Abstract
Eukaryotic proliferating cell nuclear antigen (PCNA) is a replication accessory protein that functions in DNA replication, repair, and recombination. The various functions of PCNA are regulated by post-translational modifications including mono-ubiquitylation, which promotes translesion synthesis, and sumoylation, which inhibits recombination. To understand how the SUMO modification regulates PCNA, we generated a split SUMO-modified PCNA protein and showed that it supports cell viability and stimulates DNA polymerase δ activity. We then determined its X-ray crystal structure and found that SUMO occupies a position on the back face of the PCNA ring, which is distinct from the position occupied by ubiquitin in the structure of ubiquitin-modified PCNA. We propose that the back of PCNA has evolved to be a site of regulation that can be easily modified without disrupting ongoing reactions on the front of PCNA, such as normal DNA replication. Moreover, these modifications likely allow PCNA to function as a tool belt, whereby proteins can be recruited to the replication machinery via the back of PCNA and be held in reserve until needed.
Keywords: DNA replication, DNA recombination, DNA repair, protein-DNA interactions, translesion synthesis
Eukaryotic proliferating cell nuclear antigen (PCNA) is a replication accessory factor that is involved in many nuclear processes including DNA replication, repair, recombination, translesion synthesis, and chromatin remodeling. PCNA is a homotrimer, and each subunit has two domains giving the PCNA complex a ring shape with pseudo-six-fold symmetry1. The PCNA ring is loaded onto DNA by an ATP-dependent clamp loader called replication factor C (RFC)2; 3. Once on DNA, PCNA functions as a sliding clamp to recruit many proteins to the DNA including DNA polymerases pol δ and pol ε, DNA ligase I, flap endonuclease Fen1, and DNA repair factor XPG4; 5; 6; 7; 8; 9. PCNA enhances the catalytic activity of some of these enzymes, and is required for the high processivity of replicative DNA polymerases. Nearly all of these proteins contain a conserved motif referred to as the PCNA-interaction protein (PIP) motif that binds to the front face of the PCNA ring in a hydrophobic pocket near the interdomain connector loop (IDCL), an extended loop connecting the two PCNA domains. Through these interactions, PCNA coordinates the complex events of DNA replication, repair, and recombination.
The various functions of PCNA are controlled by post-translational modifications10; 11; 12; 13; 14. PCNA is mono-ubiquitylated on Lys-164 by the E2 ubiquitin-conjugating enzyme Rad6 and the E3 ubiquitin ligase Rad18 in a DNA damage-dependent manner15; 16. PCNA ubiquitylation facilitates translesion synthesis by recruiting non-classical DNA polymerases, such as pol η, pol ι, and pol κ, which all possess ubiquitin-binding motifs17; 18. The single ubiquitin moiety on Lys-164 of PCNA can be converted into a poly-ubiquitin chain through Lys-63 linkages by the E2 ubiquitin conjugating enzyme Mms2-Ubc13 and the E3 ubiquitin ligase Rad519; 20; 21. PCNA poly-ubiquitylation facilitates a poorly characterized, error-free damage bypass pathway. PCNA is also sumoylated on Lys-164 by the E2 SUMO conjugating enzyme Ubc9 and the E3 SUMO ligase Siz115; 16. PCNA sumoylation prevents unwanted DNA recombination during DNA replication by recruiting the Srs2 helicase22; 23, which contains a SUMO binding motif and catalyzes the disruption of Rad51 nucleoprotein filaments24; 25.
Biochemical and structural studies of PCNA with post-translational modifications has progressed slowly because of the difficulty obtaining sufficient quantities of the modified protein. Recently, several approaches to producing large quantities of ubiquitin-modified PCNA (UbiPCNA) have been developed involving intein chemistry and chemical crosslinking26; 27. Previously, we developed a very efficient strategy to produce large quantities of UbiPCNA by co-expressing the modified protein as two polypeptide fragments that self-assemble in vivo28. We showed that the resultant split UbiPCNA functions to support translesion synthesis both in vivo and in vitro. Moreover, we determined the X-ray crystal structure of UbiPCNA28. This structure showed that the ubiquitin moiety sits on the back face of the PCNA ring and does not alter the structure of PCNA. Instead, it provides an additional binding surface to which the non-classical polymerases can be recruited.
