to have an antilithogenic influence individually, and these spices are generally consumed in combination as spice mix, it would be interesting to evaluate the combination of such spices for a beneficial effect on health. Hence evaluated this study the antilithogenic influence of two hypocholesterolemic spices, fenugreek seed and onion when consumed together for a possible additive or synergistic effect. These two test spices were included in the mice diet both individually at the doses that are previously observed to produce biological effect and in combination during the induction of CGS by high-cholesterol diet (HCD) feeding.
Materials and methods
Chemicals
Cholesterol, dipalmitoyl phosphatidyl choline, triolein, heparin, ammonium thiosulphate, ferrous chloride, BAs, 3α-hydroxysteroid dehydrogenase, isocitrate dehydrogenase, glucose-6-phosphate dehydrogenase, cholesterol oxidase, thiobarbutric acid, tetramethoxy propane (TMP), t-butyl hydroperoxide, triethanolamine hydrochloride, tris-(hydroxymethyl)aminomethane, chlorodinitrobenzene (CDNB), nicotinamide adenine dinucleotide (NAD), nicotinamide adenine dinucleotide phosphate (NADP), reduced nicotinamide adenine dinucleotide phosphate (NADPH), glucose-6-phosphate, dithiothreitol, sodium arsenite, coenzyme-A, 3-hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA), 7β-hydroxycholesterol, Triton X-100, commassie brilliant blue, hydrazine hydrate, sodium metaperiodate, bovine serum albumin (BSA), ethylene diamine tetraacetic acid (EDTA) and 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) were purchased from Sigma-Aldrich (St. Louis, CA, USA). All other chemicals and solvents used were of analytical grade. Edible grade casein was procured from Nimesh Corporation (Mumbai, India). AIN-76 mineral mix was from SISCO Research Labs (Mumbai, India). All vitamins, DL-methionine and choline chloride were from Himedia (Mumbai, India). Cane sugar, fenugreek seeds, maize starch, refined groundnut oil and onion were purchased from the local market. Sugar and fenugreek were powdered. Onions were shredded and dehydrated in a freeze-dryer.
Animal treatment
Animal experiments were approved by the Institutional Animal Ethics Committee. Six-week-old male albino mice [OUTB/Swiss Albino/Ind/Cft(2c)] produced in our Experimental Animal Production Facility weighing 27 ± 1.0 g were grouped and housed in polypropylene cages with four to five mice per cage and maintained in a room at 22 ± 2 °C, with relative humidity of about 60 ± 5% and having regular 12 h cycle of day and night. The animals had free access to food and water. Animal weights were recorded every week throughout the duration of treatments.
Animals were divided into five diet intervention groups (18 mice each) and fed ad libitum AIN-76 pelleted custom-made diets. The five diet groups were the following: basal diet, lithogenic diet, test diet 1, test diet 2 and test diet 3. Basal AIN-76 semipurified diet consisted of the following: sucrose, 65%; casein, 20%; cellulose, 5%; AIN-76 mineral mix, 3.5%; AIN-76 vitamin mix, 1%; DL-methionine, 0.3%; choline chloride, 0.2%; and refined groundnut oil, 5% (Vidyashankar et al. 2009). Lithogenic diet was prepared by supplementing 0.5% cholesterol and 0.125% bile salts (1:1 mixture of sodium cholate and sodium deoxycholate) to the AIN-76 basal diet substituting same quantity of sucrose. The three test diets were prepared by incorporating the following into lithogenic diet at the expense of sucrose: (i) fenugreek seed powder (12%) (test diet 1), (ii) onion powder (2%) (test diet 2) and (iii) fenugreek powder (12%) and onion powder (2%) (test diet 3). These diets were prepared by mixing the ingredients in a mechanical mixer and pelletizing using hand-operated pelletizer. Diets were stored at 4 °C in airtight containers.
Collection of gallbladder and scoring of CGS
At the end of the feeding regimen, the animals were fasted overnight and killed under ether anaesthesia. Blood was drawn immediately by cardiac puncture, and the serum was separated by centrifugation for further analysis. Cholecystectomy was performed, and gallbladders were carefully collected and trimmed off any extraneous tissue. Liver was quickly excised, washed with ice-cold saline and blotted dry, weighed and stored at −20 °C till further analysis. The weight of gallbladder along with stones was measured. The gallbladders were placed on an illuminator screen under magnifying lens to evaluate the presence/absence of CGS and their size. The grading of stones was performed on a five-point scale (Akiyoshi et al. 1986). The volume of bile was noted, and the bile from gallbladders was appropriately pooled and stored at −20 °C till further analysis.
Lipid profile
Serum and liver lipids were extracted according to Folch et al. (1957). Biliary lipids were extracted by the method of Bligh and Dyer (1959). The upper methanolic phase was used for the estimation of total BAs using 3α-hydroxysteroid dehydrogenase (Turley & Dietschy 1970). The lower chloroform layer was used for the analysis of cholesterol and phospholipid. Cholesterol levels were quantified by the method of Searcy and Bergquist (1960). The HDL cholesterol and non-HDL (LDL + VLDL) cholesterol in serum were estimated by adapting the protocol given by Warnick and Albers (1978). HDL cholesterol in the supernatant was also estimated with the same method. The HDL2 and HDL3 fractions were estimated as described by Hirano et al. (2008). Phospholipids were measured by ferrous ammonium thiocyanate method using di-palmitoyl phosphatidyl choline as reference standard as described by Stewart (1980). Triglycerides were estimated according to the method described by Fletcher (1968), using triolein as standard. Cholesterol saturation index (CSI) of the bile was calculated using the cholesterol, phospholipids, total lipids and BAs values in bile (both relative and total lipid concentrations) as described by Carey (1978).
Reactive oxygen species (ROS), lipid peroxides (LPO) and antioxidant molecules in liver
Thiobarbituric acid reactive substances in the liver homogenate were measured fluorimetrically after extraction with butanol and were compared with the standard TMP as described by Ohkawa et al. (1979). Reactive oxygen species was estimated in the liver homogenate as described by Driver et al. (2000). Total thiols in liver homogenates were measured as described by Sedlak and Lindsay (1968). Glutathione was estimated according to the protocol described by Beutler et al. (1963). Ascorbic acid was determined according to the method described by Omaye et al. (1973).
Antioxidant enzymes in serum and liver
Catalase activity was assayed by following the rate of decomposition of hydrogen peroxide as described by Aebi (1984). Glutathione reductase activity was assayed by measuring the oxidation of NADPH by oxidized glutathione according to Carlberg and Mannervik (1985). Glutathione-S-transferase activity was measured as described by Warholm et al. (1985) using CDNB and by measuring the CDNB–glutathione complex formed. Glutathione peroxidase activity was measured as described by Flohe and Gunzler (1984). Superoxide dismutase (SOD) activity was measured by quantifying the inhibition of cytochrome-C reduction in xanthine–xanthine oxidase system as described by Flohe and Otting (1984).
