Abstract
Stem cell therapies offer great promise for many diseases, especially those without current effective treatments. It is believed that noninvasive imaging techniques, which offer the ability to track the status of cells after transplantation, will expedite progress in this field and help to achieve maximized therapeutic effect. Today’s biomedical imaging technology allows for real-time, noninvasive monitoring of grafted stem cells including their biodistribution, migration, survival, and differentiation, with magnetic resonance imaging (MRI) of nanoparticle-labeled cells being one of the most commonly used techniques. Among the advantages of MR cell tracking are its high spatial resolution, no exposure to ionizing radiation, and clinical applicability. In order to track cells by MRI, the cells need to be labeled with magnetic nanoparticles, for which many types exist. There are several cellular labeling techniques available, including simple incubation, use of transfection agents, magnetoelectroporation, and magnetosonoporation. In this overview article, we will review the use of different magnetic nanoparticles and discuss how these particles can be used to track the distribution of transplanted cells in different organ systems. Caveats and limitations inherent to the tracking of nanoparticle-labeled stem cells are also discussed.
THE IMPORTANCE OF TRACKING STEM CELLS
Stem cell therapies have great promise for treatment of many debilitating diseases. Clinical trials evaluating the safety of cell-based therapies are currently under way. However, there is still much to be learned about stem-cell-based approaches. One important aspect is to identify transplantable cells that are capable of surviving, integrating with the host tissue, and undertaking the desired cellular differentiation. Crucial to further therapeutic success is the treating physician being able to answer the following questions: (1) what is the optimal cell delivery route for a particular condition? (2) What is the initial engraftment and distribution pattern of injected cells? and (3) How effectively do injected cells migrate toward the affected pathological sites? In order to address these questions, noninvasive cellular imaging is currently a very active field of research. It is far more efficient than traditional histopathological techniques and it offers unique information about cell behavior over time. The need for cellular imaging is even greater in the clinical setting where information about the location and persistence of the cells can be acquired only through invasive biopsy or postmortem analysis. Noninvasive imaging techniques are needed to evaluate the migration and function of cells and to help guide treatment for maximized therapeutic effect.
Several imaging modalities are available for cell tracking including computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI), single photon emission computer tomography (SPECT), optical imaging, and ultrasound imaging. Advantages of MRI include its high spatial resolution, widespread availability in most clinics, and that it does not expose the patient to ionizing radiation, which is present in CT, PET, and SPECT. MRI is particularly useful in imaging transplanted stem cells since it can provide additional anatomical and pathological information on the surrounding tissue, including edema or inflammation surrounding the transplantation site,1,2 thus providing further information for clinicians and helping to understand all aspects of the particular cellular therapy.
In order to visualize transplanted stem cells by MRI, the cells need to be labeled with a contrast agent prior to transplantation. There are several available magnetic nanoparticles that can be used for cellular labeling and result in cell detection as positive or negative contrast on MR images. There are two MR relaxation time constants, T1 and T2. T1 characterizes the relaxation of the nuclear spin to its longitudinal equilibrium following a radiofrequency pulse. T2 measures the loss of coherency among adjacent nuclear spins. Both time constants are affected by the local microenvironment, which may include magnetic inhomogeneities leading to a new time constant T2*. Magnetic nanoparticle contrast agents function by altering one or more of these time constants because of their magnetic properties, in order to distinguish transplanted stem cells from endogenous tissue so that their position can be visualized.
In this overview article, we will review the types of magnetic nanoparticles that are used for cell labeling and tracking by MRI and we will elaborate on the different methods used for cellular labeling. Applications of magnetically labeled cells transplanted into different organ systems as well as in clinical studies will be discussed. Finally, we will outline the limitations and difficulties associated with cell tracking by magnetic nanoparticles as well as their future applications.
TYPES OF MAGNETIC NANOPARTICLES
Magnetic nanoparticles can be classified into a few groups—superparamagnetic, paramagnetic, ferrimagnetic, and ferromagnetic nanoparticles. Elements such as iron, manganese, and gadolinium have paramagnetic properties due to unpaired electrons and have strong effects on the local magnetic field, which allows them to be utilized as contrast agents for MRI. Normally, coupling forces in magnetic materials cause the magnetic moments of neighboring atoms to align, resulting in very large internal magnetic fields. At temperatures above the Curie temperature (or the Neel temperature for antiferromagnetic materials), the thermal energy is sufficient to overcome the coupling forces, causing the atomic magnetic moments to fluctuate randomly. Because there is no longer any magnetic order, the internal magnetic field no longer exists and the material exhibits paramagnetic behavior.
Superparamagnetism occurs when the material is composed of very small crystallites (1–10 nm). In this case, even though the temperature is below the Curie or Neel temperature and the thermal energy is not sufficient to overcome the coupling forces between neighboring atoms, the thermal energy is sufficient to change the direction of magnetization of the entire crystallite. The resulting fluctuations in the direction of magnetization cause the magnetic field to average to zero. The material behaves in a manner similar to paramagnetism, except that instead of each individual atom being independently influenced by an external magnetic field, the magnetic moment of the entire crystallite tends to align with the magnetic field.
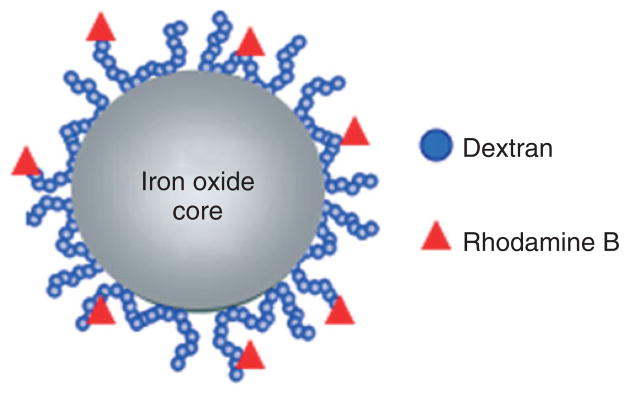
Ferromagnetic materials exhibit parallel alignment of all the individual moments and have a net magnetic moment without the presence of an external field.3 Ferrimagnetism occurs in ionic compounds such as oxides and is a complex form of magnetic ordering that results in a net magnetic moment of two sublattices. Iron oxide nanoparticles have been widely used for cellular imaging due to their strong T2* effects.4–7 There are several types of iron oxide nanoparticles that are utilized to label stem cells including superparamagnetic iron oxide (SPIO) nanoparticles, 50–200 nm diameter, ultra small superparamagnetic iron oxide (USPIO) nanoparticles, approximately 35 nm diameter, and micron-sized paramagnetic iron oxide nanoparticles (MPIO) that are approximately 1 μm. These particles locally reduce the T2 and T2* relaxation by inducing strong field inhomogeneities. When imaged with T2 or T2*-weighted pulse sequences, they produce a hypointense signal on the MRI, enabling the visualization of labeled, transplanted cells. Iron oxide nanoparticles are usually synthesized as an iron oxide core of inverse spinel structure containing mixed valence Fe+2 and Fe+3 ions (Figure 1), coated with different substrates, including dextran,8,9 carboxydextran,8,9 polyethylene glycol (PEG),10 polystyrene,11 or silica8 that aid in stability and solubility. The substrates also keep the particles from aggregating together which may cause toxicity to the cells.12 The magnetic and MRI contrast-enhancing properties of iron oxide nanoparticles can be manipulated by controlling the size of the core and the coating surface,9 offering flexibility in optimal design for particular applications.
