Abstract
Oesophageal exposure to duodenogastro-oesophageal refluxate leads to reflux oesophagitis and is implicated in the development of Barrett's metaplasia (BM). NF-κB signalling in epithelial cells is associated with the activation of transcription factors believed to be central to BM development, whilst NF-κB activation in fibroblasts plays a critical role in matrix remodelling. Our aim was to study the effects of acid exposure on NF-κB activation in primary human oesophageal fibroblasts (HOFs) and primary and immortalized oesophageal squames and to investigate any epithelial/stromal interactions in the response of these cells to acid. Primary HOFs and primary and immortalized oesophageal epithelial cells were exposed to acid (pH 7 – pH 4 ≤ 120 min) in single or pulsed treatments. Conditioned medium from epithelial cells following acid exposure was also applied to fibroblasts. Cell viability was determined by MTT-ESTA. NF-κB activation was determined by cellular localization of NF-κB/p65 visualized by immunofluorescence. Conditioned medium from oesophageal epithelial cells, subjected to pH 5 pulsatile exposure, activated NF-κB in fibroblasts, with some inter-patient variability, but these conditions did not directly activate NF-κB in the epithelial cells themselves. Significant NF-κB activation was seen in the epithelial cells but only with greater acidity and exposure times (pH 4, 60–120 min). Our findings show that acid exposure can cause indirect activation of stromal cells by epithelial–stromal interactions. This may contribute to the pathogenesis of oesophageal diseases, and the inter-patient variability may go some way to explain why some patients with reflux oesophagitis develop BM and others do not.
Keywords: acid, Barrett's Metaplasia, NF-κB, oesophageal adenocarcinoma, paracrine, reflux
Gastro-oesophageal reflux disease (GORD) is a key risk factor for the development of Barrett's metaplasia (BM). In turn, BM places an individual at risk of developing oesophageal adenocarcinoma (OAC), and the incidence of both OAC and BM is rising throughout the Western world. OAC typically has a poor prognosis, and unlike some cancers, there has been very little improvement in survival in recent years. Understanding the impact of duodenogastric reflux on oesophageal mucosa is therefore essential if we are to develop rational strategies to reduce mortality from OAC.
The histological manifestations of GORD include the presence of inflammatory cells within the epithelium, an increase in the thickness of the basal layers of the epithelium and elongation of the papillae. It has been thought that these reflect epithelial regeneration after caustic injury (e.g. erosion or ulceration), although this view has been challenged by recent data demonstrating the presence of these features very soon after acid/bile exposure, apparently without intervening ulceration (Souza et al. 2009). The mechanism by which GORD predisposes to the development of BM remains unclear, although aberrant activation of the intestine-specific caudal-related homeobox2 (CDX2) gene in oesophageal squamous cells seems critical (Eda et al. 2003; Moons et al. 2004; Lord et al. 2005). NF-κB is known to be upregulated in BM (Konturek et al. 2004), and activation of the NF-κB signalling pathway is also implicated in the aberrant expression of CDX2, putatively driving its expression via NF-κB binding sites in the CDX2 promoter region (Debruyne et al. 2006; Kazumori et al. 2006).
Several studies have examined the impact of acid exposure on different oesophageal cells. However, the results obtained vary, with the differences presumably in part reflecting the variation in patterns of acid exposure (Fitzgerald et al. 1997) and cell lines employed. For example, two 3-min exposures of a telomerase-immortalized Barrett's epithelial cell line to pH 4 cause a decrease in cell proliferation (Feagins et al. 2007); 1-h exposure to pH 3.5 induces hyperproliferation in BM mucosal biopsies (Fitzgerald et al. 1998); 1-h exposure to pH 4 causes an increase in proliferation for an immortalized normal epithelial cell line and an OAC cell line (Fu et al. 2006); and pH 4.5 exposure for 30 min induces DNA damage in two immortalized oesophageal epithelial cell lines (Jolly et al. 2004). In addition, exposure to acid induces OAC cell lines and immortalized squamous cells to express a number of genes involved in inflammation, proliferation, differentiation and the stress response (Duggan et al. 2006), whilst work with other OAC lines has shown that exposure to an increasingly acidic environment results in increased NF-κB activation, reaching a maximum at pH 4 (Abdel-latif et al. 2004). The relevance of this data from immortalized or established OAC cell lines to the initial development of BM is questionable, but published data from primary cells or other systems more closely reflecting the environment within the native oesophagus are limited.
Epithelial–stromal interactions are known to play important roles in many areas, such as wound healing (Werner et al. 2007), organogenesis (Thesleff et al. 1995), morphogenesis and maintenance of differentiated epithelia (Sharpe & Ferguson 1988) and progression to cancer (Potter 2001). There has been only limited investigation of the role of such phenomena in the pathogenesis of oesophagitis and BM. However, the finding that histological manifestations of oesophagitis occur in the absence of preceding erosion or ulceration has led to the proposal that some elements of the morphological appearances of oesophagitis, namely basal cell and papillary hyperplasia and inflammatory cell infiltration, represent a response to cytokines produced by oesophageal squamous cells following acid insult (Souza et al. 2009). Fibroblasts are the predominant cellular component of connective tissue and play a key role in tissue remodelling in response to injury, and it is likely that they would participate in the papillary elongation seen in GORD. Fibroblast activation would therefore seem to occur without direct exposure to refluxate, presumably through signalling from acid/bile-exposed epithelial cells. NF-κB signalling has a distinctive role in governing fibroblast function, being specifically implicated in the regulation of matrix degradation and remodelling (Xu et al. 1998; Ferri et al. 2003), cell motility (Palumbo et al. 2007) and myofibroblastic differentiation (Ichikawa et al. 2008). Recent studies have demonstrated changes in the expression of inflammatory mediators by intra-lesional stromal cells during the evolution of Barrett's adenocarcinoma – underscoring the potential importance of such stromal–epithelial signalling (Saadi et al. 2010).
To our knowledge, no study has specifically investigated the impact of acid insult on NF-κB activation in oesophageal fibroblasts, nor sought evidence for crosstalk between acid-exposed epithelial cells and fibroblasts. Our aims in this study are twofold. First, we have sought to provide baseline data on the impact of acid exposure on primary human oesophageal fibroblasts (HOFs), and immortalized (Het-1A) and primary (HOS) human oesophageal squamous epithelial cells, focusing on cell viability and NF-κB activation. Second, we have sought evidence for paracrine interactions between epithelial cells and fibroblasts, which may be of relevance to the overall tissue response to acid exposure.
Methods
All materials were purchased from Sigma Aldrich (Dorset, UK) unless otherwise stated.