Liver function enzymes in serum
The activities of alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) were assayed using standard commercial test kits (Span Diagnostics, Surat, India) and were expressed as Units/l. Liver protein was analysed according to Lowry et al. (1951) using BSA as standard protein.
Activities of enzymes of cholesterol metabolism
3-hydroxy-3-methylglutaryl Coenzyme A reductase activity in liver was assayed by following the formation of CoA as described by Hulcher and Oleson (1973). Activities of cholesterol-7α-hydroxylase and sterol-27-hydroxylase in liver were assayed as described by Petrack and Latario (1993) and Li et al. (2006) using HPLC system (Shimadzu – Model LC 10AVP; Shimadzu Corporation, Kyoto, Japan) equipped with a PDA detector.
Microscopic observation of cholesterol gallstones and histopathological evaluation
Selected CGS from each diet group were taken and observed under the scanning electron microscope (LEO – 435VP), in order to study the size and the crystal surface. Sections of fresh liver and gallbladder were taken for microscopic examination of liver histopathological changes and for the evaluation of gallbladder wall inflammation degree using light microscope (Leitz Laborlux POL, Wetzlar, Germany).
Statistical analysis
Statistical analysis was carried out using graphpad prism statistical software. Results were analysed by one-way anova, and the significance level was calculated using Tukey Kramer multiple comparison test; results were considered significant at P<0.05.
Results
Effect of dietary fenugreek seeds, onion and their combination on high-cholesterol diet-induced CGS incidence
Table 1 summarizes data on food intake, body weight, liver weight and gallbladder weights in the five diet groups. There was no difference in body weights and food intake between the groups, except for a higher food intake in HCD + O group. There was a significant difference in the liver and gallbladder weights as a result of addition of spices individually as well as in combination. The increases in liver and gallbladder weights produced by HCD were significantly countered by dietary fenugreek as well as dietary onion. This effect on liver weight was higher in the combination of fenugreek and onion as compared to the individual spices. In fact, the reduction in liver weight in HCD + F + O was 29% compared with HCD + F (26%) and HCD + O (12.5%). The reduction in gallbladder weights were 56%, 53% and 56% respectively.
Table 1.
Effect of dietary fenugreek and onion combination on animal weight, food intake, liver and gallbladder weight in HCD-fed mice
| Animal group | Initial animal weight (g) | Final animal weight (g) | Food intake (g/day/mice) | Gallbladder weight (mg) | Liver weight (per 100 g body weight) |
|---|---|---|---|---|---|
| Basal control | 26.9 ± 0.06 | 38.6 ± 0.75 | 4.62 ± 0.03 | 17.5 ± 2.8* | 3.58 ± 0.08† |
| HCD | 26.9 ± 0.06 | 38.3 ± 0.78 | 4.49 ± 0.12 | 39.4 ± 0.7 | 6.40 ± 0.14 |
| HCD + F | 26.9 ± 0.06 | 36.1 ± 0.44 | 4.95 ± 0.12 | 18.4 ± 1.26† | 4.75 ± 0.14† |
| HCD + O | 27.0 ± 0.10 | 37.6 ± 0.74 | 5.06 ± 0.17* | 29.2 ± 2.1* | 5.60 ± 0.12† |
| HCD + F + O | 27.0 ± 0.15 | 39.0 ± 0.92 | 4.98 ± 0.12 | 17.4 ± 3.0† | 4.54 ± 0.06† |
HCD, high-cholesterol diet; F, fenugreek; O, onion.
Values are mean ± SEM of 18 animals in each group.
Statistically significant compared with HCD group
P<0.05
P<0.01.
The incidence of HCD-induced CGS in each diet group is reported in Figures 1 and 2. There was a significant decrease in the incidence of CGS in all diet groups, the per cent incidence being 25% in HCD + F, 73% in HCD + O and 23.8% in HCD + F + O, as compared with 100% in HCD.
Figure 1.
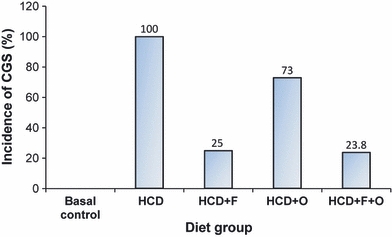
Effect of dietary fenugreek and onion combination on the incidence cholesterol gallstone (both incidence and extent of reduction) in mice maintained under lithogenic condition. HCD, high-cholesterol diet; F, fenugreek; O, onion.
Figure 2.
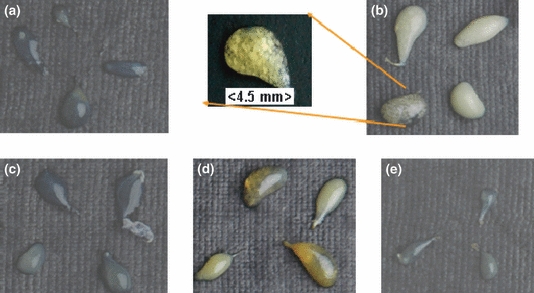
Incidence of cholesterol gallstone in different animal groups. (a) Basal control, (b) high-cholesterol diet (HCD), (c) HCD + F, (d) HCD + O, (e) HCD + F + O.
The HCD group had a gallstone size of about 1 mm, while others had gallstones of comparatively smaller size (Figure 3). The morphological observation of gallstones showed the presence of flakes with clear cut edges only in the HCD group (Figure 3).
Figure 3.

Scanning electron microscopic photographs of gallstones and gallstone surface crystals in different dietary groups. Top row: Gallstones (WD = 12nm): (a) high-cholesterol diet (HCD) (×250), (b) HCD + F (×500), (c) HCD +O (×100); Bottom row: Gallstone surface crystals (WD = 12 nm): (a) HCD (×1680), (b) HCD + F (×5000), (c) HCD + O (×2000).