FIGURE 1.
Schematic structure of a representative superparamagnetic iron oxide (SPIO) nanoparticle that is composed of an iron oxide core, dextran coating, and rhodamine as fluorescent marker.
Among the iron oxide nanoparticles, Feridex/Endorem (Berlex Pharmaceuticals, US/Guerbet, France) has been the most commonly used contrast agent for cell labeling. Feridex is an SPIO coated with dextran, and has a particle size of 50–180 nm with an overall negative charge of about −70 mV. Dextran-coated iron oxide particles such as Feridex are advantageous for animal and clinical studies as the particles are biocompatible and biodegradable through normal biochemical pathways for iron metabolism through initial uptake by Kupffer cells and macrophages.13 Feridex was Food and Drug Administration (FDA)-approved for clinical use as a liver contrast agent in 1996. Feridex (or its equivalent Endorem) is the only pharmaceutical-grade MR contrast agent that has been used for clinical cell tracking.14,15 Unfortunately, Feridex and Endorem were discontinued from production at the end of 2008 and are no longer commercially available. New types of iron oxide nanoparticles have since then been explored, although they are currently not approved for clinical use. BioPAL (Worcester, MA) produces several iron oxide nanoparticles including FeREX (USPIO, 50–150 nm) and Molday ION products (approximately 30 nm) that are available with different functional surface groups, including amino groups, carboxyl groups, and fluorescent molecules, so that the particles can be directly visualized using fluorescent microscopy.16–20
Several other types of SPIOs have been used for labeling stem cells. This includes Resovist (Bayer Schering, Berlin, Germany), which has an iron oxide core coated with carboxydextran.21,22 However, this formulation has now also been taken off the market. Although MPIO particles are not clinically approved, they are the most sensitive MR label due to their large size and high iron content.23,24 MPIOs are coated with polystyrene, which is not biodegradable. In addition, the formulations cannot be purchased with a guarantee of sterility, making clinical applications impossible.
Other groups of nanoparticle contrast agents used for cell tracking include manganese oxide nanoparticles and particles containing gadolinium chelates. These paramagnetic agents predominantly reduce the T1 relaxation time and are therefore called T1 agents. They create a positive or bright signal on the MR images. Among the metal ions, gadolinium has the most (7) unpaired electrons and therefore is the most effective paramagnetic contrast agent, but its overall relaxivity is far less than that of SPIOs. Gadolinium contrast agents include gadolinium diethylenetriamine pentaacetic acid (Gd-DPTA), gadolinium tetraazacyclododecanetertraacetic acid (Gd-DOTA), and trade names Vasovist (Bayer Health-care, Wayne, NJ, USA), Gadovist (Bayer Healthcare), and Magnevist (Bayer Healthcare).23,25–28 Manganese oxide also has been used to label cells with positive contrast nanoparticles.29–31
TECHNIQUES FOR CELL LABELING WITH MAGNETIC NANOPARTICLES
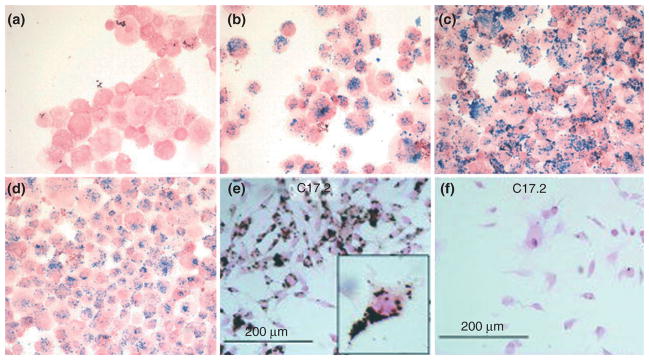
Magnetic cell labeling relies on shuttling of the particles from the extracellular environment to the intracellular compartments. The efficiency of magnetic labeling depends on cell membrane properties as well as the size of the cells of interest. Some cell types allow for efficient uptake of the nanoparticles and cell labeling is achieved by mere incubation with the nanoparticles simply suspended in culture medium.32,33 Other cell types require additional methods to take up the label. Initially, for these non-phagocytosing cells, techniques such as viral capsid incorporation,34 where a virus envelope encapsulates the iron oxide particles and then is used to infect cells, and antibody-mediated uptake, where the SPIOs can be covalently attached to antibodies,35,36 were employed. Use of viral capsid elements as well as antibody coating is quite complex and not versatile; therefore, researchers sought alternative methods and found success with coating the negatively charged SPIO nanoparticles with poly-cations to facilitate particle binding to the anionic cell membrane, therefore increasing uptake.37–40 Poly-cationic transfection agents are now widely used to increase the efficiency of nanoparticle cellular labeling. There are several commercially available transfection agents that aid in the uptake of nanoparticles, including poly-L-lysine (MW = 350–400 kDa), protamine sulfate, and lipofectamine (Figure 2). The labeling procedure involves mixing the nanoparticle solution with the cell culture medium, then adding the transfection agent, and allowing that mixture to incubate in order to form stable complexes through electrostatic interactions. When the labeling solution is added to the cells, the nanoparticle–transfection agent complexes electrostatically interact with the cell membrane and facilitate endocytosis or macropinocytosis of the contrast agent nonspecifically into endosomes within the cells over a 24–48-h labeling period.41,42 Alternative methods for cell labeling are based on temporary disruption of cell membrane stability with the use of electroporation43,44 or ultrasound45,46 pulses. Transient changes of cell membrane permeability allow for nanoparticles to pass through the membrane and into the cytosol. The primary advantage of magnetoelectro-/sonoporation is that it is instantaneous and does not require prolonged incubation of cells, which is of concern when using some sensitive, primary cell types. However, cell death can be significant for this method and the parameters need to be optimized for each cell type.
FIGURE 2.
Prussian blue staining of mesenchymal stem cells (MSCs) labeled with Feridex at 25 μg of iron per milliliter of culture medium for 2 h (40× magnification): Feridex only (a); Feridex-PLL (1200 ng/mL PLL) (b); Feridex-Superfect (2400 ng/mL Superfect) (c); Feridex-PLUS/lipofectamine (1:1250/1:2500 dilution from stock solution, Invitrogen, Carlsbad, CA) (d). Diamino-benzidine-enhanced Prussian blue staining of C17.2 mouse neural stem cells labeled with Feridex (2 mg/mL) with (e) and without magnetoelectroporation (f). (a–d, Reprinted with permission from Ref 38. Copyright 2003 Radiological Society of North America. E–F, Reprinted with permission from Ref 43. Copyright 2005 John Wiley & Sons, Inc)
Regardless of the labeling method used, the efficiency of labeling must be verified for each cell type prior to transplantation. The cells also must be thoroughly washed to remove all of the excess nanoparticles as residual extracellular contrast agent may lead to false-positive signals in vivo. It is also recommended that labeled cells are evaluated in vitro to ensure that there is no or minimal impact on cell viability and function.