Here we describe the production of large quantities of SUMO-modified PCNA (SUMOPCNA) by co-expressing the protein as two self-assembling polypeptides. We demonstrate that the split SUMOPCNA supports cell viability as well as the activity of classical pol δ in vitro. We have also determined the X-ray crystal structure of SUMOPCNA to a resolution of 2.8 Å. We found that the attachment of SUMO to PCNA, like the attachment of ubiquitin, does not alter the structure of PCNA. Both the ubiquitin moiety in the UbiPCNA structure and the SUMO moiety in the SUMOPCNA structure are located on the back face of the PCNA ring and interact with the same loop of PCNA. Despite these similarities, however, the ubiquitin and SUMO modifications occupy distinctly different positions on PCNA. We propose that the back face of PCNA has evolved to be a site of regulation that can be easily modified without disrupting ongoing reactions on the front face of the ring, such as normal DNA replication. Moreover, these modifications likely allow PCNA to function as a tool belt, whereby proteins can be recruited to the replication machinery via the back face of PCNA and be held in reserve until needed.
Production and characterization of SUMO-modified PCNA
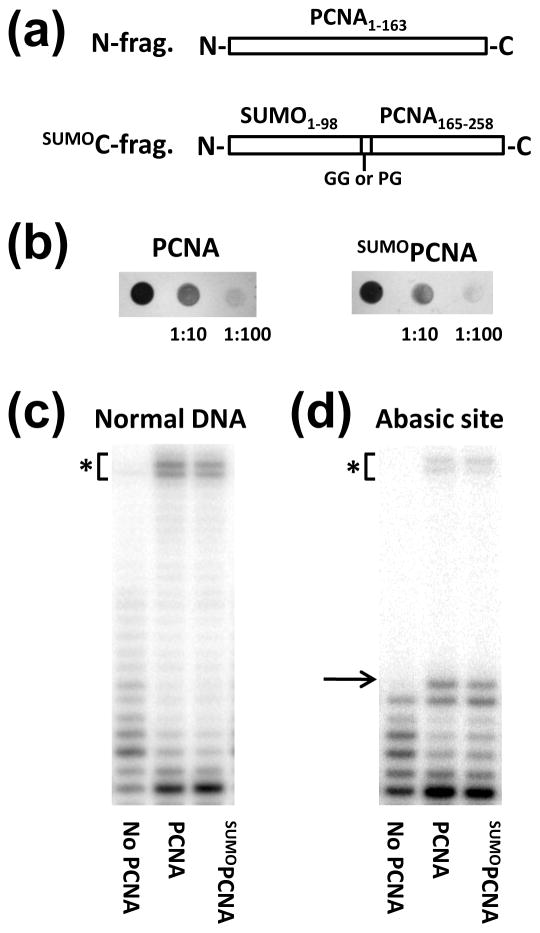
To produce SUMOPCNA, we used a strategy similar to one previously used to generate UbiPCNA28. The SUMOPCNA was made by splitting the protein into two fragments and co-expressing the genes for these fragments in either bacteria for over-expression and protein purification or yeast for in vivo functional studies (Fig. 1a). The N-fragment comprised yeast PCNA residues 1 to 163, and the SUMOC-fragment comprised yeast PCNA residues 165 to 258 fused in frame following the yeast SUMO protein. For expression in bacteria, a two-glycine linker was placed between the SUMO and PCNA portions of the SUMOC-fragment, because it is a good mimic of the Lys-164 side chain and the isopeptide bond to the C-terminus of SUMO. For expression in yeast, a proline-glycine linker was used instead to prevent the removal of the SUMO moiety by isopeptidases. When produced in either bacteria or yeast, the N-fragment and the SUMOC-fragment self-assembled to yield split SUMOPCNA (see below).
Figure 1. Production and characterization of SUMO-modified PCNA.
(a) Diagram of the two polypeptides used to make split SUMOPCNA. (b) SUMOPCNA supports cell viability. The coding regions of full length PCNA or the N-fragment were cloned into pRS315 (CEN LEU2) with a 500 base pair upstream region containing the native PCNA promoter. The coding region of the SUMOC-fragment with the proline-glycine linker was cloned into pRS313 (CEN HIS3) under control of the native PCNA promoter. These constructs were transformed into an EMY74.7 yeast strain with a POL30 gene deletion supported by the wild-type POL30 gene in pTB366 (URA3) under control of its native promoter as described28. Cell growth following counterselection with 5′-fluoroorotic acid showed that the SUMOPCNA constructs support cell viability. Shown are the results of spotting 10 μl of saturated yeast cultures, 1:10 dilutions, and 1:100 dilutions on selective growth media overnight. (c) SUMOPCNA confers high processivity to pol δ. SUMOPCNA was expressed in Rosetta-2 (DE3) cells from a pET-DUET-1 vector in which the Flag-tagged N-fragment was inserted into multi-cloning site 1 and the His6-tagged SUMOC fragment with the glycine-glycine linker was inserted into multi-cloning site 2. SUMOPCNA was purified using an NTA-agarose affinity chromatography column (Qiagen), anti-Flag M2 affinity chromatography column (Sigma), and a Superose 6 size exclusion chromatography column (Pharmacia GE Healthcare). We obtained ∼2–3 mg of pure protein per L of cells. Polymerase assays were carried out in the absence of PCNA and in the presence of unmodified PCNA (100 nM), or SUMOPCNA (100 nM) as described previously31. The reactions contained 50 nM of trimeric pol δ (exo+), which was purified as described42, 25 nM DNA, and 100 μM dNTP and were stopped after 30 min. The asterisk indicates gel bands corresponding to full length products. (d) SUMOPCNA increases the ability of pol δ to incorporate nucleotides opposite a template abasic site. Reactions were carried out as described above. The arrow indicates the gel band corresponding to incorporation opposite the abasic site, and the asterisk indicates gel bands corresponding to full length products.
