Abstract
Amniotic membrane (AM) used as a temporary or permanent graft for ocular surface reconstruction has a potent anti-inflammatory effect. We would like to investigate the mechanism whereby AM induces macrophage apoptosis in vitro. Mouse macrophages, Raw 264.7 cells, were cultured on plastic, type I collagen, corneal stromal slice or AM stromal matrix in serum-free medium with or without interferon-γ (IFN-γ). Cells were stained by LIVE/DEAD assay, Hoechst-33342, and TUNEL assay for cell death and apoptosis. Cell lysates and conditioned media were analysed by Cell Death Detection ELISA assay for quantitation of apoptosis. Conditioned media were also analysed by Griess assay for the nitrite concentration and ELISA assay for tumour necrosis factor alpha (IFN-α) concentration. Lysates of cells were subjected to Western blot analyses of IKK-α, IKK-β, p65 (RelA) subunit of nuclear factor κB (NF-κB), total Akt, phospho-Akt (Ser473), and phospho-FKHR (Thr24)/phosphor-FKHRL1 (Thr32). At 48 hr after cultivation, cells showed a low level of apoptosis when cultured on plastic, type I collagen and corneal stromal slice with or without IFN-γ and on AM without IFN-γ. Nevertheless, cells showed a significant increase of apoptosis when cultured on AM with IFN-γ activation, and this phenomenon became apparent only after 48 hr. IFN-γ-activated macrophages on plastic continuously produced nitric oxide (NO) and IFN-α during 72 hr culturing. In contrast, there was no NO and IFN-α production after 48 hr culture on AM. NO inhibitors, L-NMMA and L-NIL, attenuated NO production of IFN-γ-activated macrophages on AM, while apoptosis was not decreased accordingly. Expression of IKK-α, IKK-β, p65 (RelA) subunit of NF-κB total Akt, phosopho-Akt (Ser473), and phospho-FKHR (Thr24)/FKHRL1 (Thr32) was all down-regulated in IFN-γ-activated macrophages cultured on AM. In conclusion, AM stromal matrix induces apoptosis of IFN-γ activated, but not non-activated macrophages, not through the generation of NO, but instead by down-regulating anti-apoptotic NF-κB and Akt-FKHR signalling pathways.
Keywords: amniotic membrane, apoptosis, interferon-γ, macrophages
1. Introduction
Amniotic membrane (AM), the innermost layer of the placental membrane, consists of a simple epithelium, a thick basement membrane and a subjacent avascular stroma. AM used as a temporary or permanent graft has become a popular surgical procedure for ocular surface reconstruction (for reviews see (Tseng, 2002; Dua et al., 2004)). One notable clinical effect is that inflammation is suppressed on AM-covered ocular surface. Experimentally, several in vivo studies have demonstrated the anti-inflammatory action of AM. In a murine model of HSV-1 necrotizing keratitis, application of human AM as a temporary graft for 2 days induces rapid regression of corneal stromal edema and inflammation, and reduces infiltration of lymphocytes and macrophages in the corneal stroma (Heiligenhaus et al., 2001). In a rabbit model of excimer laser ablation, human AM as a temporary graft for 24 hr reduces infiltration of polymorphonuclear (PMN) cells in the ablated stroma while PMNs adherent to AM stroma undergo apoptosis (Park and Tseng, 2000; Wang et al., 2001). Clinically, Shimmura et al. (2001) removed AM one week after being placed as a temporary AM graft for treating persistent corneal epithelial defects or in conjunction with keratolimbal allograft, and disclosed that most monocytes and macrophages trapped by AM exhibit characteristics of apoptosis.
Therefore, it is tempting to speculate that one of the mechanisms to explain AM’s anti-inflammatory action is mediated by targeting macrophages. Activated macrophages play a pivotal role in initiating, maintaining and resolving host inflammatory responses (for reviews see (Singer and Clark, 1999)). Although known to kill viruses, bacteria and parasites, and to act as scavenger cells, macrophages also exert deleterious effects on the host by producing excessive free radicals, lytic enzymes and inflammatory cytokines, collectively aggravating tissue damage and being responsible for many of the systemic symptoms associated with acute and chronic inflammation (for reviews see (Duffield, 2003)). In excimer laser ablated corneas, macrophages participate in the inflammatory response contributing to subsequent corneal haze (O’Brien et al., 1998). In the model of cauterisation-induced corneal inflammation, corneal edema, opacity, and vascularization are significantly reduced when macrophages are selectively inhibited (Sonoda et al., 1998).
In this study, we investigate the mechanism of how AM may exert an anti-inflammatory action by facilitating apoptosis of interferon-γ (IFN-γ)-activated mouse macrophage cell line (Raw 264.7 cells). We showed that such an action is not mediated by producing nitric oxide (NO), but instead by down-regulating anti-apoptotic NF-κB and Akt-FKHR signalling pathways. The significance of these findings is further discussed.