Human oesophageal squamous (HOS) cell isolation and culture
Oesophageal tissue samples were obtained with informed patient consent and appropriate ethical approval (SSREC 165/03 & 07/1309/138) from patients undergoing gastric or oesophageal surgery. Samples were taken from disease-free background tissue proximal to any macroscopic pathology. Tissue was transported in sterile PBS containing 100 IU/ml penicillin, 100 μg/ml streptomycin and 0.625 μg/ml amphotericin B and stored overnight at 4 °C prior to cell isolation. This overnight storage step was necessary because of the surgical schedules; however, during the optimization of this protocol, it was found that in all cases, viable cells could be isolated from tissue that had been stored for 12 h at 4 °C, indeed viable cells could very often be obtained following up to 60 h storage.
The tissue was cut into 0.5-cm strips and incubated in 0.1% w/v trypsin (Gibco, Carlsbad, CA, USA) for 1 h at 37 °C. Foetal calf serum (FCS) was then added to inhibit the trypsin and the epithelial cells removed by gentle scraping with a scalpel blade. Cells were collected into Green's medium (Rheinwald & Green 1975) (consisting of Dulbecco's modified Eagle medium (DMEM) and Ham's F12 in 3:1 ratio, supplemented with 10% FCS, 2 × 10−3 M glutamine, 10 ng/ml EGF, 0.4 μg/ml hydrocortisone, 1.8 × 10−4 M adenine, 5 μg/ml insulin, 5 μg/ml transferrin, 1 × 10−10 M cholera toxin, 2 × 10−9 M triiodothyronine, 100 IU/ml penicillin, 100 μg/ml streptomycin and 0.625 μg/ml amphotericin B). The resulting cell suspension was centrifuged (200 g, 5 min), and the cells were resuspended in 12 ml Green's medium and cultured in the presence of a feeder layer of lethally irradiated mouse 3T3 fibroblasts (1 × 106/12 ml culture). Cell counts were performed using a haemocytometer, and trypan blue exclusion was used to assess cell viability. HOS cells were used between passage 1 and 4 because it is known that epithelial cells up to passage 4 retain most of the characteristics of the cells from which they were isolated but after this they rapidly differentiate and their functional biology changes significantly.
Human oesophageal fibroblast (HOF) cell isolation and culture
Oesophageal stromal tissue resulting from HOS isolation was finely minced with a scalpel and incubated overnight at 37 °C in 10 ml 0.5% (w/v) collagenase A solution. The digest was then centrifuged (200 g, 10 min) and the pellet resuspended in 12 ml fibroblast culture medium (DMEM, 10% FCS, 2 × 10−3 M glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin and 0.625 μg/ml amphotericin B). Fibroblasts were used between passages 4 and 9, because this ensures the absence of contaminating HOS cells.
Het-1A culture
An SV-40 immortalized human oesophageal epithelial cell line, Het-1A (ATCC-LGC, Middlesex, UK), was cultured in serum-free BRFF-EPM2 media (Axxora, Nottingham, UK). The medium was replaced every 3–4 days, and the cells were passaged once they had reached 80% confluency.
MTT-ESTA assay
The MTT-ESTA assay was performed to determine the metabolic activity of cells following exposure to acidified medium. Cells were seeded at 5 × 104/well in 24-well tissue culture plates. After 24 h, they were exposed to fresh pH 7.4 medium for the control or HCl-acidified medium at pH 5, pH 4 or pH 3 for 3–60 min, washed in PBS and returned to pH 7.4 medium for a further 24 h at 37 °C. Cells were washed with PBS and incubated with MTT-ESTA (0.5 mg/ml in PBS) for 40 min at 37 °C. Acidified isopropanol (200 μl) was used to solubilize the formazan, and absorbance was measured at 540 nm with a protein reference of 630 nm subtracted. Activity was calculated as a percentage of the control response.
Acid treatment regimes
Direct exposure of primary human oesophageal cells to acid
Monocultures of HOS and HOF cells were seeded in 24-well plates at 5 × 104/well. After 24 h, the medium was removed and replaced with fresh culture medium. In all cases, the medium used was the normal culture medium for that cell type, i.e. DMEM 10% FCS for HOFs and Green's 10% FCS for HOS cells. The medium was used at pH 7.4, pH 6, pH 5 or pH 4. Cells were exposed for between 3 and 120 min. Positive controls were treated with lipopolysaccharide (LPS, 1 μg/ml) for 2 h. After exposure, the medium was removed, and the cells were washed in PBS and fixed in formalin for 15 min. Fixed cells were washed ×3 in PBS and stored in PBS at 4 °C prior to immunostaining.
Pulsatile exposure of primary human oesophageal cells to acid and the effect of the conditioned media produced
Monocultures of HOS cells were seeded in 24-well plates at 5 × 104/well. After 24 h, the medium was removed and the cells exposed to Green's 10% FCS at pH 7.4, pH 5 or pH 4 for 30 min or treated by three pulses of acidified medium punctuated by periods of recovery in Green's 10% FCS at pH 7.4. An acid exposure time of 3 min (short pulse) and two intervals of 5 min (short recovery) or 30 min (long recovery) were employed. The duration of the acid exposure was selected to reflect that encountered by patients during an episode of gastro-oesophageal reflux (Richter et al. 1992; Dvorak et al. 2006). After the final acid exposure, cells were washed in PBS and placed in fresh Green's 10% FCS for 2 h to produce conditioned medium. After 2 h, the conditioned medium was removed and placed on monocultures of the HOF cells (24-well plates seeded 24 h previously at 5 × 104/well) and left for 3 h. The negative control was untreated HOF cells that remained in DMEM 10% FCS, and the positive control was HOF cells treated for 2 h with LPS (1 μg/ml). The medium was then removed, and the cells were washed in PBS, fixed in formalin, washed ×3 in PBS and stored in PBS at 4 °C prior to immunostaining.
Acid exposure of Het-1A cells
Het-1A cells were seeded in 6-well plates at 2 × 105/well in BRFF-EPM2 serum-free culture medium. After 48 h, the medium was removed and replaced with fresh BRFF-EPM2. The medium was either used without being first acidified (pH 7.4) or at pH 5 or pH 4 through the addition of 1 M HCl. Cells were exposed for 30 min or pulsed for three 3-min exposures separated by 5-min recovery periods in pH 7.4 medium. Positive controls were treated with LPS (1 μg/ml) for 2 h. After exposure, the acidified medium was removed, fresh BRFF-EPM2 (pH 7.4) added and the cells cultured for a further 2 h. The conditioned medium was collected, the Het-1A cells washed in PBS and fixed in formalin for 15 min. Fixed cells were washed ×3 in PBS and stored in PBS at 4 °C prior to immunostaining.
The conditioned medium was split into two aliquots. Half was stored at −20 °C prior to bead array analysis, and the remainder was placed on monocultures of the HOF cells (6-well plate seeded 48 h previously at 2 × 105/well) and left for 3 h. The medium was then removed from these cells, the cells washed in PBS, fixed in formalin, washed ×3 in PBS and stored in PBS at 4 °C prior to immunostaining. Positive controls were incubated with LPS (1 μg/ml) for 2 h, and negative controls remained in DMEM 10% FCS.