We first produced split SUMOPCNA in yeast cells by replacing the endogenous PCNA protein with the N-fragment and SUMOC-fragment by plasmid shuffle. Expression of the two SUMOPCNA fragments was driven by the native PCNA promoter. We found that SUMOPCNA supported cell viability, and cell growth was unaffected when it was the only form of PCNA in the cell (Fig. 1b). These results show that the split SUMOPCNA self-assembles into functional rings in yeast cells and supports normal DNA replication and cell cycle progression in vivo.
We next over-expressed both fragments of split SUMOPCNA in bacterial cells and found that the two fragments of SUMOPCNA co-purified. Size exclusion chromatography showed that SUMOPCNA formed stable trimers with a Stokes radius equal to 50 Å, which is the same as the Stokes radius of UbiPCNA28. For comparison, the Stokes radius of unmodified PCNA is 45 Å. To determine if SUMOPCNA enhanced the processivity of classical pol δ in vitro, we used RFC to load either unmodified, full length PCNA or split SUMOPCNA onto a DNA primer-template substrate. The primer strand was 32P end labeled, and both ends of the template strand were blocked with biotin-streptavidin to prevent PCNA from sliding off of the DNA ends. After PCNA or SUMOPCNA was loaded, the reactions were initiated by the addition of pol δ and all four nucleotides, and the products were analyzed by gel electrophoresis. Fig. 1c shows that in the absence of PCNA, pol δ synthesizes DNA with low processivity forming only short DNA products. In the presence of either PCNA or SUMOPCNA, the processivity of pol δ is greatly enhanced, and ∼50% of the extended products are full length.
We also examined whether SUMOPCNA enhanced the ability of pol δ to incorporate nucleotides opposite a template abasic site, as has been shown previously for unmodified PCNA29. We carried out the experiment as described above, except that the DNA substrate contained an abasic site in the template strand at the sixth position from the primer terminus. Fig. 1d shows that in the absence of PCNA, pol δ incorporates up to five nucleotides, but does not efficiently incorporate opposite the abasic site. In the presence of either PCNA or SUMOPCNA, efficient incorporation opposite the abasic site was observed, and ∼5% of the extended products are full length. Taken together, these results demonstrate clearly that the SUMOPCNA produced in this manner is loaded onto DNA by RFC and supports the activity of classical pol δ in vitro.
Structure of SUMO-modified PCNA
Given that SUMOPCNA was previously shown to interact with the Srs2 helicase22; 23, we fully expect that split SUMOPCNA will interact with Srs2. While a thorough characterization of split SUMOPCNA’s interactions and functions will be left for the future, we have shown here that split SUMOPCNA supports cell viability and DNA synthesis by pol δ. Thus, we proceeded to determine the X-ray crystal structure of SUMOPCNA to a resolution of 2.8 Å (Table 1). The SUMOPCNA protein formed cubic crystals that resembled those obtained previously with unmodified PCNA and UbiPCNA1; 28; the space group for the SUMOPCNA crystals, however, was different from the space group of the crystals of these other PCNA forms. Phases were determined by molecular replacement using the structure of unmodified, full-length PCNA1. Following molecular replacement, the electron density of the SUMO moiety was clear and considerably stronger than the density for the ubiquitin moiety observed previously with UbiPCNA28. This suggests that the SUMO moiety in SUMOPCNA may not be as flexible and mobile as is the ubiquitin moiety in UbiPCNA.
Table 1.