Effect of dietary fenugreek, onion and their combination on serum lipids during CGS induction
The serum lipid profile of animals fed spices during the induction of CGS is presented in Table 2. Feeding of HCD caused hypercholesterolemia where there was an increase in serum cholesterol concentration (300.5 mg/dl), which was 1.8-fold compared with basal control (168.3 mg/dl). Addition of fenugreek, onion and their combination to HCD significantly prevented the increase in serum cholesterol concentration. The decrease in serum total cholesterol brought about in HCD + F + O was 42.2% compared with 38.3% in HCD + F and 39.6% in HCD + O. The decreases in the LDL cholesterol concentration among the treatments were similar, viz., 46% in HCD + O, 44% in HCD + F and 41% in HCD + F + O. Fenugreek and onion addition to HCD did not affect the total HDL content when compared with HCD group. At the same time, dietary fenugreek and onion decreased the HDL3 fraction by 40–80% with proportionate increase in the HDL2 (15–22%) fraction. The decrease in serum phospholipids brought about by HCD feeding was significantly countered by inclusion of fenugreek, onion or their combination in the diet, the extent of reversal being highest in case of combination spices. Serum phospholipid content was 40.6% higher in HCD + F + O, while it was 18.7% and 12.3% higher in HCD + F and HCD + O, respectively, as compared with HCD group. Serum triglyceride content was decreased by 15–20% in all the three test groups, as compared with HCD group.
Table 2.
Effect of dietary fenugreek and onion combination on serum lipid profile in HCD-fed mice
| Cholesterol (mg/dl) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Animal group | Total | LDL | Total HDL | HDL2 | HDL3 | Phospholipids (mg/dl) | Triglycerides (mg/dl) | C:P ratio |
| Basal control | 168.3 ± 4.5† | 122.4 ± 4.6† | 48.7 ± 4.2 | 33.3 ± 3.4† | 15.3 ± 1.7† | 343.7 ± 18.7† | 143.3 ± 5.6† | 0.49 ± 0.02† |
| HCD | 300.5 ± 12.6 | 263.9 ± 12.8 | 52.5 ± 3.1 | 43.6 ± 2.7 | 5.76 ± 0.6 | 220.4 ± 10.6 | 197.6 ± 4.2 | 1.32 ± 0.08 |
| HCD + F | 185.4 ± 7.3† | 147.9 ± 11.5† | 45.3 ± 3.9 | 36.8 ± 3.9 | 8.48 ± 1.6 | 261.6 ± 11.9† | 158.3 ± 4.6† | 0.71 ± 0.04† |
| HCD + O | 181.5 ± 4.2† | 140.5 ± 5.6† | 44.5 ± 3.0 | 34.0 ± 3.2* | 10.5 ± 1.1* | 247.4 ± 10.8† | 168.9 ± 6.2† | 0.75 ± 0.05† |
| HCD + F + O | 173.7 ± 7.0† | 157.3 ± 16.3* | 46.9 ± 1.9 | 37.7 ± 1.6 | 9.18 ± 0.5† | 309.8 ± 12.0† | 161.9 ± 9.2† | 0.62 ± 0.06† |
HCD, high-cholesterol diet; F, fenugreek; O, onion; C:P ratio, cholesterol:phospholipid ratio.
Values are mean ± SEM of six samples, each sample being pooled from three animals.
Statistically significant compared with HCD group
P<0.05
P<0.01.
Effect of dietary fenugreek, onion and their combination on hepatic lipid profile during CGS induction
Table 3 summarizes liver lipid profiles of the test groups during CGS induction. Hepatic cholesterol content was significantly reduced with the addition of fenugreek, onion or their combination to HCD diet. The extent of decrease in hepatic cholesterol brought about in HCD + F + O was 72%, while it was 70.5% in HCD + F and 50% in HCD + O. The decrease in hepatic phospholipid content was significantly reversed in HCD + F (26%) and HCD + F + O (32%). Triglyceride content of the liver was significantly reduced with the dietary incorporation of fenugreek, onion and fenugreek + onion, the reduction being 39.5%, 10% and 54.0% respectively. Thus, the combination of fenugreek and onion produced greater reduction than the individual spices. Total lipids in the liver were reduced by 56.6%, 16% and 61.3%, respectively, in HCD + F, HCD + O and HCD + F + O treatments, when compared with HCD group. Cholesterol/phospholipid (C/P) ratio was decreased to 0.69 (HCD + F), 1.5 (HCD + O) and 0.64 (HCD + F + O), while it was 2.81 in case of HCD.
Table 3.
Influence of fenugreek and onion combination on liver lipid profile in HCD-fed mice
| Animal group | Cholesterol (mg/g) | Phospholipids (mg/g) | Triglycerides (mg/g) | Total lipids (mg/g) | C:P ratio |
|---|---|---|---|---|---|
| Basal control | 5.17 ± 0.16† | 24.3 ± 0.68† | 40.8 ± 3.2† | 75.1 ± 2.50† | 0.22 ± 0.01† |
| HCD | 50.6 ± 1.60 | 17.0 ± 1.11 | 112.4 ± 2.8 | 243.8 ± 12.9 | 2.81 ± 0.24 |
| HCD + F | 14.8 ± 0.90† | 21.4 ± 0.89† | 68.0 ± 6.9† | 105.7 ± 6.24† | 0.69 ± 0.04† |
| HCD + O | 25.1 ± 0.71† | 17.4 ± 0.48 | 100.1 ± 3.2* | 205.9 ± 9.16† | 1.50 ± 0.06† |
| HCD + F + O | 14.2 ± 1.23† | 22.4 ± 0.80† | 51.7 ± 5.0† | 94.4 ± 6.57† | 0.64 ± 0.06† |
HCD, high-cholesterol diet; F, fenugreek; O, onion; C:P ratio, cholesterol:phospholipid ratio.
Values are mean ± SEM of six samples, each sample being pooled from three animals.
Statistically significant compared with HCD group
P<0.05
P<0.01.
Effect of dietary fenugreek, onion and their combination on biliary lipid profile during CGS induction
Biliary lipid profile of the test groups is tabulated in the Table 4. Biliary cholesterol, total lipids, C/P ratio, cholesterol/BA (C:BA) ratio and CSI decreased significantly with dietary incorporation of fenugreek, onion and fenugreek + onion into HCD diet compared with HCD, as a result of improved content of total BAs and decreased cholesterol concentration. A decrease of 78%, 61% and 81% in cholesterol content 66.5%, 43.7% and 67% in CSI value was noticed with the addition of fenugreek, onion and their combination to HCD respectively. There was a significant favourable effect on BA content with the incorporation of fenugreek, onion and their combination. Bile acid levels were increased by 12%, 27% and 12% in HCD + F, HCD + O and HCD + F + O, respectively, as compared with HCD group. The C/P and C/BA ratios, which were elevated by HCD, were significantly countered by the incorporation of fenugreek, onion or their combination into the HCD. The decreases in C/P ratio were 71.5%, 41.5% and 66.5%, while the decreases in C/BA ratio were 80%, 70% and 83%, in HCD + F, HCD + O and HCD + F + O groups, respectively, as compared with HCD group.
Table 4.