Inhibition of NF-κB translocation
Human oesophageal squamous cells were treated with Green's 10% FCS medium supplemented with an NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC, 10 μM). After 1 h, cells were washed and treated with pH 7.4 or pH 4 medium, containing 10 μM PDTC for 60 min. Cells were then washed in PBS, fixed in formalin for 15 min, washed ×3 in PBS and stored in PBS at 4 °C prior to immunostaining.
NF-κB/p65 immunostaining
Fixed cells were permeabilized by incubating in 0.1% (v/v) Triton in PBS (20 min, room temperature). Cells were washed in PBS and blocked in 10% (w/v) milk protein for 1 h before being incubated in anti-NF-κB/p65 rabbit polyclonal antibody (Autogen Bioclear, Wiltshire, UK, 1:100 in 5% milk protein, overnight, 4 °C). After PBS washing, biotinylated secondary antibody (goat anti-rabbit, Vector Labs, Peterborough, UK, 1:1000 in 5% milk protein, 1 h, room temperature) was added. After further washing, cells were co-labelled with fluorescein–streptavidin (Vector Labs, 1:100 in PBS) and DAPI (1:1000) for 40 min at room temperature. Cells were washed in PBS and imaged using an epifluorescence microscope (ImageXpress, Molecular Devices, Union City, CA, USA) at λex495 nm/λem515 nm for FITC and λex385 nm/λem461 nm for DAPI localization or a confocal microscope (Zeiss LSM 510Meta, Welwyn Garden City, UK) at λex488 nm/λem500–550 nm for FITC localization and an HBO mercury lamp and Zeiss filter set 01 (excitation filter – bandpass (BP) 365/12 nm, emission filter – long pass (LP) 397 nm) for DAPI. Identical fields of view were captured and overlaid for analysis. NF-κB translocation was scored according to a previously published and validated protocol (Moustafa et al. 2002; Sun et al. 2004; Canton et al. 2010). Briefly, cells were scored 0 if NF-κB staining was stronger in the cytoplasm than in the nucleus, 0.5 if there was no difference in staining intensity between cytoplasm and nucleus and 1 if the nucleus stained more strongly than the cytoplasm. Samples were scored blind, the experiment was performed in triplicate, a minimum of 100 cells was scored for each replicate, and the percentage score was determined.
Cytometric bead array
Aliquots of conditioned medium (50 μl) were analysed using the Cytometric Bead Array Flex Sets (BD Biosciences, Oxford, UK) to quantify the levels of specific soluble proteins. After incubation of the media with the appropriate capture beads followed by a phycoerythrin-tagged capture antibody, beads were distinguished and quantified using the BDFACSArray. Known standards were also quantified according to manufacturer's instructions. The proteins assayed in this way were FAS ligand (soluble), basic FGF, IFN-γ, IFN-α, IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-10, TNF, GM-CSF, CD14, IL12p70 and VEGF.
Statistical analysis
Experiments were repeated a minimum of three times and the means determined and the data analysed by anova and Dunnett's post-test, with significant differences indicated by ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05. Where the use of different patient cells resulted in highly variable responses, chi-squared test was used to compare the response of each patient after acid exposure to that observed at pH 7.4.
Results
The effect of acid on primary oesophageal cell viability
The MTT-ESTA data (Figure 1) reveal that neither HOS nor HOF cells show any change in viability following exposure to pH 5 for up to 120 min. Sixty-min exposure to a pH 4 environment was necessary to induce a significant reduction in viability in HOS cells to 19.5 ± 21.5% (P ≤ 0.05), whilst HOF cells were less resistant to increases in hydrogen ion concentrations, showing a significant loss of viability to 59.5 ± 22.5% after 30 min (P ≤ 0.05). At pH 3, a significant loss in viability was observed after 30 min (16.9 ± 1.6%, P ≤ 0.001) for HOS cells and 10 min for HOF cells (29.7 ± 18.6%, P ≤ 0.001). This slight increase in resistance to acid for the HOS cells is in keeping with the barrier role of the squamous epithelium, providing some protection for the underlying stromal tissue from the effects of acid exposure encountered during reflux.
Figure 1.
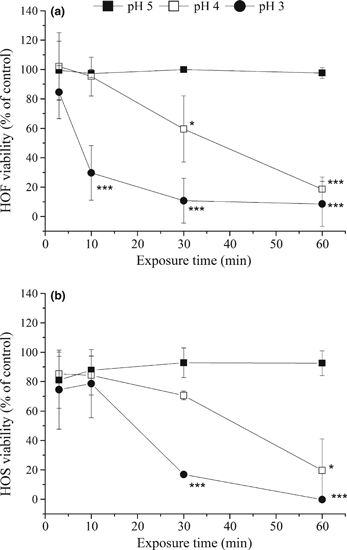
The effect of exposure to acid on cell metabolic activity. Cultures of human oesophageal fibroblasts (HOF) (a) or Human oesophageal squamous (HOS) (b) cells were directly exposed to control media (pH 7.4) or acidified medium at pH 5, pH 4 or pH 3 for 3–60 min followed by a 24-h recovery period, prior to determination of metabolic activity by MTT-ESTA assay. Sixty-min exposure to pH 5 has no impact on cell viability for either HOF or HOS cells. HOF cultures show a significant loss of viability after exposure to pH 3 acidified medium for 10 min or pH 4 for 30 min, whilst HOS cultures require pH 4 for 60 min or pH 3 for 30 min before viability is significantly affected. Results were calculated as a percentage of the control and are presented as the mean ± SD of three experiments. Significant differences from control are indicated by ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05.
The effect of acid on NF-κB translocation in primary oesophageal cells
Because pH 3 exposure resulted in a rapid reduction in viability, this was not used to study NF-κB translocation, and instead HOS and HOF cells were exposed to less toxic acid environments (pH 7.4 to pH 4) for up to 120 min. Figure 2 shows NF-κB/p65 immunostained images of HOF and HOS cells that have undergone exposure to culture medium at pH 7.4, pH 6, pH 5 or pH 4 for 30 min together with LPS-positive control images. It can be seen that NF-κB is located mainly in the cytoplasm of the cells in all cases, except for the pH 4-exposed HOS cells and the HOF and HOS LPS-treated positive controls where NF-κB is located in the nucleus for many of the cells. DAPI staining was used to confirm nuclear localization (images not shown). Similar images were obtained for each pH at each time point and the extent of translocation determined. The images obtained from HOF cells following prolonged exposure to pH 4 and, to a much lesser extent, pH 5 showed some changes in morphology and reduced adhesion, which reflect a reduction in cell viability.
Figure 2.