Data collection and refinement statistics.
| Data collection | |
| Space group | F432 |
| Cell dimensions (Å) | a = b = c = 268.82 |
| Resolution (Å) | 45.4 - 2.8 (2.9 - 2.8) |
| Rmerge (%) | 8.9 (43.3) |
| I/σI | 14.8 (4.4) |
| Completeness (%) | 100 (99.5) |
| Redundancy | 12.6 (8.8) |
| Refinement | |
| Resolution (Å) | 45.5 – 2.8 |
| Number of reflections | 20832 |
| Rwork/Rfree (%) | 23.1/25.3 |
| Number of atoms | |
| Protein | 2649 |
| Water | 0 |
| B-factors (Å2) | 78.7 |
| r.m.s.d. | |
| Bond lengths (Å) | 0.012 |
| Bond angles (°) | 1.450 |
Crystallization was performed manually using the hanging drop method. The best diffracting crystals were obtained by combining equal volumes of protein (20 mg/ml) with a reservoir solution containing 2.0 M ammonium sulfate and 0.1 M sodium citrate (pH 5.6) at 18°C for 5 days. Protein crystals were presoaked in a mother liquor containing 25% (v/v) ethylene glycol before flash cooling in liquid nitrogen. Data was collected at 100 K at the 4.2.2. beamline at the Advanced Light Source in the Lawrence Berkeley National Laboratory with a crystal-to-detector distance of 150 mm. The data were processed and scaled using d*TREK36. Molecular replacement was performed using PHASER37 first with the structure of the full length, unmodified PCNA (PDB 1PLQ)1 and then with the structure of a SUMO mimic (PDB 3GOE)38. Simulated annealing was performed to remove any structural bias using PHENIX39 before refinement with REFMAC5 from the CCP4 package40. Model building was performed using Coot41. Ramachandran analysis showed that 93% of the residues were in favored conformations and 7% were in allowed conformations. Data collection values in parentheses are for the highest resolution shell.
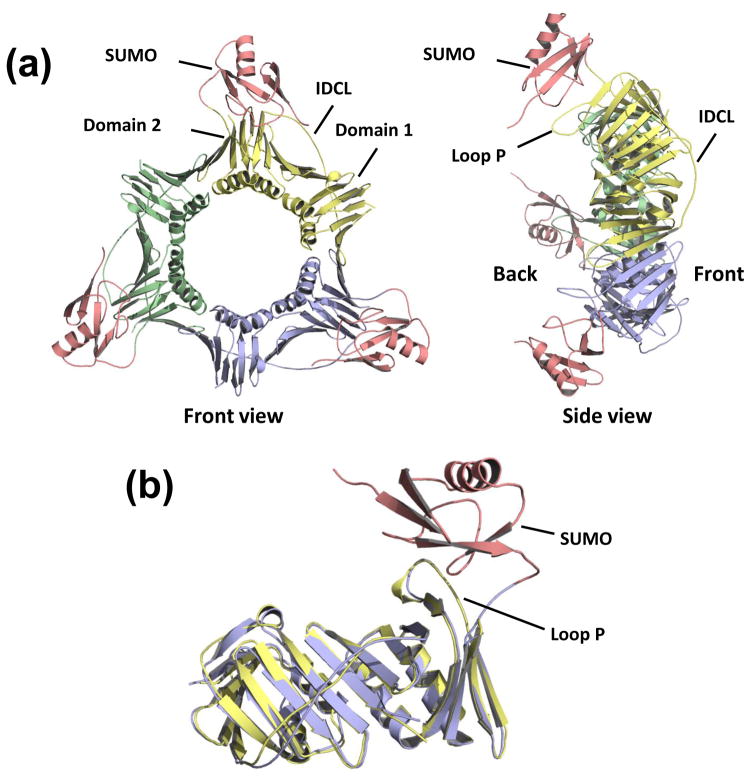
The overall structure of the PCNA portion of the SUMOPCNA is the same as that of unmodified PCNA (Fig. 2a). The PCNA subunits contain two domains (domain 1 comprising residues 1–118 and domain 2 comprising residues 135–258) connected by the long interdomain connector loop (IDCL; residues 119–134) on the front side of the ring. The SUMO moiety sits on the back face of the PCNA ring and interacts exclusively with domain 2 of PCNA. Most of the residues of PCNA contacting the SUMO are in an extended loop in domain 2 called loop P (residues 184–196). Incidentally, the extended N-terminal tail of the SUMO moiety (residues 1–18) is disordered and has not been modeled. To determine if the attachment of SUMO to PCNA alters the structure of PCNA, we overlaid the structures of SUMOPCNA and unmodified, full length PCNA (Fig. 2b). The r.m.s. deviation between these two structures was 0.8 Å over 254 Cα atoms indicating that no significant structural changes to PCNA resulted when SUMO was attached. Careful comparisons of these structures also revealed no substantial local changes in the PCNA conformation.
Figure 2. Structure of SUMO-modified PCNA.
(a) Front and side view of the structure of the SUMOPCNA trimer with the individual PCNA subunits colored yellow, blue, and green. The SUMO moieties are colored red. (b) The structure of a single subunit of SUMOPCNA, which is colored blue (PCNA) and red (SUMO) is shown superimposed on the structure of full length, unmodified PCNA, which is colored yellow.
