Abstract
Platelet-derived growth factor (PDGF) and its receptors (PDGFRs) are strongly involved in the normal development of several organs, tumour angiogenesis and malignant progression and metastasis. Few studies concerning their expression, distribution and role in normal and pathological human thymus are available in the literature. The aim of this study has been to analyse the immunohistochemical expression of PDGF and PDGFR-α in prenatal and postnatal normal human thymus and thymomal biopsy specimens. The results demonstrated immunoreactivity to both PDGF and PDGFR-α in all specimens, but the intensity, distribution and number of positive cells were different in normal thymus and thymomas, and also among different tumour types. PDGF and PDGFR-α were weakly expressed in foetal and postnatal humans with a different distribution between cortex and medulla in both blood vessels and epithelial cells, whereas they were overexpressed in thymoma, especially in type B2 and B3, in the tumour epithelial cells. Overall, these data suggest that PDGF and PDGFR-α may be involved in the pathophysiology of the human thymus.
Keywords: platelet-derived growth factor, platelet-derived growth factor receptor-α, thymoma, thymus
The thymus is crucial for the normal development of the immune system, and its peculiar microenvironment consisting of epithelial cells, connective tissue and blood vessels contributes to T cell differentiation (Gameiro et al. 2010). Much data have been accumulated in the past decades concerning the differentiation and maturation of T cells, including the contribution of the thymic stroma (Heng et al. 2010). Prenatal and postnatal human thymuses have been little investigated in terms of normal histological variants (Raica et al. 2006) and of the presence of multipotent mesenchymal stromal cells in foetal and adult human thymus (Mouiseddine et al. 2008; Siepe et al. 2009). Little data is available about the molecular mechanisms of the human thymus development and the role of different growth factors and their receptors in the normal organogenesis of this gland and its malignant transformation. Vascular endothelial growth factor (VEGF), VEGF receptors 1 and 2 (VEGFR-1 and -2), and fibroblast growth factor-2 (FGF-2) expression have been studied in the embryonic thymus (Tomita et al. 2002; Müller et al. 2005; Cimpean et al. 2008; Cuddihy et al. 2009). Parrens et al. (1999) reported that nerve growth factor (NGF) and its receptors are involved in the formation of the thymus stroma and proliferation of epithelial cells. These data were supported by Lee et al. (2008), which suggested that tropic NGF activity enhances thymus regeneration and improves host immunity in patients with immunodeficiency caused by thymus involution. Moreover, epidermal growth factor receptor (EGFR), which is strongly expressed in neoplastic cells of well-differentiated thymic carcinoma (Pedersini et al. 2008), stimulates proliferation of thymic epithelial cells.
The involvement of platelet-derived growth factor and its receptor alpha (PDGF and PDGFR-α) in the development of murine thymus was first described by Morrison-Graham et al. (1992). More recently, Jenkinson et al. (2007) proved that PDGFR-α-positive mesenchymal cells play a crucial role in early development stages of thymus in mice through proliferative signals to the thymic epithelium. Depletion of PDGFR-α-positive mesenchymal cells from embryonic thymus resulted in the formation of functional, but hypoplastic thymic gland. Moreover, Foster et al. (2008) demonstrated the presence of PDGFR-α/PDGFR-β-positive neural crest-derived mesenchymal cells, able to differentiate into perivascular cells beginning from early embryonic stages of murine thymus development. These cells also persist around blood vessels in adult thymus and may contribute to formation of the thymic blood barrier, providing structural support to vessels and regulation of endothelial cell function.
The aim of this study has been to analyse the immunohistochemical expression of PDGF and PDGFR-α in prenatal and postnatal human normal thymus and thymomas bioptic specimens, because of the lack of literature data in human specimens.
Materials and methods
Tissue specimens
Two foetal thymuses obtained from two foetuses of 24 and 28 weeks of gestation, five postnatal normal human thymuses obtained from children aged 1 month–16 year operated for cardiac malformations and 10 thymomal biopsy specimens, obtained from adult patients aged 25–78 years, have been included in this study. Specimens were fixed in buffered formalin for 24 h and then embedded in paraffin. Five micrometre sections were stained with haematoxylin and eosin for conventional diagnosis, and thymomas were classified according to the World Health Organization System (Rosai 1999) as type A (n = 2), AB (n = 2), B1 (n = 1), B2 (n = 2) and B3 (n = 3). Three micrometre slides were utilized for the immunohistochemical procedure.