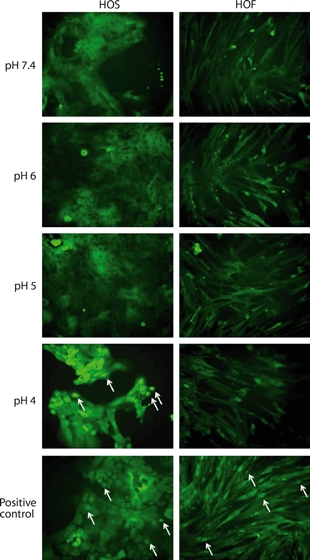
Immunohistochemical localization of NF-κB after exposure of cells to acid. Human oesophageal squamous (HOS) and human oesophageal fibroblasts (HOF) cells were exposed to pH 7.4, pH 6, pH 5 or pH 4 medium for 30 min. lipopolysaccharide-treated positive control cells (2-h treatment) are also shown. Positive control HOS and HOF cells and pH 4 exposed HOS cells show nuclear localization of NF-κB (arrows). Following all other treatments, NF-κB is detected in the cytoplasm, demonstrating no NF-κB activation. The HOF cells at pH 4 show an abnormal morphology and reduced adhesion, which can be attributed to a reduction in cell viability.
Human oesophageal squamous cells when exposed to a pH 4 insult responded with NF-κB translocation (Figure 3). After 10-min exposure, NF-κB activation (28.8 ± 8.9%) was significantly greater than the control (P ≤ 0.05), and by 30-min exposure, NF-κB activation increased to 32.5 ± 13.2%; however, the larger standard deviation (possibly a reflection of inter-patient variability in the magnitude of the response) meant this was not significant when compared to the control. Following 60-min exposure to pH 4, NF-κB activation rose to 50.4 ± 5.5%, which was significantly greater than that observed at pH 7.4 (P ≤ 0.01) and remained significantly increased to the end of the experiment (52.2 ± 7.9%, P ≤ 0.01). No effect was observed for up to 120-min exposure to either pH 5 or pH 6.
Figure 3.
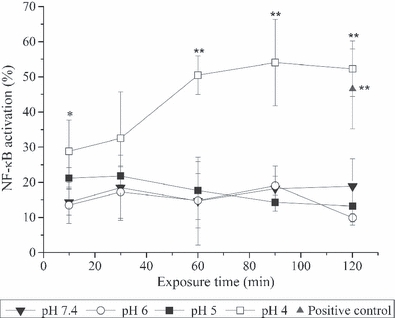
Translocation of NF-κB after direct exposure of Human oesophageal squamous (HOS) cells to acidified medium. Cells were exposed to medium between pH 7.4 and pH 4 for up to 120 min and NF-κB activation scored as described in the materials and methods section. No significant NF-κB activation was detected in HOS cells exposed to pH 7.4, pH 6 or pH 5 conditions for up to 120 min. However, exposure to pH 4 resulted in significant NF-κB activation, which remained significantly higher to the end of the experiment. Results are presented as the mean ± SD of three experiments. Significant differences from pH 7.4 control are indicated by **P ≤ 0.01, *P ≤ 0.05.
In contrast, no NF-κB activation was observed with the HOF cells exposed to acid for up to 120 min compared to cells exposed to pH 7.4 medium for the same time period (data not shown). This indicates that oesophageal fibroblasts do not respond to a short single acid insult by activating NF-κB.
Cells were also incubated in PDTC, an anti-oxidant that is a non-specific inhibitor of NF-κB activation, for 1 h prior to acid exposure and throughout the acid exposure period. Figure 4 shows that although cells in the absence of inhibitor translocated NF-κB at pH 4 but not at pH 7.4, cells in PDTC showed no translocation at either pH, indicating that the increase in nuclear staining observed at pH 4 was a result of NF-κB activation.
Figure 4.
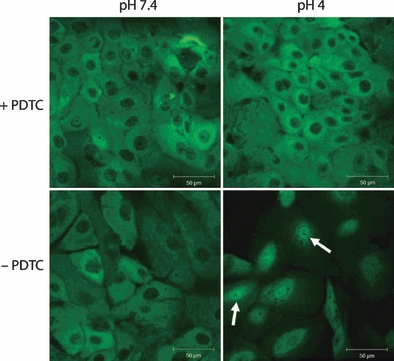
Inhibition of NF-κB translocation. NF-κB translocation in Human oesophageal squamous cells after 60-min exposure to pH 7.4 or pH 4. The top panels show cells that were pretreated for 1 h with the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) and incubated with PDTC during acid exposure, whilst the bottom panels did not receive PDTC treatment. NF-κB is detected in the nucleus of cells exposed to pH 4 in the absence of PDTC (arrows); however, in the presence of the inhibitor, NF-κB is located in the cytoplasm. NF-κB is also located in the cytoplasm of cells exposed to pH 7.4 with or without PDTC.
Pulsatile acid exposure of primary oesophageal cells and paracrine translocation of NF-κB in primary oesophageal fibroblasts
These experiments aimed to mimic the clinical situation of pulsatile acid exposure during acid reflux and also explored epithelial–stromal interactions as a response to acid exposure. Cells received either a single 30-min exposure or three consecutive 3-min exposures to pH 7.4, pH 5 or pH 4. The cells were allowed to recover in a pH 7.4 environment for either 5 or 30 min between treatments, and cells were examined for evidence of NF-κB translocation. To eliminate any effects arising from the repeated media exchanges, the results were calculated as a percentage of the response using the same treatment regime at pH 7.4.
Figure 5 shows that NF-κB translocation was significantly increased in these HOS cells exposed to pH 4 for 30 min compared to pH 5 and 7.4 (342.1 ± 39.2% of the pH 7.4 response, P ≤ 0.001). In addition, it was seen that HOS cells that encountered three short pulses of acid with either interval length did not show any significant increase in NF-κB translocation.
Figure 5.
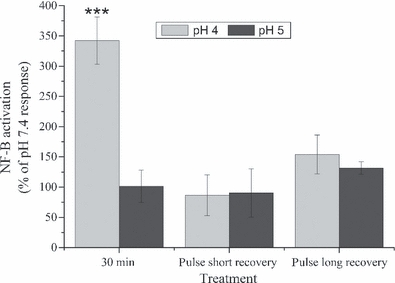
The effects of pulsatile acid exposure on NF-κB translocation. The activation of NF-κB in Human oesophageal squamous (HOS) cells following exposure to pH 5 or pH 4 for 30 min or three pulsed exposures with either a short (5 min) or long (30 min) recovery period. Results have been calculated as a percentage of the response after pH 7.4 treatment using the same regime. After treatment, the cells were cultured in pH 7.4 media for 2 h prior to fixing and immunostaining. NF-κB activation was detected in HOS cells exposed to pH 4 for 30 min; however, no significant activation was detected for any other treatment regime. Results are presented as the mean ± SD of three experiments. Significant differences from pH 7.4 control are indicated by ***P ≤ 0.001.