IMPACT OF MAGNETIC LABELING ON STEM CELLS
Several studies have been conducted to analyze the effects of magnetic nanoparticle labeling on the viability and function of stem cells. Nearly all studies published so far have not shown any detrimental effect of Feridex labeling at currently used incubation doses, including the first clinical study using Feridex-labeled cells.47 However, this contrast agent was found to inhibit chondrogenesis, although the adipogenesis and osteogenesis pathways were not affected.48,49 In addition, a study of SPIO labeling with and without the use of transfection agents reported that the labeling may cause a decrease in the migration capacity and colony-formation ability of human MSCs.50 The decrease in migration capacity was only apparent in the first two passages, but the colony-formation ability effects remained.50 A study on human MSCs labeled with Ferucarbotran, an ionic SPIO, found that the SPIO had an inhibitory effect on osteogenic differentiation and its signaling mechanism.51 Ferucarbotran SPIO caused a dose-dependent inhibition of osteogenic differentiation, abolished the differentiation at high concentration, and promoted cell migration.51 However, except for these rare cases, overall SPIO labeling appears safe, justifying its clinical use.14 In order to further support this claim, detailed gene expression profiling using micro-array analysis and quantitative polymerase chain reaction (PCR) did reveal only minor early changes in a small set of genes that largely returned back to normal levels over the course of 1 week post-labeling.52
APPLICATIONS OF STEM CELL TRACKING
Stem-cell-based therapy relies on the delivery of cells to organs that are affected by the disease. Depending on the pathology, various routes of cell delivery are being considered, including intraparenchymal injections, intravenous injections, and injections into natural cavities (such as the peritoneal space or ventricular system of the brain). MRI is particularly valuable for evaluating the cell distribution and migration and determining the best delivery route for transplantation. There are many examples of tracking stem cells by MRI with applications in different fields, including two key applications where stem-cell-based therapies are widely studied, cardiovascular and neurological diseases.
Cardiovascular Applications
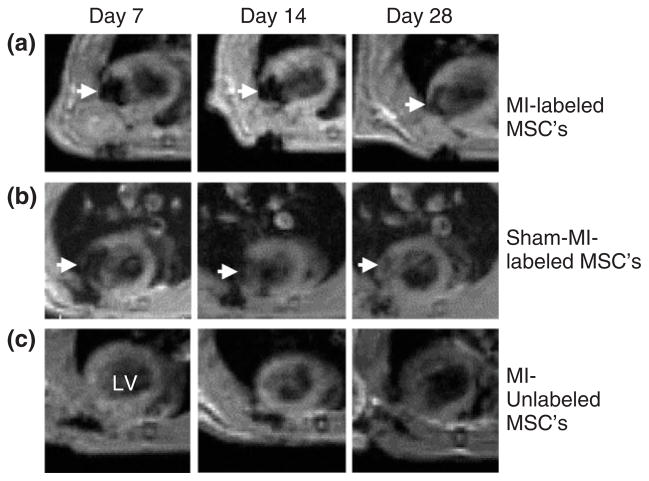
Stem-cell-based therapy is considered highly promising for treatment of heart infarct as well as other cardiovascular diseases, such as peripheral arterial occlusive disease53 or heart valve dysfunction.54 The heart muscle has limited regeneration capacity, so stem cells offer the potential for regrowth of heart tissue and restoration of function. Several studies have been performed involving tracking magnetically labeled stem cells in order to follow their migration after transplantation in cardiac disease models. Cardiac imaging is highly valuable in the clinic for diagnostic purposes, i.e., determining the extent of the infarct and for performing interventional radiology procedures. The establishment of a method for noninvasive imaging of transplanted stem cells will provide clinicians with more options in treating heart disease. The beating heart is an inherently dynamic organ and motion artifacts make MR imaging challenging.55 Nevertheless, both cardiac and respiration gating utilized together make it possible to acquire high-quality images, allowing for visualization of SPIO-labeled cells. Engraftment of MSCs and their distribution in cardiac tissue were successfully imaged in rodents56,57 (Figure 3) and in swine models of myocardial infarction.58,59 Other groups have transplanted embryonic stem cells (ESCs) or ESC-derived cardiac precursor cells (ES-CPCs) labeled with iron oxide in a mouse model of myocardial infarction.55,57,60 Feridex and protamine sulfate labeled ES-CPCs were injected into the border zone of heart infarct in mice. One week after transplantation, the graft distribution imaged by MRI correlated well with histology.55 This study did not report on monitoring long-term migration. Most importantly, transplantation of SPIO-labeled ESCs by direct intra-myocardial injection to infarcted myocardium was reported to result in improvement of heart function.60 This study showed the feasibility of in vivo ESC tracking using SPIO labeling and cardiac MRI without altering the cardiac differentiation potential and functional properties of the stem cells.
FIGURE 3.