The local research ethic committee approved the protocol of the study, and informed consent was obtained from all subjects according to the World Medical Association (WMA) Declaration of Helsinki.
Immunohistochemistry and image analysis
After heat-induced epitope retrieval in citrate buffer pH 6 for 20 min (with PT link modules; DakoCytomation, Glostrup, Denmark), the slides were incubated with primary antibodies (rabbit polyclonal anti-PDGF and anti-PDGFR-α antibodies, both ready-to-use (Labvision/Neomarkers, Fremont, CA, USA) for 30 min. The working system for both antibodies was Ultravision HRP System (Labvision/Neomarkers), followed by 3,3′diaminobenzidine dihydrochloride as chromogen. Nuclei were stained with Lillie's modified haematoxylin (Dako, Glostrup, Denmark). The entire immunohistochemical procedure was performed with DakoAutostainer Plus (DakoCytomation). Image acquisition and analysis were performed using Nikon Eclipse E600 (Nikon Corporation, Tokyo, Japan) microscope and Lucia G software (Nikon Corporation, Tokyo, Japan) for microscopic image analysis.
Results
PDGF/PDGFRα in normal foetal and postnatal thymus
In normal specimens, no differences were found in PDGF expression between foetal and postnatal thymus. A strong immunoreactivity was detectable in the middle layer of large blood vessels, in the connective tissue surrounding the normal thymus and in the medullary epithelial cells (Figure 1a,b), while weak immunoreactivity was observed in the cytoplasm of scattered epithelial cells in the cortex (Figure 1b). In the foetal thymus cortex, two types of cells positive for PDGF were recognizable: flattened cells around small capillaries and scattered stromal epithelial cells. Both were localized in the deep cortex. No positive cells were identified in the subcapsular region of the foetal thymus. In the medulla of the foetal and postnatal thymus, a network of stromal epithelial cells with strong immunoreactivity to PDGF were recognizable (Figure 1c), while Hassall corpuscles showed a positive reaction with a granular pattern (Figure 1d).
Figure 1.
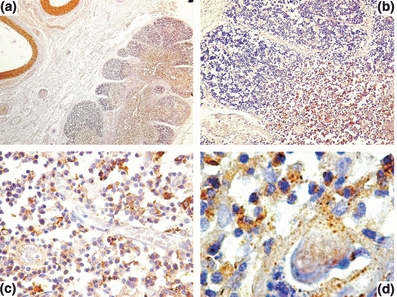
Platelet-derived growth factor (PDGF) expression in normal prenatal human thymus. In (a) a strong immunoreactivity to PDGF in the media of large vessels and in the medullary epithelial cell. In (b) a detail of the cortico-medullary junction showing PDGF expression in the connective tissue septae and a strong immunoreactivity in the medulla. A strong immunoreactivity to PDGF in the epithelial network of the medulla (c) and in Hassall corpuscles (d). Original magnification: a, ×40; b, ×100; c, ×400; d, ×1000.
Platelet-derived growth factor receptor-α had the same distribution of PDGF with a lower intensity of reaction in both foetal and postnatal thymus (Figure 2).
Figure 2.
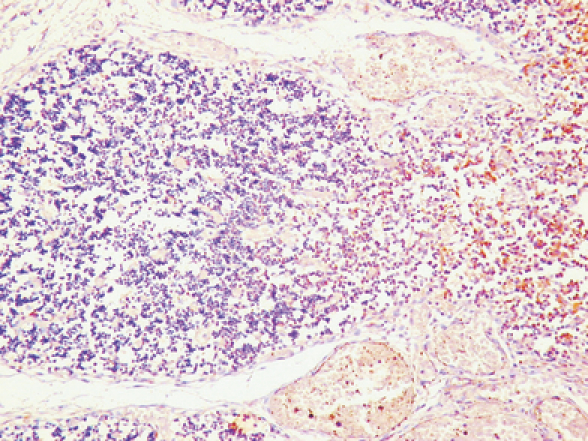
Differences in platelet-derived growth factor receptors (PDGFR)-α expression between the cortex and the medulla in normal human thymus. Note a higher number of immunoreactive epithelial stromal cells in the medulla as compared with the cortex. Original magnification: ×400.