Influence of fenugreek and onion combination on biliary lipid profile in mice fed HCD
| Animal group | Cholesterol (mM) | Phospholipids (mM) | Bile acids (mM) | Total lipids (g/dl) | CSI | Cholesterol:phospholipid ratio | Cholesterol:bile acid ratio |
|---|---|---|---|---|---|---|---|
| Basal control | 7.88 ± 0.44† | 16.9 ± 0.47† | 165.6 ± 1.43† | 8.69 ± 0.45† | 0.91 ± 0.03† | 0.47 ± 0.03† | 0.045 ± 0.003† |
| HCD | 59.6 ± 0.62 | 29.8 ± 0.41 | 162.4 ± 2.18 | 11.7 ± 0.21 | 4.14 ± 0.02 | 2.00 ± 0.02 | 0.365 ± 0.004 |
| HCD + F | 13.2 ± 0.37† | 23.0 ± 0.40† | 182.3 ± 4.10* | 10.7 ± 0.20 | 1.39 ± 0.37† | 0.57 ± 0.02† | 0.073 ± 0.003† |
| HCD + O | 23.0 ± 0.48† | 19.6 ± 0.14† | 206.7 ± 3.50† | 11.3 ± 0.25 | 2.33 ± 0.06† | 1.17 ± 0.03† | 0.111 ± 0.003† |
| HCD + F + O | 11.5 ± 0.37† | 17.4 ± 0.68† | 182.8 ± 2.98* | 10.3 ± 0.40* | 1.37 ± 0.04† | 0.67 ± 0.02† | 0.063 ± 0.002† |
HCD, high-cholesterol diet; F, fenugreek; O, onion; CSI, cholesterol saturation index.
Values are mean ± SEM of six samples, each sample being pooled from three animals.
Statistically significant compared with HCD group
P<0.05
P<0.01.
Effect of dietary fenugreek, onion and their combination on hepatic cholesterol metabolizing enzymes during CGS induction
The activity of hepatic HMG-CoA reductase in different test groups of animals is presented in Figure 4, while the activities of hepatic cholesterol 7α-hydroxylase (CYP7A) and sterol 27α-hydroxylase (CYP27A) are shown in Figure 5. With the addition of fenugreek, onion or their combination to HCD, there was a significant increase in the activity of all these three enzymes involved in cholesterol metabolism. The activity of HMG-CoA was threefold higher in HCD + F group, while it was 1.2-fold higher in HCD + O group, as compared with HCD group. The activity of hepatic CYP7A was increased by 17%, 12% and 11.5% in HCD + F, HCD + O and HCD + F + O respectively. The activity of CYP27A was increased by 41.8%, 21.7% and 22% in HCD + F, HCD + O and HCD + F + O, respectively, as compared with HCD group.
Figure 4.
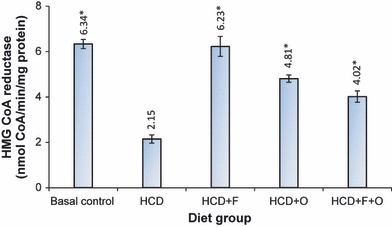
Effect of dietary fenugreek and onion combination on the activity of hepatic 3-hydroxy-3-methylglutaryl Coenzyme A reductase in mice under lithogenic diet. HCD, high-cholesterol diet; F, fenugreek; O, onion. *Statistically significant compared with HCD group (P<0.05).
Figure 5.
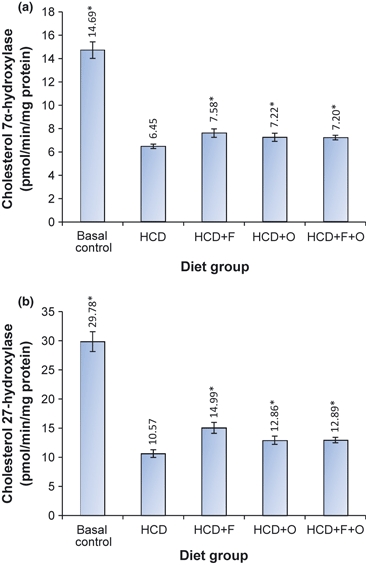
Effect of dietary fenugreek and onion combination on the activity of cholesterol 7α-hyderoxylase (a) and sterol 27α-hyderoxylase (b) in the liver of mice under lithogenic diet. HCD, high-cholesterol diet; F, fenugreek; O, onion.* Statistically significant compared with HCD group (P<0.05).
Effect of dietary fenugreek, onion and their combination on serum antioxidant enzymes during CGS induction
The activity of antioxidant enzymes in the serum of various treatment groups is given in Table 5. There was an improvement in the activity of glutathione-S-transferase by 24%, 24% and 19%, respectively, compared with HCD, while the activity of glutathione reductase was improved by 34% in HCD + F + O. No significant changes were detected for the other enzymes. There was also a significant reduction in lipid peroxide levels with the addition of spices to HCD, which was 23–33.7% in the spice incorporated groups.
Table 5.
Effect of dietary fenugreek and onion combination on the activity of serum antioxidant enzymes in HCD-fed mice
| Animal group | Catalase (mmol/min/ml) | Glutathione-S-transferase (mmol/min/ml) | Glutathione reductase (mmol/min/dl) | Glutathione peroxidase\(mmol/min/dl) | Superoxide dismutase (Units/ml) | Lipid peroxides (mmol MDA per mg protein) |
|---|---|---|---|---|---|---|
| Basal control | 0.35 ± 0.03* | 4.38 ± 0.35 | 2.24 ± 0.22* | 23.2 ± 0.78 | 0.42 ± 0.04 | 171.6 ± 5.5† |
| HCD | 0.48 ± 0.04 | 3.76 ± 0.22 | 1.66 ± 0.12 | 20.9 ± 0.31 | 0.45 ± 0.04 | 220.0 ± 8.4 |
| HCD + F | 0.38 ± 0.03 | 4.66 ± 0.27* | 1.86 ± 0.15 | 23.8 ± 0.89 | 0.37 ± 0.04 | 169.9 ± 8.3† |
| HCD + O | 0.44 ± 0.03 | 4.66 ± 0.21* | 1.85 ± 0.15 | 22.7 ± 0.95 | 0.50 ± 0.05 | 145.9 ± 5.2† |
| HCD + F + O | 0.38 ± 0.04 | 4.48 ± 0.30* | 2.16 ± 0.16* | 23.2 ± 0.92 | 0.46 ± 0.03 | 167.7 ± 5.7† |
HCD, high-cholesterol diet; F, fenugreek; O, onion.
Values are mean ± SEM of six samples, each sample being pooled from three animals.
Statistically significant compared with HCD group
P<0.05
P<0.01.