Serial in vivo magnetic resonance (MR) tracking of Endorem-labeled MSCs (25 μg/mL iron and 0.375 μg/mL PLL). (a) Injection of 2 × 106 SPIO-labeled MSCs 7 days after left coronary artery ligation creates a wide intramural area of hypointensity (arrows) at the anterior lateral ventricle (LV) wall. Positive signals are still visible after 28 days. (b) Similar magnetic signals (arrows) were produced by labeled cells injected to normal hearts. (c) Injection of unlabeled MSCs did not alter the magnetic signal of the myocardium. (Reprinted with permission from Ref 56. Copyright 2007 American Heart Association, Inc)
Neurological Applications
The central nervous system (CNS) is the most complex system in the body with very limited regenerative abilities. Transplantation of exogenous cells offers an alternative with potential to enhance restorative capacity of the brain. Due to the complexity of the CNS, cell-based therapy is challenging, and tools for monitoring and guiding these approaches are highly desired. While tissue biopsies can be considered for evaluation of cell therapy in other organs, it is rather not an option in the case of brain. Therefore, access to a method for evaluating the status of transplanted cells and their contribution to therapeutic effect noninvasively over time is critical. MR cellular imaging seems ideally suited for that purpose as it can be used to monitor cell delivery as well as report on cell migration over time. Imaging of SPIO-labeled cells is now widely used for monitoring cell-based therapy in animal models of neurological disease. In the study by Kim et al., Feridex-labeled human MSCs were transplanted into the brain of stroked rats and their migration was imaged using a 4.7-T MRI scanner. Cells were shown to migrate extensively and were capable of reaching the infarcted area from both ipsilateral and contralateral injections.61
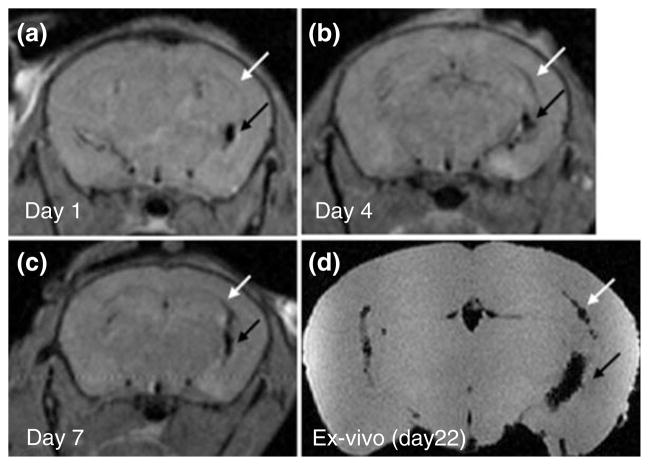
Multiple sclerosis (MS) is another type of disease where cell therapy and cellular imaging have been explored widely in animal models.62–65 It is expected that stereotaxically transplanted cells will migrate toward lesion sites that emit inflammatory cues. In experimental autoimmune encephalomyelitis (EAE), a rodent model of MS, MRI has visualized the migration of SPIO-labeled neurospheres from the ventricles into the periventricular white matter.62 A recent study evaluated the effect of Feridex labeling on the survival, differentiation, migration, and immunomodulatory properties of multipotential neurospheres (Figure 4).64 Following intracerebroventricular (ICV) transplantation in EAE mice, Feridex-labeled neurospheres responded to inflammatory cues in a similar fashion as unlabeled cells. Labeled cells inhibited lymph node cell proliferation similar to unlabeled cells, which suggests a preserved immunomodulatory function despite the presence of intracellular Feridex. These labeled neurospheres also migrated over comparable distances in white matter tracts and differentiated equally into the glial lineages. This study is an important demonstration that labeling with magnetic nanoparticles does not affect the function of the neurospheres, providing key evidence that these labels can be used to serially track stem cells in an EAE model of demyelinating disease.
FIGURE 4.
Serial in vivo magnetic resonance imaging (MRI) tracking of intracerebroventricular (ICV)-transplanted neurospheres in experimental autoimmune encephalomyelitis (EAE). Feridex-labeled neurospheres were transplanted into the right ventricle of EAE mice (black arrow). At day 1 after ICV transplantation (a), cells featuring hypointense (black) signals are found exclusively within the cerebral ventricles and are absent within the corpus callosum (white arrow). At 4 (b) and 7 (c) days after ICV transplantation, some cells had migrated into the corpus callosum (white arrow). Ex vivo MRI at day 22 posttransplantation confirmed this pattern of migration (d). (Reprinted with permission from Ref 64. Copyright 2009 John Wiley & Sons, Inc)
As for human cells, SPIO-labeled neural stem cells (NSCs) were followed up for 1 month after transplantation into adult murine brains. The study found that the cell survival, proliferation, self-renewal, and multipotency were not impaired by the intracellular presence of SPIO.66 Human NSCs, labeled with Feridex and protamine sulfate and transplanted into an adult rodent stroke model, were shown to migrate through an intraparenchymal migration pathway from the cortical transplantation site.67 Labeled cells survived, migrated, differentiated, and responded to microenvironment cues without altering the neuronal electrophysiological characteristics.67 A recent study of Feridex-labeled NSCs transplanted into normal adult rat brains described a quantitative method for determining the migratory capacity of stem cells by co-registration of serial images and parametric analysis to determine the migration speed.68 Quantification as well as location relative to key anatomical features will be beneficial in clinical studies in determining the therapeutic effect of stem cells.
Clinical Applications
Several clinical studies utilizing magnetic nanoparticles to label cells have been conducted.15,47,69–71 The first report involving magnetically labeled stem cells was from a patient with brain trauma who received an autologous transplant of Feridex-labeled NSCs into the damaged temporal lobe.69 NSCs were isolated from exposed neural tissue (brain injury region) from the patient. Cells were cultured to select for neural progenitor cells and labeled with Feridex prior to stereotactical transplantation. The patient was then imaged with a 3.0-T MR scanner weekly for 10 weeks after transplantation. Hypointense signals at the injection sites were only observed after transplantation (Figure 5). This signal persisted for 7 weeks, after which it became undetectable, which the authors attributed to the dilution of the label during cellular proliferation. Although not directly proven, since the MRI also revealed increased hypointensity at the periphery of the lesion over time, the authors believed that the cells were migrating to the border of the damaged tissue. This validated another major goal of magnetically labeled cells—not only to detect initial distribution of the cells, but also to follow their migration. A control patient receiving unlabeled NSCs revealed only slight hypointense signal at the injection site, which did not significantly change over time. This initial clinical study involving two patients clearly demonstrates that MRI can be utilized for the noninvasive detection of the engraftment and migration of stem cells. The findings are consistent with the preceding data in animal models, and no unwanted side effects were encountered in this preliminary study. A clinical study was conducted recently to evaluate the feasibility, safety, and immunological effects of autologous MSCs in patients with MS and amyotrophic lateral sclerosis (ALS).15 While several patients received unlabeled cells, nine patients received Feridex-labeled MSCs administered intrathecally or intravenously. Patients were followed for up to 25 months following administration of the cells, and MRI was utilized to track the labeled cells and determine if there were other related pathology changes. Hypointense signals in T2-weighted images indicated the presence of SPIO-positive cells in the meninges of the spinal cord and nerve roots, in the sub-arachnoid space, and in the spinal cord parenchyma. The study concluded that transplantation of MSCs into patients with MS and ALS is clinically feasible and safe, while resulting in immediate immunomodulatory effects.15 Other clinical studies on MRI cell tracking have used other cell types, including dendritic cells in melanoma patients,47 bone marrow CD34+ stem cells in chronic spinal cord injury patients,70 and pancreatic islets for type 1 diabetes patients.71
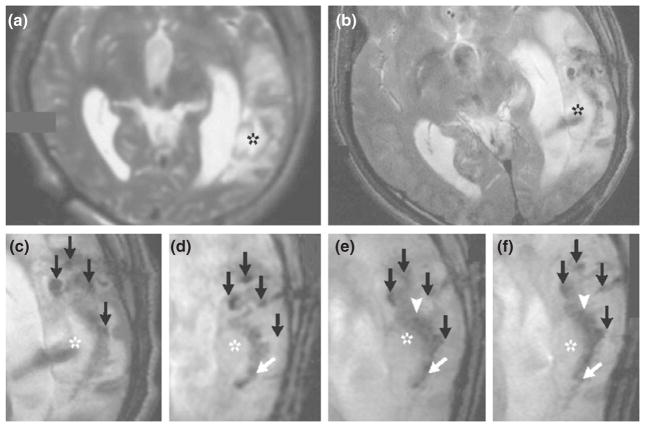
FIGURE 5.