Comparison of SUMO-modified and ubiquitin-modified PCNA
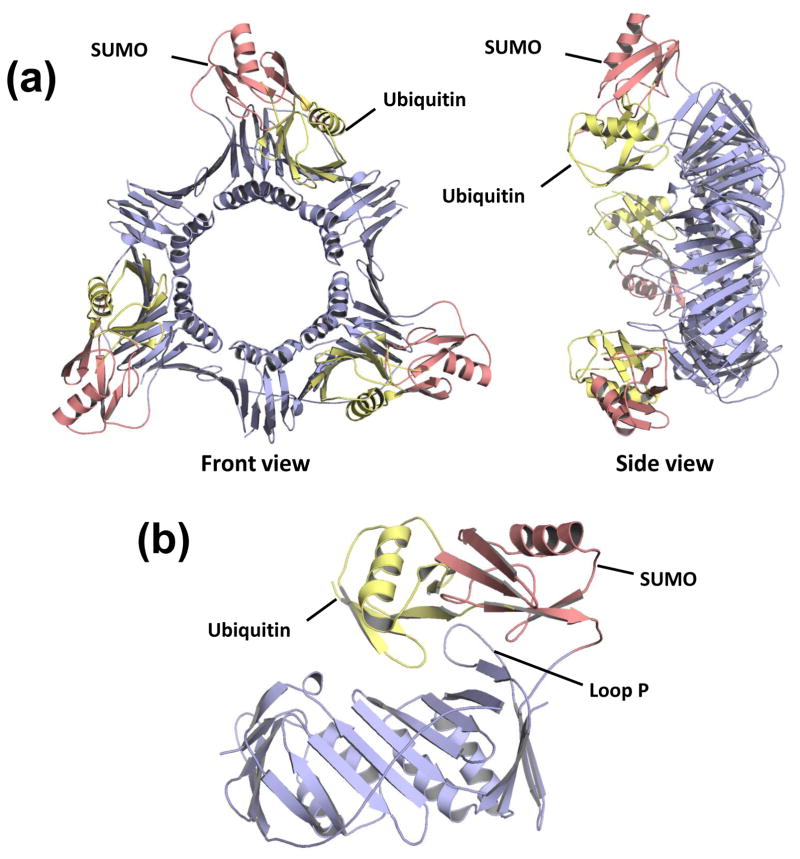
Both ubiquitin and SUMO are attached to PCNA on Lys-164, and these modifications result in PCNA forms with different functions. To better understand the similarities and differences between these modified forms of PCNA, we overlaid the structures of UbiPCNA and SUMOPCNA (Fig. 3a). In both cases, the ubiquitin and SUMO modifiers sit on the back face of the PCNA ring interacting with domain 2. In fact, both modifiers interact predominantly with loop P of PCNA (Fig. 3b). The positions of the modifiers, however, are quite distinct. The SUMO in the SUMOPCNA structure is situated in a more radial position with respect to the PCNA ring than is the ubiquitin in the UbiPCNA structure, which essentially points straight back from the ring. This difference in position may be due in part to the longer flexible linker at the C-terminus of ubiquitin compared to SUMO that allows the ubiquitin to occupy positions further from the point of attachment at Lys-164 on PCNA.
Figure 3. Comparison of SUMO-modified PCNA with ubiquitin-modified PCNA.
(a) Front and side view of the SUMOPCNA trimer, which is colored blue (PCNA) and red (SUMO), is shown superimposed on the structure of the ubiquitin moieties from UbiPCNA, which are colored yellow. (b) The structure of a single subunit of SUMOPCNA, which is colored blue (PCNA) and red (SUMO), is shown superimposed on the structure of ubiquitin from UbiPCNA, which is shown in yellow.