We then investigated paracrine signalling between the two cell types by exposing HOF cells to conditioned medium. In all cases, the acid-exposed epithelial cells were maintained for a further 2 h in a pH 7.4 environment following the final acid exposure to produce conditioned medium.
Exposure of HOF cells to the HOS conditioned medium revealed that the epithelial cells did, under certain conditions, produce a soluble factor capable of paracrine stimulation of NF-κB (Figure 6), although the degree of response varied considerably with cells derived from different patients. Again, the results were calculated as a percentage of the response observed at pH 7.4 for the same treatment regime (Figure 7).
Figure 6.
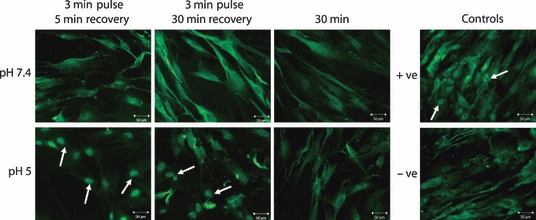
The effect of Human oesophageal squamous (HOS) conditioned medium on human oesophageal fibroblasts (HOF) cells using patient set 1 cells. NF-κB translocation in HOF cells following exposure to conditioned medium taken from HOS cells after treatment with medium at pH 7.4 or pH 5. Conditioned medium was taken after the following treatments: three 3-min treatments with a 5-min interval, three 3-min treatments with a 30-min interval or 30 min. Positive and negative controls are also shown. NF-κB translocation to the nucleus is observed in the cells treated with conditioned medium from cells exposed to pH 5 for 3 min with either a 5- or 30-min interval. Translocation is also observed in the positive control, but in all other cells, NF-κB remains in the cytoplasm.
Figure 7.
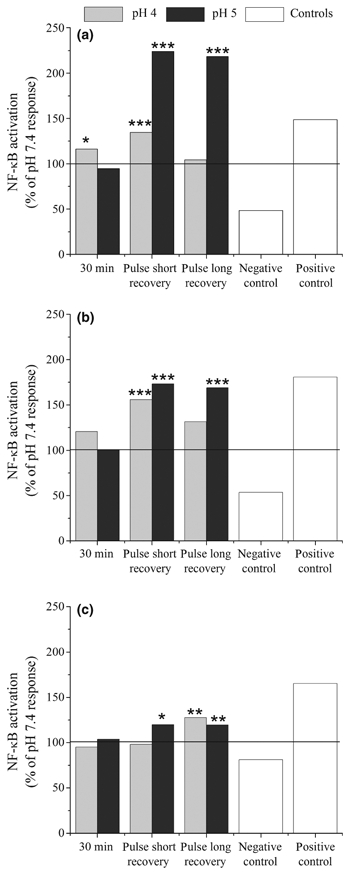
Activation of NF-κB in human oesophageal fibroblasts (HOF) cells after 3-h exposure to human oesophageal squamous (HOS) conditioned medium. NF-κB translocation in HOF cells following exposure to conditioned medium taken from acid-treated HOS cells. Results are calculated as a percentage of the response at pH 7.4 using the same regime and are shown for cells from three different patients (a, b and c). The solid line at 100% indicates the response seen for the pH 7.4 control. In all three experiments, HOF cells show increased NF-κB activation over the pH 7.4 control after exposure to short pulses of pH 5 with both long (30 min) and short (5 min) intervals at pH 7.4, with higher levels of activation when the recovery period was reduced. Significant differences from pH 7.4 treatment are indicated by ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05.
No significant NF-κB translocation was observed with any patient's cells when fibroblasts were exposed to conditioned medium from epithelial cells after 30 min at pH 5. However, brief pulsatile exposure of epithelial cells to acid did, in some circumstances, induce NF-κB activation in the fibroblasts (Table 1). This effect was statistically significant for epithelial cells from all three patients when exposed to pH 5 pulses (Figure 7), although the magnitude of the response varied from 119.5% to 224.1% of the response observed for the same patient at pH 7.4.
Table 1.
Paracrine activation of NF-κB in human oesophageal fibroblasts (HOF) cells following exposure to Human oesophageal squamous (HOS) conditioned medium
| Paracrine NF-κB activation in HOF cells | |||||||
|---|---|---|---|---|---|---|---|
| HOS exposure regime | Patient 1 | Patient 2 | Patient 3 | ||||
| Insult | Interval (minutes) | % of pH 7.4 response | P-value | % of pH 7.4 response | P-value | % of pH 7.4 response | P-value |
| pH 5 | 0 (single treatment) | 94.6 | 0.3286 | 100.6 | 0.2376 | 103.7 | 0.2776 |
| 5 | 224.1 | <0.0001*** | 173.1 | <0.0001*** | 119.9 | 0.0106* | |
| 30 | 218.5 | <0.0001*** | 168.8 | <0.0001*** | 119.5 | 0.0034** | |
| pH 4 | 0 (single treatment) | 116.2 | 0.0116* | 120.8 | 0.0629 | 95.1 | 0.0893 |
| 5 | 134.7 | <0.0001*** | 155.8 | 0.0001*** | 98.0 | 0.1243 | |
| 30 | 1.0 | 0.5162 | 131.4 | 0.0812 | 127.8 | 0.0014** | |
Conditioned medium experiments demonstrated variable but statistically significant activation of NF-κB in fibroblasts in response to exposure to culture medium conditioned by HOS cells following an acidic insult. Acid treatments were either pulsatile, with three 3-min exposures separated by 5- or 30-min intervals at pH 7.4, or a single 30-min exposure. The response and P-values are relative to the internal control experiment (HOS cells from the same patient exposed to pH 7.4 using the same treatment regime). Results in bold indicate those which are significantly greater (***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05).
For patients 1 and 2, significant NF-κB translocation in HOFs was also detected after epithelial exposure to pH 4 pulses when the interval was restricted to 5 min (patient 1 – 134.7% of pH 7.4 response P < 0.0001, patient 2 – 155.8% of pH 7.4 response P = 0.0001). However, in both cases, this was significantly less than that observed at pH 5 (patient 1 –P < 0.0001, patient 2 –P = 0.035). A small response was also observed for patient 1 after 30 min at pH 4 (116.2% of pH 7.4 response, P = 0.0116) and patient 3 with pulsatile pH 4 treatment and a 30-min interval (127.8% of pH 7.4 response, P = 0.0014). In general, the cells from patient 1 appeared to show the greatest level of epithelial–stromal interactions following acid exposure whilst patient 3 was the least responsive.