MRI scans from a patient who received neural stem cells labeled with Feridex. The scan obtained before implantation of the labeled neural stem cells (a) did not show a pronounced hypointense signal around the lesion (asterisk) in the left temporal lobe, whereas circular areas of hypointense signal were visible at the injection sites 1 day after implantation (b). Four hypointense signals (black arrows) were observed at injection sites around the lesion on day 1 (c), day 7 (d), day 14 (e), and day 21 (f). On day 7 (d), dark signals (white arrow) posterior to the lesion were observed, a finding that is consistent with the presence of the labeled cells. By day 14 (e), the hypointense signals at the injection sites had faded, and another dark signal (white arrowhead) had appeared and spread along the border of the damaged brain tissue. By day 21 (f), the dark signal had thickened and extended further along the lesion (white arrow). (Reprinted with permission from Ref 69. Copyright 2006 Massachusetts Medical Society)
Limitations
There are several limitations to exogenous cellular labeling with magnetic nanoparticles for long-term MR imaging. Immature cells such as stem or progenitor cells frequently continue to proliferate after transplantation, as they are not terminally differentiated. In such cases, the label is diluted as the cells divide. Dilution of the iron oxide label is a major limitation for long-term tracking, as the MR signal is lost over time due to cellular proliferation, especially with rapidly dividing cells.43,66,72–74 Upon staining, Prussian blue negative cells have been detected as a result of dilution of the label following cellular division. Therefore, tracking proliferating cells long term by MRI and nanoparticle labeling is challenging. There are also concerns that stem cells may divide asymmetrically, leading to an unequal distribution of label with one daughter cell receiving most of the nanoparticles.75 This results in less gradual dilution of the contrast agent than if there was symmetric division. Asymmetric division of stem cells would quickly dilute the label from some daughter cells, leaving these cells undetectable by MRI even early on after transplantation. There are also detection limits and difficulties in quantifying the number of iron-oxide-labeled cells that are transplanted. The detection threshold for cells labeled with magnetic nanoparticles is affected by a number of factors including field strength, signal-to-noise ratio, pulse sequence, type of particle, and voxel size. In typical settings, detection limits are approximately a few hundred cells. The quantification of SPIO-labeled cells by MRI is an indirect technique. Signal change is the result of the overall concentration of magnetic nanoparticles, and not the actual total number of cells.76 Another complication is that certain endogenous conditions can also result in hypointense MR signals, which can be confused with the presence of magnetic contrast agents. Macrophages loaded with hemosiderin from hemorrhage can often be found in infarcted myocardium, and their hypointense signals may be indistinguishable from labeled cells.56,77
Magnetic nanoparticles also do not allow for the discrimination by MRI between live and dead cells. If the transplanted cells die, magnetic nanoparticles could persist in the tissue and produce signal that is detected by MRI.78 Another potential difficulty with magnetic nanoparticle labeling is that endogenous cells such as macrophages can take up particles.79 Immune cells such as macrophages can engulf dead cells containing nanoparticles. In the brain, microglia have been shown to co-localize with the nanoparticles. Recent studies in the heart and leg indicate that the magnetic nanoparticles are not cleared quickly after cell death, with the cellular viability being confirmed by other methods, indicating that in certain cases the persistent MR signal may be misleading (Figure 6).80–82 If macrophages phagocytize dead or dying SPIO-labeled cells, the endogenous macrophages can be mistaken for transplanted stem cells. There can also be extracellular iron that remains in the tissue instead of being removed or taken up by endogenous cells. Also, nanoparticles biodegrade over time, which further hinders imaging over extended time periods.30,83 One option would be to slow down the metabolism of iron oxides, such as that occurs in iron oxide nanoparticles coated with phospholipids. After cellular internalization, the nanoparticles reach the endosomes, where the chemoabsorbed phospho-lipid layer shields the core from degradation, resulting in a persistent MRI signal for at least 1 month even in a continuously proliferating cell culture like 3T3 fibroblasts.84 But MRI remains unable to discern whether MR hypointensity is a result of live, transplanted cells or nanoparticles that are no longer within transplanted cells.
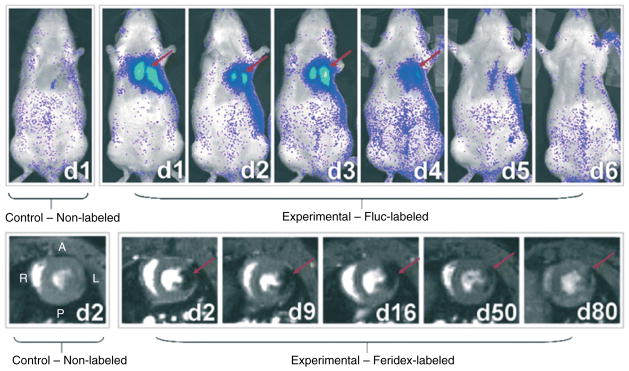
FIGURE 6.
Serial optical bioluminescence imaging and magnetic resonance imaging (MRI) of H9c2 cells after transplantation. Optical bioluminescence imaging of a representative rat intramyocardially injected with 2 × 106 Fluc-labeled cells (top panel, right) shows a robust distinct heart signal on day 1 (red arrow), compared to no discernable signal in a representative control rat having received non-labeled cells (top panel, left). The signal increases slightly on day 3 but decreases rapidly to near-background levels by day 6. MRI of a representative rat injected with 2 × 106 Feridex-labeled cells (bottom panel, right) shows a large hypointense signal (red arrow) in the anterolateral wall of myocardium when viewed in short axis. The size of the signal decreases slightly over time, and the signal persists for at least 80 days post cell injection, even though the cells have died by day 6. No corresponding signal is observed on the short-axis image of a representative control rat having received non-labeled cells (bottom panel, left). A, P, R, and L indicate anterior, posterior, right, and left anatomical orientations, respectively. (Reprinted with permission from Ref 81. Copyright 2008 Academy of Molecular Imaging)
Conclusion
Noninvasive MRI of tracking stem cells is likely to become an integral part of clinical cellular therapies. In order to successfully monitor the accuracy of injection and the distribution of cells throughout the course of treatment, the high-resolution cellular imaging technology is of crucial importance. SPIO magnetic nanoparticles are biocompatible, have low toxicity, high MR sensitivity without exposing the patient to radiation, and are clinically applicable. The magnetic nanoparticles discussed in this article are valuable for labeling and tracking of stem cells; however, there are certain limitations that cannot be ignored. The ideal cellular MR contrast for labeling of cells has a low toxicity, is biocompatible, provides stable contrast beyond the background level, provides high sensitivity allowing for single-cell detection, does not dilute during cellular proliferation, and exhibits minimum transfer to endogenous host cells. While currently available magnetic nanoparticles do not meet all of these criteria, they are well suited for real-time, MR-guided delivery of stem cells into patients. This will ensure that the initial transplantation and distribution of stem cells are anatomically accurate to provide the desired treatment. For subsequent long-term stem-cell tracking, an approach based on the use of MR reporter genes85–89 that can report on the survival of transplanted cells and will not be diluted out with cellular divisions may become ultimately the method of choice, or a combination of magnetic nanoparticles and reporter genes may be utilized.