Effect of dietary fenugreek, onion and their combination on hepatic antioxidant molecules, antioxidant enzymes and lipid peroxides during CGS induction
The levels of antioxidant molecules glutathione and ascorbic acid, LPO, ROS in the liver of different test groups are reported in Table 6. There was a significant increase in total thiols, glutathione and ascorbic acid content, which was accompanied by a significant decrease in the ROS and LPO with incorporation of fenugreek, onion and combination into HCD. The decrease in ROS brought about by dietary spices ranged from 39% to 45%, and the decrease in LPO was from 19% to 22%. There was a favourable increase of 43–66% in glutathione content, 46–71% in total thiols and 19–23.5% in ascorbic acid content, but there was no additive effect when fenugreek and onion were incorporated together in HCD.
Table 6.
Influence of fenugreek and onion combination on antioxidant molecules in the liver of HCD-fed mice
| Animal group | ROS (mmol MDA/mg protein) | LPO (μmol MDA/mg protein) | Glutathione (nmol/mg protein) | Total thiols (nmol/mg protein) | Ascorbic acid (μg/mg protein) |
|---|---|---|---|---|---|
| Basal control | 0.55 ± 0.06† | 166.3 ± 22.9† | 53.4 ± 3.0† | 0.36 ± 0.02† | 9.26 ± 0.58† |
| HCD | 1.20 ± 0.09 | 264.9 ± 13.1 | 30.2 ± 1.9 | 0.28 ± 0.01 | 7.35 ± 0.25 |
| HCD + F | 0.67 ± 0.04† | 212.3 ± 10.5† | 43.3 ± 3.0* | 0.48 ± 0.01† | 8.91 ± 0.32† |
| HCD + O | 0.73 ± 0.02† | 207.2 ± 10.9† | 50.2 ± 3.7† | 0.41 ± 0.01† | 9.08 ± 0.28† |
| HCD + F + O | 0.66 ± 0.08† | 214.5 ± 10.6† | 49.3 ± 2.2† | 0.45 ± 0.01† | 8.78 ± 0.21† |
HCD, high-cholesterol diet; F, fenugreek; O, onion; LPO, lipid peroxides; ROS, reactive oxygen species.
Values are mean ± SEM of six samples, each sample being pooled from three animals.
Statistically significant compared with HCD group
P<0.05
P<0.01.
Activities of hepatic antioxidant enzymes in various treatment groups are presented in Table 7. There was a favourable effect on the activity of these enzymes by the inclusion of fenugreek, onion or their combination into HCD. The activity of hepatic glutathione-S-transferase was increased by 64% and 45% in HCD + F and HCD + F + O, respectively, when compared with HCD group. The activity of hepatic glutathione reductase was increased by 39% and 32% by dietary onion and combination of fenugreek and onion respectively. The activity of glutathione peroxidase was increased by 51% and 64% by dietary onion and combination of fenugreek and onion respectively. Incorporation of fenugreek, onion and fenugreek + onion into HCD did not affect the activities of catalase and SOD in liver.
Table 7.
Effect of dietary fenugreek and onion combination on the activity of liver antioxidant enzymes in HCD-fed mice
| Animal group | Catalase (mmol/min/mg protein) | Glutathione-S-transferase (μmol/min/mg protein) | Glutathione reductase (μmol/min/mg protein) | Glutathione peroxidase (μmol/min/mg protein) | Superoxide dismutase (Units/min/mg protein) |
|---|---|---|---|---|---|
| Basal control | 121.4 ± 6.04 | 0.15 ± 0.02 | 25.9 ± 2.64 | 2.04 ± 0.20 | 11.6 ± 0.4* |
| HCD | 132.9 ± 9.07 | 0.11 ± 0.01 | 18.3 ± 1.18 | 1.67 ± 0.12 | 14.1 ± 0.4 |
| HCD + F | 114.5 ± 8.31 | 0.18 ± 0.03* | 22.9 ± 0.78 | 1.90 ± 0.21 | 12.8 ± 0.9 |
| HCD + O | 114.6 ± 7.46 | 0.13 ± 0.01 | 25.4 ± 1.64† | 2.52 ± 0.17* | 13.1 ± 0.9 |
| HCD + F + O | 116.6 ± 5.85 | 0.16 ± 0.01* | 24.2 ± 1.93† | 2.74 ± 0.16† | 13.2 ± 0.5 |
HCD, high-cholesterol diet; F, fenugreek; O, onion.
Values are mean ± SEM of six samples, each sample being pooled from three animals.
Statistically significant compared with HCD group
P<0.05
P<0.01.
Effect of dietary fenugreek, onion and their combination on liver enzymes during CGS induction
There was a significant decrease in the activities of liver enzymes in the serum of animal groups with the addition of fenugreek, onion or their combination to HCD (Table 8). The combination of the two spices with HCD did not have any additive effect when compared to the individual effects of fenugreek and onion alone. Alanine aminotransferase activity was decreased by 54.7%, 42.9% and 60.1% in HCD + F, HCD + O and HCD + F + O groups compared with HCD group. Similarly, ASAT was decreased by 42.3%, 33.4% and 44.8%, in the respective groups when compared with HCD. With the incorporation of fenugreek, onion or their combination, the activity of LDH was decreased by 34%, 30% and 36.8%, while that of ALP was decreased by 32.4%, 33.3% and 38.7%, respectively, when compared with HCD.
Table 8.
Effect of dietary fenugreek and onion combination on the activity of serum aminotransferases and ALP in HCD-fed mice
| Animal group | ALAT | ASAT | LDH | ALP |
|---|---|---|---|---|
| Basal control | 18.2 ± 0.97† | 53.4 ± 2.48† | 1333.7 ± 64.0† | 53.2 ± 3.9† |
| HCD | 50.2 ± 1.39 | 95.8 ± 4.04 | 2157.1 ± 51.9 | 122.8 ± 4.8 |
| HCD + F | 22.8 ± 1.71† | 55.3 ± 2.95† | 1424.7 ± 81.3† | 83.0 ± 6.4† |
| HCD + O | 28.7 ± 1.09† | 63.8 ± 4.12† | 1506.8 ± 61.8† | 82.0 ± 9.0† |
| HCD + F + O | 20.0 ± 1.18† | 52.9 ± 8.91† | 1362.6 ± 30.5† | 75.3 ± 2.5† |
HCD, high-cholesterol diet; F, fenugreek; O, onion; ALAT, alanine aminotransferase; ALP, alkaline phosphatase; ASAT, aspartate aminotransferase; LDH, lactate dehydrogenase.
Values are expressed as Units/l and are mean ± SEM of six samples, each sample being pooled from three animals.
Statistically significant compared with HCD group
P<0.01.