The effect of acid exposure on Het-1A cells
The Het-1A cell line is an SV-40 immortalized human oesophageal cell line commonly used as an experimental model, and we explored whether the results observed using primary oesophageal cells were reflected in these cells. NF-κB activation was assessed in Het-1A cells following 30 min or three 3-min pulsatile exposures to an acidic environment (pH 4–pH 7.4). Results were again calculated as a percentage of the equivalent regime at pH 7.4.
Significantly greater NF-κB activation in the epithelial cells was observed only when the Het-1A cells were exposed to 30 min at pH 4 (457.8 ± 45.9% of pH 7.4 response, P ≤ 0.001) or the positive control (427.4 ± 114.1% of pH 7.4 response, P ≤ 0.001). In all other cases, no significant activation was observed (Figure 8a).
Figure 8.
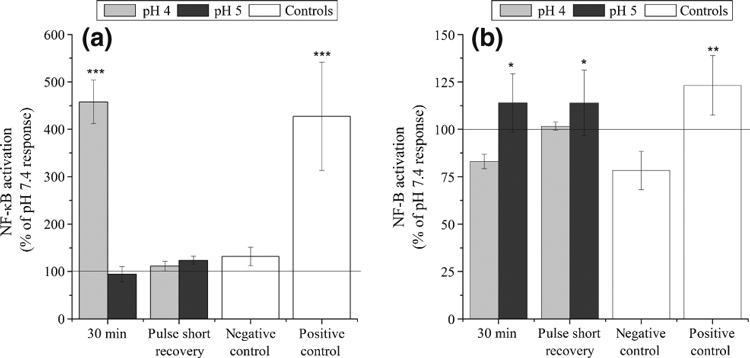
Activation of NF-κB in Het-1A and human oesophageal fibroblasts (HOF) cells following Het-1A exposure to acid. The activation of NF-κB in Het-1A cells after (a) a single 30-min treatment or three pulsed exposures to pH 7.4, pH 5 or pH 4 followed by (b) the paracrine activation in HOF cells after exposure to Het-1A conditioned media. Results have been calculated as a percentage of the response following pH 7.4 treatment using the same regime. After acid treatment, the Het-1A cells produced conditioned medium for 2 h, and HOF cells were then exposed to this for 3 h. NF-κB activation was detected in Het-1A cells exposed to pH 4 for 30 min, and in HOF cells after Het-1A exposure to pH 5 (both single or pulsatile treatments). No significant activation was detected for any other treatment regime. Results are presented as the mean ± SD of three experiments. Significant differences from pH 7.4 control are indicated by ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05.
Following acid exposure, the Het-1A cells produced conditioned media for 2 h, and primary oesophageal fibroblasts were exposed to this conditioned media for 3 h. NF-κB translocation within the fibroblasts was then determined (Figure 8b). HOF cells demonstrated relatively high NF-κB activation in the negative control. This may be the result of exposing the HOF cells, usually cultured in 10% FCS, to the BRFF-EPM2 serum-free medium required for Het-1A culture. To account for this, activation was once again calculated as a percentage of the response observed for the same treatment at pH 7.4. This demonstrated a significant increase in NF-κB activation in HOF cells exposed to conditioned media from Het-1A cells treated with pH 5 for 30 min (114.0 ± 15.4% of pH 7.4 response, P ≤ 0.05) or pulse treated with pH 5 media (114.0 ± 17.2% of pH 7.4 response, P ≤ 0.05).
We sought to identify the specific soluble mediator of this paracrine effect by screening the conditioned medium for a panel of 15 soluble proteins. We selected potential mediators based on the previous reports of likely candidate molecules; however, no significant differences were observed for any of the proteins assayed (results not shown).
Discussion
The aim of this study was to examine how acidic conditions affect viability and NF-κB activation in oesophageal epithelial and stromal cells and to investigate whether there is any evidence of paracrine signalling between these cell types in response to acid exposure. Our results suggest an acid-induced paracrine activation of NF-κB in oesophageal stromal cells following exposure of the epithelial cells to acid. It is entirely plausible that such crosstalk would operate in oesophageal tissue in response to GORD. Because the number of primary cell cultures employed was limited (because of the technical and logistical challenges associated with these cells), we sought to replicate the results in one of the commonly used oesophageal cell lines, Het-1A, and saw comparable results.
We demonstrated that acid insults affect the viability of primary HOS and HOF cells to a slightly different extent, with oesophageal epithelial cells proving a little more resistant to acid conditions than the oesophageal fibroblasts. It was the aim of the first part of this work to determine the extent of acid insult which cells could tolerate without a significant loss of viability. We considered environments that were directly cytotoxic to be of less clinical relevance because cells exposed to such conditions are unlikely to be capable of transformation to a BM phenotype. We showed that primary oesophageal epithelial cells are capable of withstanding 30-min acid exposure at pH 4 or above without a significant loss in viability, and accordingly, we consider that further studies should focus on the impact of exposure to acid in this range.
We also demonstrated a clear difference in NF-κB activation between the two cell types in response to acid exposure. Both primary and immortalized epithelial oesophageal cells responded to even a relatively brief exposure to a pH 4 insult by translocating and thus activating NF-κB, whereas fibroblasts exposed to a similar regime showed no NF-κB translocation. NF-κB activation has a recognized anti-apoptotic effect in many circumstances, enabling cells to withstand exposure to stimuli that would normally induce cell death (Beg & Baltimore 1996), and it may be that the increased acid resistance of epithelial cells is because of NF-κB activation.
Our second key finding is that brief pulsatile exposure of primary or immortalized epithelial cells to a pH 5 acidified environment results in a paracrine signal that activates NF-κB in HOF cells, even though this exposure regime was insufficient to activate NF-κB in the epithelial cells themselves. This is in agreement with work performed recently in our laboratory (Canton et al. 2010) where certain environments induced the paracrine activation of NF-κB in human dermal fibroblasts exposed to dermal keratinocyte conditioned media whilst not directly activating NF-κB in the exposed keratinocytes.
NF-κB activity is regulated by interactions with inhibitory IκB proteins. When IκBa is bound to NF-κB, DNA binding is prevented and the complex shuttles continuously between nucleus and cytoplasm. However, because of a strong nuclear export signal on IκBa, the complex is primarily located in the cytoplasm. Meanwhile, binding to an alternative inhibitory protein, IκBb, retains NF-κB in the cytoplasm. Thus, in most cells, NF-κB is located in the cytoplasm as an inactive complex bound to IκB. It is only when the cell receives an NF-κB activation signal that IκB is unbound and NF-κB translocates to the nucleus to activate target gene expression. Consequently, most NF-κB activation assays use the translocation of NF-κB from the cytoplasm to the nucleus as the measure of NF-κB activation, although methods have been developed recently where cells are transfected with plasmid-based NF-κB reporter genes. Unfortunately, primary epithelial cells are particularly difficult to transfect, with the process frequently resulting in terminally differentiated cells, making this latter approach unsuitable for this study. Similarly, assays that require the production of nuclear or whole cell extracts to quantify NF-κB levels in specific subcellular locations typically require high volumes of material, and primary cells cannot be passaged sufficiently to generate such volumes.