References
- 1.Anderson SA, Glod J, Arbab AS, Noel M, Ashari P, Fine HA, Frank JA. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105:420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- 2.Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, Yamasaki M, Momoshima S, Ishii H, Ando K, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182–190. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- 3.Focke A, Schwarz S, Foerschler A, Scheibe J, Milosevic J, Zimmer C, Schwarz J. Labeling of human neural precursor cells using ferromagnetic nanoparticles. Magn Reson Med. 2008;60:1321–1328. doi: 10.1002/mrm.21745. [DOI] [PubMed] [Google Scholar]
- 4.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990;175:494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D, Hong KS, Song J. The present status of cell tracking methods in animal models using magnetic resonance imaging technology. Mol Cells. 2007;23:132–137. [PubMed] [Google Scholar]
- 9.Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, Poremba C, Ebert W, Heindel W, Bremer C. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005;235:155–161. doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]
- 10.Basly B, Felder-Flesch D, Perriat P, Billotey C, Taleb J, Pourroy G, Begin-Colin S. Dendronized iron oxide nanoparticles as contrast agents for MRI. Chem Commun (Camb) 2010;46:985–987. doi: 10.1039/b920348f. [DOI] [PubMed] [Google Scholar]
- 11.Seymour L, Schacht E, Duncan R. The effect of size of polystyrene particles on their retention within the rat peritoneal compartment, and on their interaction with rat peritoneal macrophages in vitro. Cell Biol Int Rep. 1991;15:277–286. doi: 10.1016/0309-1651(91)90166-g. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Mascheri N, Dharmakumar R, Fan Z, Paunesku T, Woloschak G, Li D. Superparamagnetic iron oxide nanoparticle-labeled cells as an effective vehicle for tracking the GFP gene marker using magnetic resonance imaging. Cytotherapy. 2009;11:43–51. doi: 10.1080/14653240802420243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrucci JT, Stark DD. Iron oxide-enhanced MR imaging of the liver and spleen: review of the first 5 years. AJR Am J Roentgenol. 1990;155:943–950. doi: 10.2214/ajr.155.5.2120963. [DOI] [PubMed] [Google Scholar]
- 14.Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009;193:314–325. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto S, Hyodo F, Subramanian S, Devasahayam N, Munasinghe J, Hyodo E, Gadisetti C, Cook JA, Mitchell JB, Krishna MC. Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J Clin Invest. 2008;118:1965–1973. doi: 10.1172/JCI34928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.JuanYin J, Tracy K, Zhang L, Munasinghe J, Shapiro E, Koretsky A, Kelly K. Noninvasive imaging of the functional effects of anti-VEGF therapy on tumor cell extravasation and regional blood volume in an experimental brain metastasis model. Clin Exp Metastasis. 2009;26:403–414. doi: 10.1007/s10585-009-9238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feigenbaum GS, Lemberg L, Hare JM. Tracking cell fate with noninvasive imaging. J Am Coll Cardiol. 2009;54:1627–1628. doi: 10.1016/j.jacc.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccaro DE, Yang M, Weinberg JS, Reinhardt CP, Groman EV. Cell tracking using nanoparticles. J Cardiovasc Transl Res. 2008;1:217–220. doi: 10.1007/s12265-008-9039-8. [DOI] [PubMed] [Google Scholar]
- 20.Ungersma SE, Pacheco G, Ho C, Yee SF, Ross J, van Bruggen N, Peale FV, Jr, Ross S, Carano RA. Vessel imaging with viable tumor analysis for quantification of tumor angiogenesis. Magn Reson Med. 63:1637–1647. doi: 10.1002/mrm.22442. [DOI] [PubMed] [Google Scholar]
- 21.Krejci J, Pachernik J, Hampl A, Dvorak P. In vitro labelling of mouse embryonic stem cells with SPIO nanoparticles. Gen Physiol Biophys. 2008;27:164–173. [PubMed] [Google Scholar]
- 22.Niemeyer M, Oostendorp RA, Kremer M, Hippauf S, Jacobs VR, Baurecht H, Ludwig G, Piontek G, Bekker-Ruz V, Timmer S, et al. Non-invasive tracking of human haemopoietic CD34(+) stem cells in vivo in immunodeficient mice by using magnetic resonance imaging. Eur Radiol. 2010;20:2184–2193. doi: 10.1007/s00330-010-1773-z. [DOI] [PubMed] [Google Scholar]
- 23.von Zur Muhlen C, Sibson NR, Peter K, Campbell SJ, Wilainam P, Grau GE, Bode C, Choudhury RP, Anthony DC. A contrast agent recognizing activated platelets reveals murine cerebral malaria pathology undetectable by conventional MRI. J Clin Invest. 2008;118:1198–1207. doi: 10.1172/JCI33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slotkin JR, Cahill KS, Tharin SA, Shapiro EM. Cellular magnetic resonance imaging: nanometer and micrometer size particles for noninvasive cell localization. Neurotherapeutics. 2007;4:428–433. doi: 10.1016/j.nurt.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modo M, Cash D, Mellodew K, Williams SC, Fraser SE, Meade TJ, Price J, Hodges H. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage. 2002;17:803–811. [PubMed] [Google Scholar]
- 26.Rudelius M, Daldrup-Link HE, Heinzmann U, Piontek G, Settles M, Link TM, Schlegel J. Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. Eur J Nucl Med Mol Imaging. 2003;30:1038–1044. doi: 10.1007/s00259-002-1110-0. [DOI] [PubMed] [Google Scholar]
- 27.Crich SG, Biancone L, Cantaluppi V, Duo D, Esposito G, Russo S, Camussi G, Aime S. Improved route for the visualization of stem cells labeled with a Gd-/Euchelate as dual (MRI and fluorescence) agent. Magn Reson Med. 2004;51:938–944. doi: 10.1002/mrm.20072. [DOI] [PubMed] [Google Scholar]
- 28.Vuu K, Xie J, McDonald MA, Bernardo M, Hunter F, Zhang Y, Li K, Bednarski M, Guccione S. Gadolinium-rhodamine nanoparticles for cell labeling and tracking via magnetic resonance and optical imaging. Bioconjug Chem. 2005;16:995–999. doi: 10.1021/bc050085z. [DOI] [PubMed] [Google Scholar]
- 29.Na HB, Lee JH, An K, Park YI, Park M, Lee IS, Nam D-H, Kim ST, Kim S-H, Kim S-W, et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles13. Angew Chem Int Ed. 2007;46:5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 30.Gilad AA, Walczak P, McMahon MT, Na HB, Lee JH, An K, Hyeon T, van Zijl PC, Bulte JW. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn Reson Med. 2008;60:1–7. doi: 10.1002/mrm.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin J, Anisur RM, Ko MK, Im GH, Lee JH, Lee IS. Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery13. Angew Chem. 2009;121:327–330. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 32.Yeh TC, Zhang W, Ildstad ST, Ho C. Intracellular labeling of T-cells with superparamagnetic contrast agents. Magn Reson Med. 1993;30:617–625. doi: 10.1002/mrm.1910300513. [DOI] [PubMed] [Google Scholar]