PDGF/PDGFR-α in thymoma
Tumour epithelial cell were immunoreactive to PDGF. In type A and AB thymomas, moderate PDGF immunoreactivity with a cytoplasmic diffuse pattern was restricted to the spindle cells (Figure 3a) and to isolated tumour blood vessels. In type B1 thymoma, PDGF immunoreactivity was recognizable into the medulla-like area and absent into the cortex-like zone (Figure 3b). Moreover, immunoreactive blood vessels were grouped at the tumour periphery and within the tumour. The highest intensity of immunoreactivity was detected in type B3 thymomas, showing an intense immunoreactivity of all tumour cells with a cytoplasmic granular pattern (Figure 3c).
Figure 3.
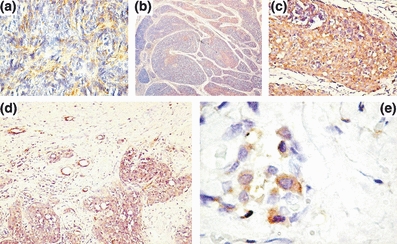
Platelet-derived growth factor (PDGF) and platelet-derived growth factor receptor (PDGFR)-α expression in thymoma bioptic specimens. In (a) a type A thymoma with moderate PDGF expression in tumour cells. In (b) a type B1 thymoma with PDGF positive cells into the medulla-like zone. In (c) a type B3 thymoma with a strong immunoreactivity to PDGF of tumour cells. In (d) a type B3 thymoma with a weaker PDGFR-α immunoreactivity of tumour cells. In (e) a type B3 thymoma with clusters of PDGFR-α-positive tumour cells inside the capsule. Original magnification: a, c, e ×400; b, ×40; d, ×200.
As concerns PDGFR-α expression, scattered positive tumour cells were recognizable in type A and AB thymoma, while positive tumour cells were more numerous in thymoma type B2 and B3 at the periphery of tumour, showing a prevalent membrane pattern of expression (Figure 3d). Clusters of positive tumour cells were also found in the capsule (Figure 3e).
Discussion
Platelet-derived growth factor was initially identified as a growth factor for fibroblasts, smooth muscle cells and glial cells (Kohler & Lipton 1974; Ross et al. 1974; Westermark & Wasteson 1976). PDGF-B is secreted by endothelial cells, presumably in response to VEGF, facilitates recruitment of mural cells, and expression of PDGFR-β in pericytes and smooth muscle cells is required for their proliferation, migration and incorporation in the vessel wall (Kazlauskas 2008).
Further evidence has clearly demonstrated involvement of PDGF and its specific receptors in different types of tumours. Within a tumour, the endothelial cells produced PDGF, and in some instances, the tumour cell also secrete PDGF. PDGF-B expressed by tumour cells increased pericyte recruitment in several in vivo tumour models, and genetic abolition of PDGFR-β expression by embryonic pericytes decreased their recruitment in tumours (Abramsson et al. 2003). More recently, Faraone et al. (2009) reported that melanoma tumour cells expressing PDGFR-α have a lower metastatic potential compared with those tumour cells that lack PDGFR-α. In breast cancer, stromal PDGFR-β expression significantly correlated with less favourable clinic–pathological parameters and shorter survival (Paulsson et al. 2009).
Platelet-derived growth factor and PDGFRs have been little studied in murine and human thymus, and no data are available concerning their expression in normal and pathological human thymus and derived tumours. During embryogenesis of murine thymus, PDGFR-α is highly expressed in mesenchymal cells derived from the neural crest (Schatteman et al. 1992). Studies performed using ph/ph mice, lacking PDGFR-α expression, showed that the development of thymic epithelial cells was impaired despite a normal migration of thymocyte precursors (Itoi et al. 2007). Odaka (2009) detected positive PDGFR-α thymic mesenchymal cells inside the capsule, septa and around blood vessels in normal murine thymus.