For these reasons, we used the immunocytochemical approach described to determine the subcellular location of the p65 subunit of NF-κB as a measure of NF-κB activation. The presence of a transactivating domain on the p65 subunit ensures that p65 nuclear localization is a positive indicator of NF-κB transcriptional activation (Baeuerle & Henkel 1994), and previous work has confirmed that results obtained using the method described in this paper are comparable to those obtained by EMSA detection of nuclear NF-κB (Moustafa et al. 2002).
Our studies on the ability of oesophageal epithelial cells to induce paracrine NF-κB activation in fibroblasts used primary cells from three different patients. The benefits, in terms of biological applicability, of using primary cells are countered by the inherent practical difficulties around obtaining tissue samples from human volunteer donors. To broaden the applicability of our results, we repeated our studies on the immortalized oesophageal cell line Het-1A. Similar results were obtained from HOS and Het-1A cells for NF-κB activation following direct exposure to acid, with both requiring a pH 4 insult to activate NF-κB. However, although the paracrine response was broadly similar, there were some slight differences observed. Exposure of Het-1A cells to a pH 5 environment for 30 min induced NF-κB activation in HOF cells, but this was not observed with primary cells. In addition, Het-1A cells exposed to pH 5 pulses with a 5-min recovery period resulted in paracrine NF-κB activation which was less than the mean response observed with primary oesophageal epithelial cells. It is worth noting that recent work in our laboratory has demonstrated that Het-1A cells grown in 3D organotypic cultures do not mature and differentiate in the same way as primary human oesophageal squames, retaining instead a proliferative phenotype (Green et al. 2010). Because NF-κB is known to play a role in many aspects of proliferation and cell differentiation, it is perhaps not surprising that the response of acid-exposed Het-1A cells differs very slightly from that of primary HOS cells. However, for both primary and immortalized epithelial cells, the conditions that resulted in paracrine activation of NF-κB in HOF cells were also ones that did not result in significant NF-κB activation in the epithelial cells themselves. Nevertheless, further studies are obviously required to fully investigate the phenomenon we report.
Attempts were made to determine the epithelial-derived factor that initiates the NF-κB response in the oesophageal fibroblasts. For this, we used immortalized Het-1A cells, because of the limited availability of primary human oesophageal cells. When the conditioned media were screened for a bank of potential initiation factors, no significant changes were detected and the factor or factors responsible for this effect remain undetermined.
Several cytokines known to activate NF-κB have been identified in Barrett's epithelia: These include TGFβ, IFNγ and TNFα (Jankowski et al. 2000); IL-1β (Jankowski et al. 2000; Fitzgerald et al. 2002a; Abdel-latif et al. 2005); IL-8 (Fitzgerald et al. 2002a; Abdel-latif et al. 2005; Jenkins et al. 2007); IL-10 and IL-4 (Fitzgerald et al. 2002b). However, because they were all observed in epithelia that had already entered the metaplasia–dysplasia–carcinoma sequence, this may not fully reflect events occurring in the initial stages of BM development. A more recent study demonstrated that 60-min exposure of primary HOS and Het-1A cells to pH 4.5 induced IL-6 and IL-8 production (Rafiee et al. 2009); however, the acid exposure time was longer than that in our study. Further research, ideally in the form of a full proteomic characterization of acid-exposed primary HOS cells from a larger number of patients, is probably required to characterize the factor responsible for the paracrine NF-κB activation.
The inter-sample variability seen in the paracrine response when using primary cells derived from different patients is unsurprising. Our laboratory has previously reported a 50-fold difference in the response of epidermal keratinocytes to chromium when using cells isolated from different individuals (Little et al. 1996). This was attributed to differences in the enzymatic capabilities of these cells for dealing with oxidative stress. It remains unclear why some patients with GORD develop BM whilst others simply develop reactive changes in the squamous epithelium, but our data raise the possibility that this clinical variation may be, in part, attributed to differences in the epithelial–stromal dialogue modulating the overall tissue response, and we suggest that this warrants further investigation, alongside studies to identify the molecular mediators of this crosstalk.
Conclusions
These results show that human oesophageal epithelial cells respond to a pulsatile acid exposure with the paracrine activation of the NF-κB in the oesophageal fibroblasts.
The limited availability of primary human oesophageal cells restricted the sample size of the study, and so further investigation into this phenomenon is required. However, these findings highlight the potential importance of epithelial–stromal interactions in the pathogenesis of oesophageal diseases. Given the role of NF-κB signalling in fibroblast functions such as matrix remodelling and cell migration, the secondary activation of NF-κB described here may well contribute to the morphological manifestations of GORD. Furthermore, the inter-patient variability observed may go some way to explain why only a minority of patients with reflux oesophagitis develop BM. Further investigation into such epithelial–mesenchymal communications, specifically the stress-coping mechanisms of the cell, is likely to add to our understanding of the pathogenesis of both reflux oesophagitis and BM. This is a novel insight into the impact of GORD, which is amenable to further investigation and which may have therapeutic implications.
Acknowledgments
We are grateful to Mr. Roger Ackroyd, Mr. Andrew Wyman and Mr. Chris Stoddard, Consultant Surgeons at Sheffield Teaching Hospitals NHS Foundation Trust, for their help in acquiring oesophageal tissue samples and their support of our work. We also thank Sue Newton from the Flow Cytometry Core Facility for performing the Cytometric Bead Array. This study was supported by grants from the Bardhan Research and Education Trust (BRET) and Yorkshire Cancer Research (YCR).
Competing interests
The authors declare that they have no competing interests.