- 33.Yeh TC, Zhang W, Ildstad ST, Ho C. In vivo dynamic MRI tracking of rat T-cells labeled with superparamagnetic iron-oxide particles. Magn Reson Med. 1995;33:200–208. doi: 10.1002/mrm.1910330209. [DOI] [PubMed] [Google Scholar]
- 34.Hawrylak N, Ghosh P, Broadus J, Schlueter C, Greenough WT, Lauterbur PC. Nuclear magnetic resonance (NMR) imaging of iron oxide-labeled neural transplants. Exp Neurol. 1993;121:181–192. doi: 10.1006/exnr.1993.1085. [DOI] [PubMed] [Google Scholar]
- 35.Norman AB, Thomas SR, Pratt RG, Lu SY, Norgren RB. Magnetic resonance imaging of neural transplants in rat brain using a superparamagnetic contrast agent. Brain Res. 1992;594:279–283. doi: 10.1016/0006-8993(92)91135-2. [DOI] [PubMed] [Google Scholar]
- 36.Bulte JW, Hoekstra Y, Kamman RL, Magin RL, Webb AG, Briggs RW, Go KG, Hulstaert CE, Miltenyi S, The TH, et al. Specific MR imaging of human lymphocytes by monoclonal antibody-guided dextran-magnetite particles. Magn Reson Med. 1992;25:148–157. doi: 10.1002/mrm.1910250115. [DOI] [PubMed] [Google Scholar]
- 37.Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 38.Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Jr, Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 39.Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 40.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 41.Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 42.Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: implications for cellular magnetic resonance imaging. NMR Biomed. 2005;18:383–389. doi: 10.1002/nbm.970. [DOI] [PubMed] [Google Scholar]
- 43.Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW. Instant MR labeling of stem cells using magnetoelectroporation. Magn Reson Med. 2005;54:769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 44.Walczak P, Ruiz-Cabello J, Kedziorek DA, Gilad AA, Lin S, Barnett B, Qin L, Levitsky H, Bulte JW. Magnetoelectroporation: improved labeling of neural stem cells and leukocytes for cellular magnetic resonance imaging using a single FDA-approved agent. Nanomedicine. 2006;2:89–94. doi: 10.1016/j.nano.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Qiu B, Xie D, Walczak P, Li X, Ruiz-Cabello J, Minoshima S, Bulte JW, Yang X. Magnetosonoporation: instant magnetic labeling of stem cells. Magn Reson Med. 63:1437–1441. doi: 10.1002/mrm.22348. [DOI] [PubMed] [Google Scholar]
- 46.Xie D, Qiu B, Walczak P, Li X, Ruiz-Cabello J, Minoshima S, Bulte JW, Yang X. Optimization of magnetosonoporation for stem cell labeling. NMR Biomed. 23:480–484. doi: 10.1002/nbm.1485. [DOI] [PubMed] [Google Scholar]
- 47.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 48.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 49.Bulte JW, Kraitchman DL, Mackay AM, Pittenger MF. Chondrogenic differentiation of mesenchymal stem cells is inhibited after magnetic labeling with ferumoxides. Blood. 2004;104:3410–3412. doi: 10.1182/blood-2004-06-2117. author reply 3412–3413. [DOI] [PubMed] [Google Scholar]
- 50.Schafer R, Kehlbach R, Muller M, Bantleon R, Kluba T, Ayturan M, Siegel G, Wolburg H, Northoff H, Dietz K, et al. Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy. 2009;11:68–78. doi: 10.1080/14653240802666043. [DOI] [PubMed] [Google Scholar]
- 51.Chen YC, Hsiao JK, Liu HM, Lai IY, Yao M, Hsu SC, Ko BS, Yang CS, Huang DM. The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol Appl Pharmacol. 2010;245:272–279. doi: 10.1016/j.taap.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Kedziorek DA, Muja N, Walczak P, Ruiz-Cabello J, Gilad AA, Jie CC, Bulte JW. Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magn Reson Med. 2010;63:1031–1043. doi: 10.1002/mrm.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawall H, Bramlage P, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 2010;103:696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]
- 54.Loh Y, Oyama Y, Statkute L, Traynor A, Satkus J, Quigley K, Yaung K, Barr W, Bucha J, Gheorghiade M, et al. Autologous hematopoietic stem cell transplantation in systemic lupus erythematosus patients with cardiac dysfunction: feasibility and reversibility of ventricular and valvular dysfunction with transplant-induced remission. Bone Marrow Transplant. 2007;40:47–53. doi: 10.1038/sj.bmt.1705698. [DOI] [PubMed] [Google Scholar]
- 55.Mani V, Adler E, Briley-Saebo KC, Bystrup A, Fuster V, Keller G, Fayad ZA. Serial in vivo positive contrast MRI of iron oxide-labeled embryonic stem cell-derived cardiac precursor cells in a mouse model of myocardial infarction. Magn Reson Med. 2008;60:73–81. doi: 10.1002/mrm.21642. [DOI] [PubMed] [Google Scholar]
- 56.Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, Ocherashvilli A, Holbova R, Yosef O, Barbash IM, et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007;116:I38–I45. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 57.Arai T, Kofidis T, Bulte JW, de Bruin J, Venook RD, Berry GJ, McConnell MV, Quertermous T, Robbins RC, Yang PC. Dual in vivo magnetic resonance evaluation of magnetically labeled mouse embryonic stem cells and cardiac function at 1.5 t. Magn Reson Med. 2006;55:203–209. doi: 10.1002/mrm.20702. [DOI] [PubMed] [Google Scholar]
- 58.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 59.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Au KW, Liao SY, Lee YK, Lai WH, Ng KM, Chan YC, Yip MC, Ho CY, Wu EX, Li RA, et al. Effects of iron oxide nanoparticles on cardiac differentiation of embryonic stem cells. Biochem Biophys Res Commun. 2009;379:898–903. doi: 10.1016/j.bbrc.2008.12.160. [DOI] [PubMed] [Google Scholar]
- 61.Kim D, Chun BG, Kim YK, Lee YH, Park CS, Jeon I, Cheong C, Hwang TS, Chung H, Gwag BJ, et al. In vivo tracking of human mesenchymal stem cells in experimental stroke. Cell Transplant. 2008;16:1007–1012. [PubMed] [Google Scholar]
- 62.Bulte JW, Ben-Hur T, Miller BR, Mizrachi-Kol R, Einstein O, Reinhartz E, Zywicke HA, Douglas T, Frank JA. MR microscopy of magnetically labeled neurospheres transplanted into the Lewis EAE rat brain. Magn Reson Med. 2003;50:201–205. doi: 10.1002/mrm.10511. [DOI] [PubMed] [Google Scholar]