Effect of dietary fenugreek, onion and their combination on the histopathological changes in liver and gallbladder during induction of CGS
The morphological variations in the histology of liver and gallbladder are shown in Figure 6. Feeding of HCD caused fat accumulation in the liver, which was significantly reduced with the addition of fenugreek, onion or their combination to HCD, while there was no fat cell in the basal control group. Gallbladder membrane morphological observation revealed that there is an increased inflammation in the HCD group, while the incorporation of fenugreek, onion or their combination into HCD could have a positive effect in the reduction of inflammation degree of gallbladder wall.
Figure 6.
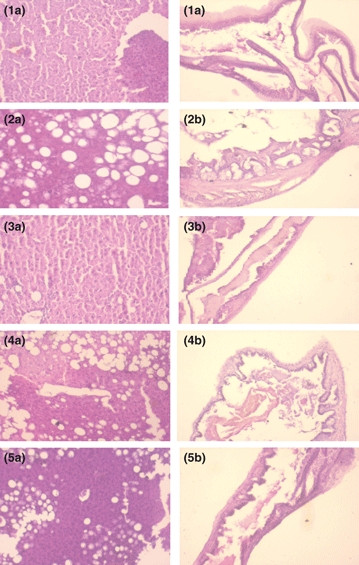
Effect of dietary fenugreek and onion combination on (a) liver histology and (b) gallbladder histology in mice maintained on lithogenic diet. 1. Basal control, 2. High-cholesterol diet (HCD), 3. HCD + F, 4. HCD + O, 5. HCD + F + O.
Discussion
In the present study, feeding of HCD for 10 weeks induced CGS in mice. Inclusion of fenugreek, onion or a combination of these along with HCD significantly lowered the incidence of CGS. Addition of fenugreek, onion or their combination to HCD had a significant hypocholesterolemic effect. In particular, these spices reduced serum concentration of LDL cholesterol, which is primarily responsible for the reduction in cholesterol/phospholipid ratio in serum. There was a significant decrease in liver weight with the incorporation of spices into HCD during CGS induction, which was a result of lowered lipid content in the hepatic tissue. There was also a significant reduction in hepatic cholesterol and triglyceride and an increase in hepatic phospholipids, thus resulting in beneficial lowering of cholesterol/phospholipid ratio in the liver. Many studies have reported hypocholesterolemic influence of various dietary components like fibre (Anderson et al. 1992; Jenkins et al. 1993) and spices — Red pepper, turmeric, fenugreek, onion and garlic have been used in several animal models (Srinivasan et al. 2004); particularly fenugreek seeds (Reddy & Srinivasan 2009a,b;) and onion in mice (Vidyashankar et al. 2009).
Diets supplemented with cholesterol have shown to produce lithogenic bile and gallstones in experimental animals including prairie dogs, squirrel monkeys and hamsters (DenBesten et al. 1974; Pearlman et al. 1979), rats and mice (Hussain & Chandrasekhara 1992, 1994; Vidyashankar et al. 2009, 2010). Continuous feeding of HCD increased cholesterol content and hence elevated the cholesterol/phospholipid ratio, cholesterol/BAs ratio and CSI in bile, which eventually resulted in cholesterol crystallization. Supplementation of fenugreek, onion or their combination resisted these changes, thus ameliorating cholesterol crystallization in gallbladder. Feeding Allium spices, especially onion, significantly reduced serum cholesterol, especially LDL cholesterol, with parallel reduction in liver cholesterol, along with a significant increase in the phospholipid content (Vidyashankar et al. 2009). Our results indicated an improvement in the HDL3 levels with the inclusion of fenugreek/onion/their combination in the diet. A positive relation between total cholesterol, LDL cholesterol and CGS has been reported (Halldestam et al. 2009). Acalovschi (2001) has reported that plasma total HDL or HDL3 cholesterol is inversely related to gallstone formation and suggested that HDL could have a protective role against CGS.
Sowmaa and Rajyalakshmi (1999) have reported that feeding diet containing fenugreek resulted in reduced level of total cholesterol with simultaneous reduction in LDL cholesterol. Among various mechanisms of hypocholesterolemic effect of fenugreek, is the increased excretion of BAs and neutral steroids through faeces. Dietary fenugreek stimulates the conversion of cholesterol to BAs (Bhat et al. 1985) and inhibits intestinal uptake of cholesterol and BAs (Oakenfull & Sidhu 1990; Sauvaire et al. 1991). Crude saponin fraction of fenugreek has been found to reduce serum cholesterol in rats (Sharma 1986a,b;). Feeding of the diet containing fenugreek significantly decreased plasma cholesterol, without affecting triglyceride levels. It is believed that large mixed micelles containing bile salts are formed and these large molecules are less efficiently absorbed because of the formation of the physical barrier (Trowell 1975; Sidhu & Oakenfull 1986).
Feeding of the diet enriched with cholesterol markedly elevated the lithogenic index of bile. Decrease in the lithogenic index with incorporation of fenugreek into HCD reflects a favourable effect: reduced precipitation of cholesterol in bile. Similar results envisaging significant reduction in biliary cholesterol, cholesterol/phospholipid ratio and CSI of the bile and a higher bile flow have been reported for curcumin and capsaicin (Hussain & Chandrasekhara 1992, 1994), onion and garlic (Vidyashankar et al. 2009, 2010) and fenugreek (Reddy & Srinivasan 2009a,b;).
Administration of spice active principles stimulated bile formation in liver and also increased BA secretion into bile. Antilithogenic effect of fibre is mainly attributed to its influence on the intestinal transit and reduction in secondary BAs (Marcus & Wheaton 1986a,b; Cuevas et al. 2004). Fibre also acts as a physical barrier for absorption (Sidhu & Oakenfull 1986). Supplementation of fibre into lithogenic diet inhibits cholesterol stone formation by reducing biliary cholesterol saturation (Schwesinger et al. 1999).
Addition of fenugreek, onion or their combination into HCD had a significant countering effect on transaminase enzymes and ALP in serum during the induction of CGS, suggesting their hepatoprotective influence. Besides, there is also a significant antioxidant effect in both serum and liver with the addition of fenugreek, onion or their combination. These spices improved the concentration of antioxidant molecules and enhanced the activity of antioxidant enzymes. Ravikumar and Anuradha (1999) have reported that supplementation of fenugreek in diet lowers lipid peroxidation.
The activity of hepatic HMG-CoA reductase, CYP7A and CYP27A increased with the addition of fenugreek, onion and their combination to HCD compared with HCD alone, thus countering the effect of HCD. Increased activity of cholesterol metabolizing enzymes: CYP7A and CYP27A have a role in cholesterol excretion and hence influence cholesterol levels. Increased activity of HMG-CoA reductase by inclusion of these specific spices in the diet is an adaptive response, where the suppressed enzyme activity during high-cholesterol feeding (feedback since no need for de novo synthesis) is countered because of accelerated excretion rate. Feeding of HCD continuously shattered the feedback mechanism and affected the balance by depositing more and more cholesterol in solution than its carrying capacity (Carey 1989). The turn around effect was observed with the incorporation of fenugreek, onion and combination of these into HCD. The overall impairment of BA absorption results in the up-regulation of cholesterol-7α-hydroxylase with a consequent increasing in BA synthesis (Matheson et al. 1995).