References
- Abdel-latif MM, O'Riordan J, Windle HJ, et al. NF-kappaB activation in esophageal adenocarcinoma: relationship to Barrett's metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann. Surg. 2004;239:491–500. doi: 10.1097/01.sla.0000118751.95179.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-latif MM, O'Riordan JM, Ravi N, Kelleher D, Reynolds JV. Activated nuclear factor-kappa B and cytokine profiles in the esophagus parallel tumor regression following neoadjuvant chemoradiotherapy. Dis. Esophagus. 2005;18:246–252. doi: 10.1111/j.1442-2050.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Canton I, Cole DM, Kemp EH, et al. Development of a 3D human in vitro skin co-culture model for detecting irritants in real-time. Biotechnol. Bioeng. 2010;106:794–803. doi: 10.1002/bit.22742. [DOI] [PubMed] [Google Scholar]
- Debruyne PR, Witek M, Gong L, et al. Bile acids induce ectopic expression of intestinal guanylyl cyclase C Through nuclear factor-kappaB and Cdx2 in human esophageal cells. Gastroenterology. 2006;130:1191–1206. doi: 10.1053/j.gastro.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Duggan SP, Gallagher WM, Fox EJ, Abdel-latif MM, Reynolds JV, Kelleher D. Low pH induces co-ordinate regulation of gene expression in oesophageal cells. Carcinogenesis. 2006;27:319–327. doi: 10.1093/carcin/bgi211. [DOI] [PubMed] [Google Scholar]
- Dvorak K, Fass R, Dekel R, et al. Esophageal acid exposure at pH < or = 2 is more common in Barrett's esophagus patients and is associated with oxidative stress. Dis. Esophagus. 2006;19:366–372. doi: 10.1111/j.1442-2050.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- Eda A, Osawa H, Satoh K, et al. Aberrant expression of CDX2 in Barrett's epithelium and inflammatory esophageal mucosa. J. Gastroenterol. 2003;38:14–22. doi: 10.1007/s005350300001. [DOI] [PubMed] [Google Scholar]
- Feagins LA, Zhang HY, Hormi-Carver K, et al. Acid has antiproliferative effects in nonneoplastic Barrett's epithelial cells. Am. J. Gastroenterol. 2007;102:10–20. doi: 10.1111/j.1572-0241.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- Ferri N, Garton KJ, Raines EW. An NF-kappaB-dependent transcriptional program is required for collagen remodeling by human smooth muscle cells. J. Biol. Chem. 2003;278:19757–19764. doi: 10.1074/jbc.M212714200. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RC, Omary MB, Triadafilopoulos G. Acid modulation of HT29 cell growth and differentiation. An in vitro model for Barrett's esophagus. J. Cell Sci. 1997;110(Pt 5):663–671. doi: 10.1242/jcs.110.5.663. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RC, Omary MB, Triadafilopoulos G. Altered sodium-hydrogen exchange activity is a mechanism for acid-induced hyperproliferation in Barrett's esophagus. Am. J. Physiol. 1998;275:G47–G55. doi: 10.1152/ajpgi.1998.275.1.G47. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RC, Abdalla S, Onwuegbusi BA, et al. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut. 2002a;51:316–322. doi: 10.1136/gut.51.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002b;50:451–459. doi: 10.1136/gut.50.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J. Biol. Chem. 2006;281:20368–20382. doi: 10.1074/jbc.M603353200. [DOI] [PubMed] [Google Scholar]
- Green N, Huang Q, Khan L, et al. The development and characterization of an organotypic tissue-engineered human esophageal mucosal model. Tissue Eng Part A. 2010;16:1053–1064. doi: 10.1089/ten.TEA.2009.0217. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Sugiura H, Koarai A, et al. Peroxynitrite augments fibroblast-mediated tissue remodeling via myofibroblast differentiation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L800–L808. doi: 10.1152/ajplung.90264.2008. [DOI] [PubMed] [Google Scholar]
- Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett's metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- Jenkins GJ, Mikhail J, Alhamdani A, et al. Immunohistochemical study of NF-kB activity and IL-8 abundance in oesophageal adenocarcinoma; a useful strategy for monitoring these biomarkers. J. Clin. Pathol. 2007;60:1232–1237. doi: 10.1136/jcp.2006.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly AJ, Wild CP, Hardie LJ. Acid and bile salts induce DNA damage in human oesophageal cell lines. Mutagenesis. 2004;19:319–324. doi: 10.1093/mutage/geh035. [DOI] [PubMed] [Google Scholar]
- Kazumori H, Ishihara S, Rumi MA, Kadowaki Y, Kinoshita Y. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett's epithelium. Gut. 2006;55:16–25. doi: 10.1136/gut.2005.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek PC, Nikiforuk A, Kania J, Raithel M, Hahn EG, Muhldorfer S. Activation of NFkappaB represents the central event in the neoplastic progression associated with Barrett's esophagus: a possible link to the inflammation and overexpression of COX-2, PPARgamma and growth factors. Dig. Dis. Sci. 2004;49:1075–1083. doi: 10.1023/b:ddas.0000037790.11724.70. [DOI] [PubMed] [Google Scholar]
- Little MC, Gawkrodger DJ, MacNeil S. Chromium- and nickel-induced cytotoxicity in normal and transformed human keratinocytes: an investigation of pharmacological approaches to the prevention of Cr(VI)-induced cytotoxicity. Br. J. Dermatol. 1996;134:199–207. [PubMed] [Google Scholar]
- Lord RV, Brabender J, Wickramasinghe K, et al. Increased CDX2 and decreased PITX1 homeobox gene expression in Barrett's esophagus and Barrett's-associated adenocarcinoma. Surgery. 2005;138:924–931. doi: 10.1016/j.surg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Moons LM, Bax DA, Kuipers EJ, et al. The homeodomain protein CDX2 is an early marker of Barrett's oesophagus. J. Clin. Pathol. 2004;57:1063–1068. doi: 10.1136/jcp.2003.015727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa M, Szabo M, Ghanem GE, et al. Inhibition of tumor necrosis factor-alpha stimulated NFkappaB/p65 in human keratinocytes by alpha-melanocyte stimulating hormone and adrenocorticotropic hormone peptides. J. Invest Dermatol. 2002;119:1244–1253. doi: 10.1046/j.1523-1747.2002.19602.x. [DOI] [PubMed] [Google Scholar]
- Palumbo R, Galvez BG, Pusterla T, et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J. Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JD. Morphostats: a missing concept in cancer biology. Cancer Epidemiol. Biomarkers Prev. 2001;10:161–170. [PubMed] [Google Scholar]
- Rafiee P, Nelson VM, Manley S, et al. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-{kappa}B. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G388–G398. doi: 10.1152/ajpgi.90428.2008. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig. Dis. Sci. 1992;37:849–856. doi: 10.1007/BF01300382. [DOI] [PubMed] [Google Scholar]
- Saadi A, Shannon NB, Lao-Sirieix P, et al. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc. Natl. Acad. Sci. U S A. 2010;107:2177–2182. doi: 10.1073/pnas.0909797107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe PM, Ferguson MW. Mesenchymal influences on epithelial differentiation in developing systems. J. Cell Sci. Suppl. 1988;10:195–230. doi: 10.1242/jcs.1988.supplement_10.15. [DOI] [PubMed] [Google Scholar]
- Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–1784. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Sun T, Haycock JW, Szabo M, Hill RP, MacNeil S. Measurement of NF-kappaB in normal and reconstructed human skin in vitro. J. Mater. Sci. Mater. Med. 2004;15:743–749. doi: 10.1023/b:jmsm.0000032812.18703.a9. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int. J. Dev. Biol. 1995;39:35–50. [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J. Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- Xu J, Zutter MM, Santoro SA, Clark RA. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J. Cell Biol. 1998;140:709–719. doi: 10.1083/jcb.140.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]