- 63.Ben-Hur T, van Heeswijk RB, Einstein O, Aharonowiz M, Xue R, Frost EE, Mori S, Reubinoff BE, Bulte JW. Serial in vivo MR tracking of magnetically labeled neural spheres transplanted in chronic EAE mice. Magn Reson Med. 2007;57:164–171. doi: 10.1002/mrm.21116. [DOI] [PubMed] [Google Scholar]
- 64.Cohen ME, Muja N, Fainstein N, Bulte JW, Ben-Hur T. Conserved fate and function of ferumoxides-labeled neural precursor cells in vitro and in vivo. J Neurosci Res. 2010;88:936–944. doi: 10.1002/jnr.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muja N, Cohen ME, Zhang J, Gilad AA, Walczak P, Ben-Hur T, Bulte JWM. Neural precursors exhibit distinctly different patterns of cell migration upon transplantation during either the acute or chronic phase of EAE: a serial MR imaging. Magn Reson Med. doi: 10.1002/mrm.22757. (published online Feb 8, 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neri M, Maderna C, Cavazzin C, Deidda-Vigoriti V, Politi LS, Scotti G, Marzola P, Sbarbati A, Vescovi AL, Gritti A. Efficient in vitro labeling of human neural precursor cells with superparamagnetic iron oxide particles: relevance for in vivo cell tracking. Stem Cells. 2008;26:505–516. doi: 10.1634/stemcells.2007-0251. [DOI] [PubMed] [Google Scholar]
- 67.Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, Capela A, Greve J, Malenka RC, Moseley ME, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flexman JA, Cross DJ, Tran LN, Sasaki T, Kim Y, Minoshima S. Quantitative analysis of neural stem cell migration and tracer clearance in the rat brain by MRI. Mol Imaging Biol. 2010;13:104–111. doi: 10.1007/s11307-010-0311-3. [DOI] [PubMed] [Google Scholar]
- 69.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 70.Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007;16:461–466. doi: 10.1089/scd.2007.0083. [DOI] [PubMed] [Google Scholar]
- 71.Toso C, Vallee JP, Morel P, Ris F, Demuylder-Mischler S, Lepetit-Coiffe M, Marangon N, Saudek F, James Shapiro AM, Bosco D, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8:701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 72.Balakumaran A, Pawelczyk E, Ren J, Sworder B, Chaudhry A, Sabatino M, Stroncek D, Frank JA, Robey PG. Superparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their “stemness”. PLoS One. 2010;5:e11462. doi: 10.1371/journal.pone.0011462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyoshi S, Flexman JA, Cross DJ, Maravilla KR, Kim Y, Anzai Y, Oshima J, Minoshima S. Transfection of neuroprogenitor cells with iron nanoparticles for magnetic resonance imaging tracking: cell viability, differentiation, and intracellular localization. Mol Imaging Biol. 2005;7:286–295. doi: 10.1007/s11307-005-0008-1. [DOI] [PubMed] [Google Scholar]
- 74.Sun R, Dittrich J, Le-Huu M, Mueller MM, Bedke J, Kartenbeck J, Lehmann WD, Krueger R, Bock M, Huss R, et al. Physical and biological characterization of superparamagnetic iron oxide- and ultrasmall superparamagnetic iron oxide-labeled cells: a comparison. Invest Radiol. 2005;40:504–513. doi: 10.1097/01.rli.0000162925.26703.3a. [DOI] [PubMed] [Google Scholar]
- 75.Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: the case of the shiverer dysmyelinated mouse brain. Magn Reson Med. 2007;58:261–269. doi: 10.1002/mrm.21280. [DOI] [PubMed] [Google Scholar]
- 76.Liu W, Frank JA. Detection and quantification of magnetically labeled cells by cellular MRI. Eur J Radiol. 2009;70:258–264. doi: 10.1016/j.ejrad.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Bos EJ, Baks T, Moelker AD, Kerver W, van Geuns RJ, van der Giessen WJ, Duncker DJ, Wielopolski PA. Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: possible interference with iron oxide-labelled cell tracking? Eur Heart J. 2006;27:1620–1626. doi: 10.1093/eurheartj/ehl059. [DOI] [PubMed] [Google Scholar]
- 78.Berman SC, Galpoththawela C, Gilad AA, Bulte JW, Walczak P. Long-term MR cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magn Reson Med. 2010;65:564–574. doi: 10.1002/mrm.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lepore AC, Walczak P, Rao MS, Fischer I, Bulte JW. MR imaging of lineage-restricted neural precursors following transplantation into the adult spinal cord. Exp Neurol. 2006;201:49–59. doi: 10.1016/j.expneurol.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 80.Baligand C, Vauchez K, Fiszman M, Vilquin JT, Carlier PG. Discrepancies between the fate of myoblast xenograft in mouse leg muscle and NMR label persistency after loading with Gd-DTPA or SPIOs. Gene Ther. 2009;16:734–745. doi: 10.1038/gt.2009.12. [DOI] [PubMed] [Google Scholar]
- 81.Chen IY, Greve JM, Gheysens O, Willmann JK, Rodriguez-Porcel M, Chu P, Sheikh AY, Faranesh AZ, Paulmurugan R, Yang PC, et al. Comparison of optical bioluminescence reporter gene and superparamagnetic iron oxide MR contrast agent as cell markers for non-invasive imaging of cardiac cell transplantation. Mol Imaging Biol. 2009;11:178–187. doi: 10.1007/s11307-008-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schar M, Gerstenblith G, Weiss RG, Marban E, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 83.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229:838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- 84.Soenen SJ, Vercauteren D, Braeckmans K, Noppe W, De Smedt S, De Cuyper M. Stable long-term intracellular labelling with fluorescently tagged cationic magnetoliposomes. Chembiochem. 2009;10:257–267. doi: 10.1002/cbic.200800510. [DOI] [PubMed] [Google Scholar]
- 85.Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11:450–454. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- 86.Gilad AA, McMahon MT, Walczak P, Winnard PT, Jr, Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007;25:217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 87.Gilad AA, Winnard PT, Jr, van Zijl PC, Bulte JW. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20:275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 88.Zurkiya O, Chan AW, Hu X. MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn Reson Med. 2008;59:1225–1231. doi: 10.1002/mrm.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldhawk DE, Lemaire C, McCreary CR, McGirr R, Dhanvantari S, Thompson RT, Figueredo R, Koropatnick J, Foster P, Prato FS. Magnetic resonance imaging of cells overexpressing MagA, an endogenous contrast agent for live cell imaging. Mol Imaging. 2009;8:129–139. [PubMed] [Google Scholar]