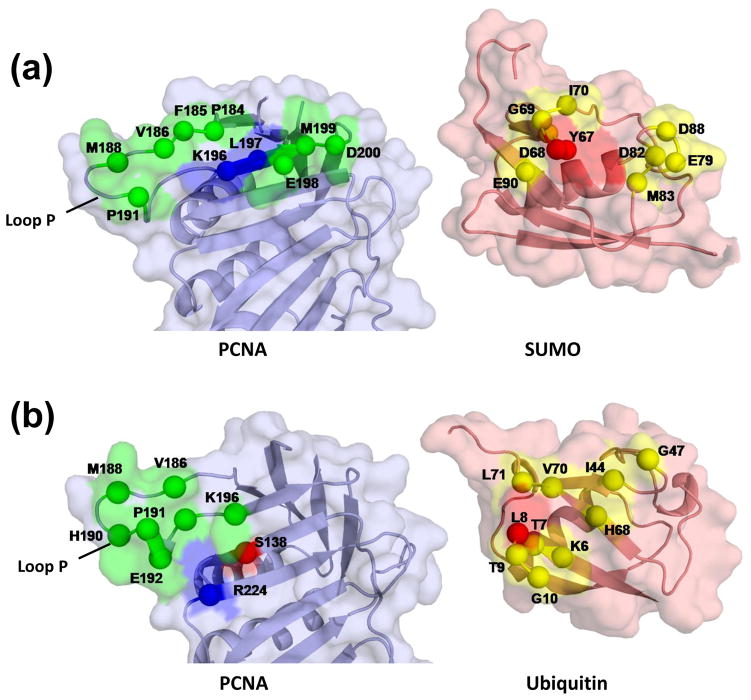
We have examined the contacts between the SUMO moiety and PCNA (Fig. 4a). The residues of PCNA that contact SUMO in the SUMOPCNA structure are part of a continuous stretch of the PCNA protein starting from the end of β strand D2 (residues 175–183) and encompassing nearly all of loop P (residues 184–195) and β strand E2 (residues 196–199). While most of these contacts are hydrophobic, there are hydrogen bonding interactions between the side chain nitrogen of Lys-196 (PCNA) and the side chain oxygen of Tyr-67 (SUMO) and between the backbone amide nitrogen of Leu-197 (PCNA) and the backbone carbonyl oxygen of Asp-68 (SUMO).
Figure 4. Interactions between the SUMO moiety and PCNA.
(a) Space-filling representation of the SUMO-PCNA interface with amino acid residues on PCNA making hydrophobic contacts with SUMO shown in green and with amino acid residues on SUMO making contacts with PCNA shown in yellow. Residues forming hydrogen bonds are shown in blue (hydrogen bond donors) and red (hydrogen bond acceptors). Both the PCNA and SUMO proteins have been rotated to make their interacting surfaces visible. (b) The analogous space-filling representation of the ubiquitin-PCNA interface, adapted from28.
Several of the residues in loop P also make contacts with ubiquitin in the UbiPCNA structure (Fig. 4b); ubiquitin, however, does not contact residues in β strands D2 or E2 as does SUMO, but it does contact other neighboring secondary structural elements in PCNA that SUMO does not. The residues of SUMO that contact PCNA in the SUMOPCNA structure are essentially those along two loops (residues 67 to 70 and residues 79 to 83). These residues are on a different surface of SUMO than the analogous residues of ubiquitin that contact PCNA in the UbiPCNA structure. Thus the orientations of the SUMO in the SUMOPCNA structure and ubiquitin in the UbiPCNA structure are quite distinct.
Implications for regulating PCNA function
The attachment of ubiquitin or SUMO to Lys-164 of PCNA recruits proteins factors to the replication machinery that influence the choice of pathways – DNA replication, repair, or recombination – in which PCNA participates10; 11; 12; 13. Ubiquitylation of PCNA recruits non-classical polymerases thereby facilitating translesion synthesis. Sumoylation of PCNA recruits the Srs2 helicase thereby preventing unwanted recombination. In both cases, it is possible that the recruitment of these factors could arise by allosteric changes in PCNA induced by the modification that enhances the binding affinity of these factors. Alternatively, the recruitment of these factors could arise by directly binding to the modifiers themselves. In both the structure of SUMOPCNA reported here and the structure of UbiPCNA reported previously28, no significant structural changes in PCNA resulting from the attachment of the modifiers is observed. Both of these results suggest that PCNA does not undergo allosteric changes in response to post-translational modifications. Instead, it seems likely that the ubiquitin and SUMO modifications simply provide additional binding sites for other factors: non-classical polymerases in the case of ubiquitin and Srs2 in the case of SUMO.
The front face of the PCNA ring is well known for its ability to interact with many proteins involved in DNA replication, repair, and recombination4; 5; 6; 7; 8; 9. The role of the back face of PCNA, by contrast, has been less appreciated. It is now becoming clear that the back face of PCNA plays an important role in regulating PCNA function. Both SUMO and ubiquitin modifiers are attached to Lys-164, which is located on the back face of PCNA near the side of the ring. In the structures of both SUMOPCNA and UbiPCNA, the modifiers sit on the back face of the PCNA ring on opposite sides of loop P. Structure-function studies have shown that residues of loop P are necessary for PCNA sumoylation, and it has been suggested that the Siz1 E3 ligase likely interacts with this loop30. Another loop on the back of PCNA, loop J (residues 105–110), is necessary for translesion synthesis31, and it has been suggested that alterations to this loop may interfere with PCNA ubiquitylation32. Taken together, this suggests a division of labor between the front face of the PCNA ring, which interacts with many proteins needed for various DNA metabolic pathways, and the back face of the PCNA ring, where post-translational modifications to PCNA occur that regulate its function.