In this study, we have demonstrated for the first time the same distribution and intensity of PDGFR-α expression in positive cells inside the normal foetal and postnatal human thymus and the presence of two types of positive cells restricted to the deep cortex, suggesting that this subpopulation of stromal cells could be responsible for PDGFR synthesis and storage. Moreover, we have found PDGFR-α positive perivascular cells only around small capillaries in the deep cortex, and we have also demonstrated a positivity in the Hassall corpuscles of normal human thymus and strong reaction for PDGF and PDGFR-α in tumour epithelial cells from thymomas, depending on the tumour type.
Pharmacological approaches have been introduced by using inhibitors that blocked PDGFRs, which reduced the pericyte number of tumour vessels and vascularity of tumours (Bergers et al. 2003). In 2005, the Food and Drug Administration granted regular marketing approval for sorafenib, an inhibitor of VEGFRs, PDGFRs, Raf and c-kit, for the treatment of patients with advanced renal cell carcinoma (Kane et al. 2006).
These data suggest that PDGF and PDGFR-α inhibitors might be used as antiangiogenic agents in the treatment of thymoma. Accordingly, motesanib diphosphonate, an angiogenesis inhibitor targeting VEGFRs, PDGFRs and c-kit, has been utilized in advanced-stage thymoma (Azad et al. 2009), sorafenib in human thymic carcinoma (Bisagni et al. 2009) and dasatinib, an inhibitor of PDGFR-β and c-kit, in a metastatic thymoma type B2 (Chuah et al. 2006).
In conclusion, this is the first study that describes the expression and distribution of PDGF and PDGFR-α in the normal prenatal and postnatal human thymus and in thymoma and indicates that the evaluation of the expression of PDGF and PDGFR-α in thymoma may be a useful tool for a more complete phenotypic characterization of this type of tumour.
Acknowledgments
This work was supported by grant IDEI 1147/2009 of Romanian Ministry of Education, Research and Innovation.
Competing interests
None to declare.
References
- Abramsson A, Lindblom P, Betshowz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A, Herbertson RA, Pook D, White S, Mitchell PL, Tebbutt NC. Motesanib diphosphate (AMG 706), an oral angiogenesis inhibitor, demonstrates clinical efficacy in advanced thymoma. Acta Oncol. 2009;48:619–621. doi: 10.1080/02841860802495362. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagni G, Rossi G, Cavazza A, Sartori G, Gardini G, Boni C. Long lasting response to the multikinase inhibitor bay 43-9006 (Sorafenib) in a heavily pretreated metastatic thymic carcinoma. J. Thorac. Oncol. 2009;4:773–775. doi: 10.1097/JTO.0b013e3181a52e25. [DOI] [PubMed] [Google Scholar]
- Chuah C, Lim TH, Tiong Lim AS. Dasatinib induces a response in malignant thymoma. J. Clin. Oncol. 2006;24:56–58. doi: 10.1200/JCO.2006.08.8963. [DOI] [PubMed] [Google Scholar]
- Cimpean AM, Raica M, Encica S, Cornea R, Bocan V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann. Anat. 2008;190:238–245. doi: 10.1016/j.aanat.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Cuddihy AR, Ge S, Zhu J, et al. VEGF-mediated cross-talk within the neonatal murine thymus. Blood. 2009;113:2723–2731. doi: 10.1182/blood-2008-06-162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone D, Aguzzi MS, Toietta G, et al. Platelet-derived growth factor-receptor alpha strongly inhibits melanoma growth in vitro and in vivo. Neoplasia. 2009;11:732–742. doi: 10.1593/neo.09408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K, Sheridan J, Veiga-Fernandes H, et al. Contribution of neural crest-derived cells in the embryonic and adult thymus. J. Immunol. 2008;180:3183–3189. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- Gameiro J, Nagib P, Verinaud L. The thymus microenvironment in regulating thymocyte differentiation. Cell Adh. Migr. 2010;4:382–390. doi: 10.4161/cam.4.3.11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng TS, Chidgey AP, Boyd RL. Getting back at nature: understanding thymic development and overcoming its atrophy. Curr. Opin. Pharmacol. 2010;10:425–433. doi: 10.1016/j.coph.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Itoi M, Tsukamoto N, Yoshida H, Amagai T. Mesenchymal cells are required for functional development of thymic epithelial cells. Int. Immunol. 2007;19:953–964. doi: 10.1093/intimm/dxm060. [DOI] [PubMed] [Google Scholar]