Histopathological observation of liver and gallbladder showed that feeding of HCD increased fat accumulation in liver and inflammation of the gallbladder membrane. These effects of fat accumulation in liver and inflammation were reduced with the addition of fenugreek, onion and their combination into HCD. Similar observation has been made by Rege and Prystowsky (1998), who have reported that feeding a lithogenic diet developed cholesterol crystals and gallstones at 2 and 6 weeks. Mucus layer was progressively increased during this period, and the inflammation is an early and necessary event in the formation of gallstones in bile. Histopathological observation revealed that feeding of lithogenic diet to gallstone susceptible mice (C57L) progressively increased the gallbladder wall thickness. In contrast, AKR mice that are resistant to gallstones displayed no or mild increase in gallbladder thickness. Both strains accumulated subepithelial inflammatory cells and oedema, which was highly significant in C57L mice. These findings suggest that during the formation of CGS, gallbladder undergoes progressive changes that ultimately result in increased oedema and decreased motility. Similar results have been reported in prairie dogs, where the inflammation was an essential event of CGS cholelithogenesis. In our study, fenugreek, onion and their combination showed a beneficial effect by reducing the accumulation of fat in the liver and reducing the inflammation of gallbladder membrane.
Thus, incorporation of fenugreek (12%), onion (2%) and their combination into HCD significantly reduced the incidence of CGS during a 10-week feeding trial. The antilithogenic influence was associated with a significant reduction in serum LDL cholesterol and cholesterol/phospholipid ratio. Incorporation of fenugreek and onion either singly or in combination beneficially moderated the CSI in bile, which was a result of reduction in cholesterol and also an increase in BA concentration in bile. These spices individually or in combination effectively reduced the accumulation of fat in the liver and also reduced the inflammation caused by feeding of HCD. Apart from this antilithogenic influence, addition of fenugreek, onion or their combination had a beneficial antioxidant and hepatoprotective effect under lithogenic condition. The results of this investigation suggested that the antilithogenic influence was maximum with fenugreek alone and the presence of onion along with it did not bring about any further increase in this health effect. Generally, there was no additive effect with respect to either hepatoprotective influence or the antioxidant molecules and the activity of antioxidant enzymes.
Acknowledgments
The first author (RLR) is grateful to the Council of Scientific and Industrial Research, New Delhi, for the award of Research Fellowship. This work was supported by a grant-in-aid from the Department of Science and Technology, Government of India, New Delhi.
References
- Acalovschi M. Cholesterol gallstones: from epidemiology to prevention. Postgrad. Med. J. 2001;77:221–229. doi: 10.1136/pmj.77.906.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Akiyoshi T, Uchida K, Takase H, et al. Cholesterol gallstones in alloxan-diabetic mice. J. Lipid Res. 1986;27:915–924. [PubMed] [Google Scholar]
- Anderson JW, Riddell-Mason S, Gustafson NJ, et al. Cholesterol lowering effects of psyllium-enriched cereal as an adjunct to a prudent diet in the treatment of mild to moderate hypercholesterolemia. Am. J. Clin. Nutr. 1992;56:93–98. doi: 10.1093/ajcn/56.1.93. [DOI] [PubMed] [Google Scholar]
- Apstein MD, Carey MC. Pathogenesis of cholesterol gallstones a parsimonious hypothesis. Eur. J. Clin. Invest. 1996;26:343–352. doi: 10.1046/j.1365-2362.1996.148287.x. [DOI] [PubMed] [Google Scholar]
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Bhat BG, Sambaiah K, Chandrasekhara N. The effect of feeding fenugreek and ginger on bile composition in the albino rats. Nutr. Rep. Int. 1985;32:1145–1151. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J. Lipid Res. 1978;9:945–955. [PubMed] [Google Scholar]
- Carey MC. Formation of cholesterol gallstones: the new paradigms. In: Paumgartner G, Stiehl A, Gerok W, editors. Trends in Bile Acid Research. Lancaster: Kluwer Academic Publishers; 1989. pp. 259–281. [Google Scholar]
- Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Cuevas A, Miquel JF, Reyes MS, et al. Review: diet as a risk factor for cholesterol gallstone disease. J. Am. Coll. Nutr. 2004;23:187–196. doi: 10.1080/07315724.2004.10719360. [DOI] [PubMed] [Google Scholar]
- DenBesten L, Safaie-Shirazi S, Connor WE, et al. Early changes in bile composition and gallstone formation induced by a high cholesterol diet in praire dogs. Gastroenterology. 1974;66:1036–1045. [PubMed] [Google Scholar]
- Driver AS, Kodavanti PR, Mundy WR. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol. Teratol. 2000;22:175–181. doi: 10.1016/s0892-0362(99)00069-0. [DOI] [PubMed] [Google Scholar]
- Ebihara K, Kiriyama S. Prevention of CGS by a water soluble fibre – Konjac Mannan in mice. Nutr. Rep. Int. 1985;32:223–229. [Google Scholar]
- Fletcher MJ. A colorimetric method for estimating serum triglycerides. Clin. Chim. Acta. 1968;22:303–307. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Halldestam I, Kullman E, Borch K. Incidence of and potential risk factors for gallstone disease in a general population sample. Br. J. Surg. 2009;96:1315–1322. doi: 10.1002/bjs.6687. [DOI] [PubMed] [Google Scholar]
- Hirano T, Nohtomi K, Koba S, et al. A simple and precise method for measuring HDL-cholesterol subfractions by a single precipitation followed by homogenous HDL-cholesterol assay. J. Lipid Res. 2008;49:1130–1136. doi: 10.1194/jlr.D700027-JLR200. [DOI] [PubMed] [Google Scholar]
- Hulcher FH, Oleson WH. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl coenzyme-A reductase by measurement of coenzyme A. J. Lipid Res. 1973;14:625–631. [PubMed] [Google Scholar]
- Hussain MS, Chandrasekhara N. Effect of curcumin on cholesterol gallstone induction in mice. Indian J. Med. Res. 1992;96:288–291. [PubMed] [Google Scholar]
- Hussain MS, Chandrasekhara N. Effect of curcumin and capsaicin on the regression of pre-established cholesterol gallstones in mice. Nutr. Res. 1994;14:1561–1574. [Google Scholar]