This division of labor between the front and back faces of PCNA is important for several reasons. First, it allows for post-translational modifications to occur on the back face of PCNA without requiring proteins to dissociate from the front face. Essentially, modification to the back face of PCNA can readily occur without disrupting ongoing reactions on the front face of the PCNA, such as normal DNA replication. Second, it provides an opportunity for PCNA to function as a tool belt, whereby multiple proteins can simultaneously bind to the PCNA ring. Specific tool belt mechanisms have been proposed for translesion synthesis33; 34; 35, but such mechanism can, in fact, be more general and apply to other PCNA-mediated processes. In translesion synthesis, for example, the non-classical polymerase could be recruited to the back face of the UbiPCNA ring via interactions with ubiquitin while the classical polymerase synthesizes DNA on the front face of the PCNA ring. In this scenario, the non-classical is held in reserve until it is needed when the replication fork encounters DNA damage. A similar model may hold for SUMOPCNA and Srs2. Srs2 could be recruited to the back face of SUMOPCNA via interactions with SUMO while the classical polymerase is bound to the front. When Rad51 nucleoprotein filaments are encountered by the replication machinery, Srs2 can be brought out of reserve to disrupt these filaments. Tool belt mechanisms such as these would allow replication forks to prepare for various contingencies by having the necessary factors in place on the back face of PCNA, yet not hinder the reactions that are ongoing on the front face of PCNA.
Acknowledgments
The project described was supported by Award Number R01GM081433 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
We thank Lynne Dieckman, John Pryor, and Marc Wold for valuable discussions. We also thank Jay Nix at the 4.2.2. beamline at Advanced Light Source at the Lawrence Berkley National Laboratory for help with diffraction data collection.
Footnotes
Accession numbers
Atomic coordinates and structure factors have been deposited in the Protein Data Bank under accession code 3PGE.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krishna TSR, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 2.Mossi R, Hubscher U. Clamping down on clamps and clamp loaders - The eukaryotic replication factor C. European Journal of Biochemistry. 1998;254:209–216. [PubMed] [Google Scholar]
- 3.Majka J, Burgers PMJ. The PCNA-RFC families of DNA clamps and clamp loaders. Progress in Nucleic Acid Research and Molecular Biology. 2004;78:227–260. doi: 10.1016/S0079-6603(04)78006-X. [DOI] [PubMed] [Google Scholar]
- 4.Warbrick E. PCNA binding through a conserved motif. Bioessays. 1998;20:195–199. doi: 10.1002/(SICI)1521-1878(199803)20:3<195::AID-BIES2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Warbrick E. The puzzle of PCNA’s many partners. Bioessays. 2000;22:997–1006. doi: 10.1002/1521-1878(200011)22:11<997::AID-BIES6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani MM, O’Donnell M. Sliding clamps: A (tail)ored fit. Current Biology. 2000;10:R25–R29. doi: 10.1016/s0960-9822(99)00252-3. [DOI] [PubMed] [Google Scholar]
- 7.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. Journal of Cell Science. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 8.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Tsurimoto T. PCNA binding proteins. Frontiers in Bioscience. 1999;4:d849–858. doi: 10.2741/tsurimoto. [DOI] [PubMed] [Google Scholar]
- 10.Ulrich HD. Regulating post-translational modifications of the eukaryotic replication clamp PCNA. DNA Repair. 2009;8:461–469. doi: 10.1016/j.dnarep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich HD, Vogel S, Davies AA. SUMO keeps a check on recombination during DNA replication. Cell Cycle. 2005;4:1699–1702. doi: 10.4161/cc.4.12.2194. [DOI] [PubMed] [Google Scholar]
- 12.Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nature Reviews Molecular Cell Biology. 2010;11:479–489. doi: 10.1038/nrm2921. [DOI] [PubMed] [Google Scholar]
- 13.Watts FZ. Sumoylation of PCNA: Wrestling with recombination at stalled replication forks. DNA Repair. 2006;5:399–403. doi: 10.1016/j.dnarep.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458:461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 15.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 16.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 17.Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, Hofmann K, Dikic I. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science. 2005;310:1821–1824. doi: 10.1126/science.1120615. [DOI] [PubMed] [Google Scholar]