- Jenkinson WE, Rossi SW, Parnell SM, Jenkinson EJ, Anderson G. PDGFR alpha-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood. 2007;109:954–960. doi: 10.1182/blood-2006-05-023143. [DOI] [PubMed] [Google Scholar]
- Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A. Platelet-derived growth factor. In: Figg WD, Folkman J, editors. “Angiogenesis. An Integrative Approach from Science to Medicine”. New York, NY: Springer Science; 2008. pp. 99–102. [Google Scholar]
- Kohler N, Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp. Cell Res. 1974;87:297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Lee HW, Na YJ, Jung PK, et al. Nerve growth factor stimulates proliferation, adhesion and thymopoietic cytokine expression in mouse thymic epithelial cells in vitro. Regul. Pept. 2008;147:72–81. doi: 10.1016/j.regpep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Morrison-Graham K, Schatteman GC, Bork T, Bowen-Pope DF, Weston JA. A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest-derived cells. Development. 1992;115:133–142. doi: 10.1242/dev.115.1.133. [DOI] [PubMed] [Google Scholar]
- Mouiseddine M, Mathieu N, Stefani J, Demarquay C, Bertho JM. Characterization and histological localization of multipotent mesenchymal stromal cells in the human postnatal thymus. Stem Cell Dev. 2008;17:1165–1174. doi: 10.1089/scd.2007.0252. [DOI] [PubMed] [Google Scholar]
- Müller SM, Terszowski G, Blum C, et al. Gene targeting of VEGF-A in thymus epithelium disrupts thymus blood vessel architecture. Proc. Natl Acad. Sci. USA. 2005;102:10587–10592. doi: 10.1073/pnas.0502752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka C. Localization of mesenchymal cells in adult mouse thymus: their abnormal distribution in mice with disorganization of thymic medullary epithelium. J. Histochem. Cytochem. 2009;57:373–382. doi: 10.1369/jhc.2008.952895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrens M, Dubus P, Groppi A, et al. Differential expression of NGF receptors in human thymic epithelial tumors. Pathol. Res. Pract. 1999;195:549–553. doi: 10.1016/S0344-0338(99)80004-1. [DOI] [PubMed] [Google Scholar]
- Paulsson J, Sjöblom T, Micke P, et al. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am. J. Pathol. 2009;175:334–341. doi: 10.2353/ajpath.2009.081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersini R, Vattemi E, Lusso MR, Mazzoleni G, Ebner H, Graiff C. Erlotinib in advanced well-differentiated thymic carcinoma with overexpression of EGFR: a case report. Tumori. 2008;94:849–852. doi: 10.1177/030089160809400613. [DOI] [PubMed] [Google Scholar]
- Raica M, Encică S, Motoc A, Cîmpean AM, Scridon T, Bârsan M. Structural heterogeneity and immunohistochemical profile of Hassall corpuscles in normal human thymus. Ann. Anat. 2006;188:345–352. doi: 10.1016/j.aanat.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Rosai J. Histological Typing of Tumours of the Thymus. Verlag: Springer; 1999. [Google Scholar]
- Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc. Natl Acad. Sci. USA. 1974;71:1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatteman GC, Morrison-Graham K, van Koppen A, Weston JA, Bowen-Pope DF. Regulation and role of PDGF receptor α-subunit expression during embryogenesis. Development. 1992;115:123–131. doi: 10.1242/dev.115.1.123. [DOI] [PubMed] [Google Scholar]
- Siepe M, Thomsen AR, Duerkopp N, et al. Human Neonatal Thymus-Derived Mesenchymal Stromal Cells (nTMSC): characterization, differentiation, and immunomodulatory properties. Tissue Eng. Part A. 2009;15:1787–1796. doi: 10.1089/ten.tea.2008.0356. [DOI] [PubMed] [Google Scholar]
- Tomita M, Matsuzaki Y, Edagawa M, et al. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J. Thorac. Cardiovasc. Surg. 2002;124:493–498. doi: 10.1067/mtc.2002.124389. [DOI] [PubMed] [Google Scholar]
- Westermark B, Wasteson A. A platelet factor stimulating human normal glial cells. Exp. Cell Res. 1976;98:170–174. doi: 10.1016/0014-4827(76)90476-6. [DOI] [PubMed] [Google Scholar]