- Jenkins DJ, Wolever TM, Rao AV, et al. Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol. N. Engl. J. Med. 1993;329:21–26. doi: 10.1056/NEJM199307013290104. [DOI] [PubMed] [Google Scholar]
- Li X, Hylemon P, Pandak MW, et al. Enzyme activity assay for cholesterol 27-hydroxylase in mitochondria. J. Lipid Res. 2006;47:1507–1512. doi: 10.1194/jlr.M600117-JLR200. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with the Folin-Phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marcus SN, Wheaton KW. Intestinal transit rate, deoxycholic acid and the cholesterol saturation of bile-three interrelated factors. Gut. 1986a;27:550–558. doi: 10.1136/gut.27.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SN, Wheaton KW. Effects of a new, concentrated wheat fiber preparation on intestinal transit, deoxycholic acid metabolism and the composition of bile. Gut. 1986b;27:893–900. doi: 10.1136/gut.27.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo MP, Rigotti A, Nervi F. Secretion of biliary lipids from the hepatocyte. Hepatology. 1990;12(Suppl.):134–142. [PubMed] [Google Scholar]
- Matheson HB, Colon IS, Story JA. Cholesterol-7-α-hydroxylase activity is increased by dietary modification with psyllium hydrocolloid, pectin, cholesterol and cholestyramine in rats. J. Nutr. 1995;125:454–458. doi: 10.1093/jn/125.3.454. [DOI] [PubMed] [Google Scholar]
- Mott GE, Jackson EM, McMohan CA. Effect of dietary cholesterol, type of fat and sex on bile lipid composition of adult baboons. Am. J. Clin. Nutr. 1992;56:511–515. doi: 10.1093/ajcn/56.3.511. [DOI] [PubMed] [Google Scholar]
- Oakenfull DG, Sidhu GS. Could saponins be a useful treatment for hyper-cholesterolemia? Eur. J. Clin. Nutr. 1990;44:79–88. [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1973;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- Pearlman BH, Bonorris GG, Phillips MJ. Cholesterol gallstone formation and prevention by chenodeoxycholic and ursodeoxycholic acids: a new hamster model. Gastroenterology. 1979;77:634–641. [PubMed] [Google Scholar]
- Petrack B, Latario BJ. Synthesis of 27-hydroxycholesterol in rat liver mitochondria: HPLC assay and marked activation by exogenous cholesterol. J. Lipid Res. 1993;34:643–649. [PubMed] [Google Scholar]
- Ravikumar P, Anuradha CV. Effect of fenugreek seeds on blood lipid peroxidation and antioxidants in diabetic rats. Phytother. Res. 1999;13:197–201. doi: 10.1002/(SICI)1099-1573(199905)13:3<197::AID-PTR413>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Reddy RLR, Srinivasan K. Fenugreek seeds reduce atherogenic diet induced cholesterol gallstone formation in experimental mice. Can. J. Physiol. Pharmacol. 2009a;87:933–943. doi: 10.1139/y09-084. [DOI] [PubMed] [Google Scholar]
- Reddy RLR, Srinivasan K. Dietary fenugreek seed regresses preestablished cholesterol gallstones in mice. Can. J. Physiol. Pharmacol. 2009b;87:684–693. doi: 10.1139/y09-062. [DOI] [PubMed] [Google Scholar]
- Rege RV, Prystowsky JB. Inflammation and a thickened mucus layer in mice with cholesterol gallstones. J. Surg. Res. 1998;74:81–85. doi: 10.1006/jsre.1997.5213. [DOI] [PubMed] [Google Scholar]
- Sauvaire Y, Ribes G, Baccon JC, et al. Implication of steroid saponins and sapogenins in the hypocholesterolemic effect of fenugreek. Lipids. 1991;26:191–197. doi: 10.1007/BF02543970. [DOI] [PubMed] [Google Scholar]
- Schwesinger WW, Kurtin WE, Page CP, et al. Soluble dietary fiber protects against cholesterol gallstone formation. Am. J. Surg. 1999;177:307–310. doi: 10.1016/s0002-9610(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Searcy RL, Bergquist LM. A new colour reaction for the quantification of serum cholesterol. Clin. Chim. Acta. 1960;5:192–199. doi: 10.1016/0009-8981(60)90035-8. [DOI] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Sharma RD. An evaluation of hypocholesterolemic factor of fenugreek seeds (T. foenum- graecum) in rats. Nutr. Rep. Int. 1986a;33:669–677. [Google Scholar]
- Sharma RD. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in heman subjects. Nutr. Res. 1986b;6:1353–1364. [Google Scholar]
- Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br. J. Nutr. 1986;55:643–649. doi: 10.1079/bjn19860070. [DOI] [PubMed] [Google Scholar]
- Sowmaa P, Rajyalakshmi P. Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Plant Food Hum. Nutr. 1999;53:359–365. doi: 10.1023/a:1008021618733. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Sambaiah K, Chandrasekhara N. Spices as beneficial hypolipidemic food adjuncts: a review. Food Rev. Int. 2004;20:187–220. [Google Scholar]
- Stewart JCM. Colorimetric estimation of phospholipids with ammonium ferrothio cyanate. Anal. Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Thornton JR, Emmett PM, Heaton KW. Diet and gallstone effect on refined and unrefined carbohydrate diets on bile cholesterol saturation and bile acid metabolism. Gut. 1983;24:2–6. doi: 10.1136/gut.24.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowell HC. Letter: dietary fibre hypothesis. Br. Med. J. 1975;13:649. doi: 10.1136/bmj.4.5997.649-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SD, Dietschy JM. Reevaluation of 3α-hydroxysteroid dehydrogenase assay for total bile acids. J. Lipid Res. 1970;19:924–928. [PubMed] [Google Scholar]
- Vidyashankar S, Sambaiah K, Srinivasan K. Dietary garlic and onion reduce the incidence of atherogenic diet-induced cholesterol gallstones in experimental mice. Br. J. Nutr. 2009;101:1621–1629. doi: 10.1017/S0007114508118748. [DOI] [PubMed] [Google Scholar]
- Vidyashankar S, Sambaiah K, Srinivasan K. Regression of preestablished cholesterol gallstones by dietary garlic and onion in experimental mice. Metabolism. 2010;59:1402–1412. doi: 10.1016/j.metabol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Von Erpecum KJ, Von Berge-Henegouwen GP. Pathogenic factors in cholesterol gallstone disease. Scand. J. Gastroenterol. 1989;24(Suppl.):83–90. [Google Scholar]
- Warholm M, Guthenberg C, Bahr CV, et al. Glutathine transferase from human liver. Methods Enzymol. 1985;113:499–504. doi: 10.1016/s0076-6879(85)13065-x. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese chloride precipitation procedure for estimating HDL-cholesterol. J. Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]