- 18.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: A possible mechanism for the polymerase switch in response to DNA damage. Molecular Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 19.Broomfield S, Chow BL, Xiao W. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5678–5683. doi: 10.1073/pnas.95.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich HD, Jentsch S. Two RING finger proteins mediate cooperation between ubiquitin-conjugating enzymes in DNA repair. Embo Journal. 2000;19:3388–3397. doi: 10.1093/emboj/19.13.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- 23.Papouli E, Chen SH, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Molecular Cell. 2005;19:123–133. doi: 10.1016/j.molcel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Krejci L, Macris M, Li Y, Van Komen S, Villemain J, Ellenberger T, Klein H, Sung P. Role of ATP hydrolysis in the antirecombinase function of Saccharomyces cerevisiae Srs2 protein. Journal of Biological Chemistry. 2004;279:23193–23199. doi: 10.1074/jbc.M402586200. [DOI] [PubMed] [Google Scholar]
- 25.Veaute X, Jeusset J, Soustelle C, Kowalczykowski SC, Le Cam E, Fabre F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature. 2003;423:309–312. doi: 10.1038/nature01585. [DOI] [PubMed] [Google Scholar]
- 26.Chen JJ, Ai YX, Wang JL, Haracska L, Zhuang ZH. Chemically ubiquitylated PCNA as a probe for eukaryotic translesion DNA synthesis. Nature Chemical Biology. 2010;6:270–272. doi: 10.1038/nchembio.316. [DOI] [PubMed] [Google Scholar]
- 27.Carlile CM, Pickart CM, Matunis MJ, Cohen RE. Synthesis of Free and Proliferating Cell Nuclear Antigen-bound Polyubiquitin Chains by the RING E3 Ubiquitin Ligase Rad5. Journal of Biological Chemistry. 2009;284:29326–29334. doi: 10.1074/jbc.M109.043885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freudenthal BD, Gakhar L, Ramaswamy S, Washington MT. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nature Structural & Molecular Biology. 2010;17:479–U123. doi: 10.1038/nsmb.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg P, Stith CM, Majka J, Burgers PMJ. Proliferating cell nuclear antigen promotes translesion synthesis by DNA polymerase zeta. Journal of Biological Chemistry. 2005;280:23446–23450. doi: 10.1074/jbc.C500173200. [DOI] [PubMed] [Google Scholar]
- 30.Yunus AA, Lima CD. Structure of the Siz/PIAS SUMO E3 Ligase Siz1 and Determinants Required for SUMO Modification of PCNA. Molecular Cell. 2009;35:669–682. doi: 10.1016/j.molcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freudenthal BD, Ramaswamy S, Hingorani MM, Washington MT. Structure of a Mutant Form of Proliferating Cell Nuclear Antigen That Blocks Translesion DNA Synthesis. Biochemistry. 2008;47:13354–13361. doi: 10.1021/bi8017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HS, Gibbs PEM, Lawrence CW. The Saccharomyces cerevisiae rev6-1 mutation, which inhibits both the lesion bypass and the recombination mode of DNA damage tolerance, is an allele of POL30, encoding proliferating cell nuclear antigen. Genetics. 2006;173:1983–1989. doi: 10.1534/genetics.106.058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Indiani C, McInerney P, Georgescu R, Goodman MF, O’Donnell M. A sliding-clamp toolbelt binds high-and low-fidelity DNA polymerases simultaneously. Molecular Cell. 2005;19:805–815. doi: 10.1016/j.molcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Sutton MD. Coordinating DNA polymerase traffic during high and low fidelity synthesis. Biochimica Et Biophysica Acta-Proteins and Proteomics. 2010;1804:1167–1179. doi: 10.1016/j.bbapap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang ZH, Ai YX. Processivity factor of DNA polymerase and its expanding role in normal and translesion DNA synthesis. Biochimica Et Biophysica Acta-Proteins and Proteomics. 2010;1804:1081–1093. doi: 10.1016/j.bbapap.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pflugrath JW. The finer things in X-ray diffraction data collection. Acta Crystallographica Section D-Biological Crystallography. 1999;55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 37.Read RJ. Pushing the boundaries of molecular replacement with maximum likelihood. Acta Crystallographica Section D-Biological Crystallography. 2001;57:1373–1382. doi: 10.1107/s0907444901012471. [DOI] [PubMed] [Google Scholar]
- 38.Prudden J, Perry JJP, Arvai AS, Tainer JA, Boddy MN. Molecular mimicry of SUMO promotes DNA repair. Nature Structural & Molecular Biology. 2009;16:509–516. doi: 10.1038/nsmb.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallographica Section D-Biological Crystallography. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 40.Bailey S. The Ccp4 Suite - Programs for Protein Crystallography. Acta Crystallographica Section D-Biological Crystallography. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 41.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallographica Section D-Biological Crystallography. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 42.Dieckman LM, Johnson RE, Prakash S, Washington MT. Pre-Steady State Kinetic Studies of the Fidelity of Nucleotide Incorporation by Yeast DNA Polymerase delta. Biochemistry. 2010;49:7344–7350. doi: 10.1021/bi100556m. [DOI] [PMC free article] [PubMed] [Google Scholar]