2. Materials and methods
2.1. Materials and reagents
Mouse monocyte/macrophage cell line Raw 264.7 cells were obtained from the ATCC (Manassas, VA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and trypsin/EDTA were from Gibco BRL (Grand Island, NY). Type I collagen was from BD Biosciences (Bedford, MA). LIVE/DEAD assay reagent was from Molecular Probes (Eugene, OR). DeadEnd™ fluorometric TUNEL system was from Promega (Madison, WI). Cell Death Detection ELISAPLUS kit was from Roche Diagnostics (Mannheim, Germany). Mouse IFN-α ELISA kit was from R&D System (Minneapolis, MN). Griess reagent was from ICN Biomedicals (Aurora, OH). Western lightning™ chemiluminescence reagent was from PerkinElmer Life Sciences (Boston, MA). Mouse monoclonal anti-IKK-α, IKK-β, p65 (RelA) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti- total Akt, phospho-Akt (Ser473), rabbit polyclonal anti-phospho-FKHR (Thr24)/FKHRL1 (Thr32) antibodies were from Cell Signalling Technology (Beverly, MA). Anti-mouse and anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies were from Bio-Rad (Hercules, CA). Cryopreserved human AM was kindly provided by Bio-Tissue, Inc. (Miami, FL), Human corneoscleral tissues from donor eyes were obtained from the Florida Lions Eye Bank (Miami, FL). All other reagents were from Sigma (St. Louis, MO).
2.2. Macrophage cultures
Raw 264.7 cells, mouse macrophage cell line established from a tumour induced by Abelson murine leukemia virus, were routinely cultured in DMEM supplemented with 10% FBS, 50 μg ml−1 gentamicin, and 1.25 μg ml−1 fungizone in 100 mm culture dishes. Upon reaching 70–80% confluence, cells were washed three times with Hanks’-based salt solution, then treated with 0.05% trypsin/0.53 mM EDTA at 37°C for 10 min to loosen the cells from plastic, then harvested by gentle pipetting, and resuspended in serum-free DMEM+ITS, i.e. phenol red-free DMEM containing 5 μg ml−1 insulin, 5 μg ml−1 human transferrin, and 5 ng ml−1 sodium selenite (ITS), 50 μg ml−1 gentamicin, and 1.25 μg ml−1 fungizone. Cell viability measured by staining with 0.4% trypan blue was in the range of 97–99%.
2.3. Subculture on different matrices
Cryopreserved human AM was epithelially-denuded (Grueterich et al., 2002). AM of the size of 1.5×1.5 cm2 was fastened to a culture plate insert with stroma side up and placed in the well of a 24-well plate as previously reported (Meller and Tseng, 1999). Type I collagen diluted in acetic acid at a concentration of 50 μg ml−1 was coated to 24 well culture dish as a thin coating according to the procedure recommended by the manufacturer. Human corneoscleral tissue was handled according to the Declaration of Helsinki. The tissue was rinsed three times with DMEM containing 50 mg ml−1 gentamicin and 1.25 mg ml−1 amphotericin B. Epithelium and endothelium were then scraped off and the remaining stroma was embedded in O.C.T. The corneal stroma was cut tangentially to get 6–8 mm diameter wide and 8 μm thick round slices, mounted to 12 mm diameter wide plastic coverslips, then sterilized with 70% ethanol for 2 hr, after that rinsed with culture medium for three times. Macrophages suspended in DMEM+ITS were seeded at the density of 2.5×105 cells per well on plastic, type I collagen, corneal stroma slice or AM inserts. Cultures were incubated at 37°C in a moist atmosphere of 5% CO2 for 1 hr to allow cell adhesion before adding 200 U ml−1 IFN-γ as final concentration, while the same amount of IFN-γ diluent solution containing 0.5% BSA was added to the control without IFN-γ. Then cells were cultured at 37°C in a moist atmosphere of 5% CO2 for different duration before being terminated for different assays.
2.4. LIVE/DEAD assay
The culture medium was removed and 200 μl of the combined LIVE/DEAD assay reagents was added to the cells, incubated for 15 min at room temperature, and then observed under a fluorescent microscope. Live cells are distinguished by the presence of ubiquitous intracellular esterase activity, determined by the enzymatic conversion of the virtually nonfluorescent cell-permeant calcein AM to green fluorescent calcein retained within live cells. Another dye ethidium homodimer-1 is excluded by the intact plasma membrane of live cells, while can enter cells with damaged membranes and undergo a 40-fold enhancement of fluorescence upon binding to nucleic acids, thereby producing a bright red fluorescence in dead cells.
2.5. Hoechst-33342 staining
To characterize the nuclear morphology as a means to assess apoptosis, Hoechst-33342 dye was added to the cell culture medium to a final concentration of 10 μg ml−1, incubated at 37°C for 15 min, and observed under a fluorescent microscope. Hoechst-33342 stains the nuclei of live cells and identifies apoptotic cells by the increased fluorescence and nuclear fragmentation or condensation.
2.6. TUNEL assay
Terminal deoxyribonucleotidyl transferase-mediated FITC-linked dUTP nick-end DNA labelling (TUNEL) assay was performed according to the manufacturer’s instructions. Briefly, cells cultured on different matrices were fixed in 4% formaldehyde for 20 min at room temperature and permeabilized with 1% Triton X-100. Samples were then incubated for 60 min at 37°C with exogenous TdT and fluorescein-conjugated dUTP for repair of nicked 3′-hydroxyl DNA ends. Cells were treated with DNase I as the positive control, while the negative control was incubated with buffer lacking rTdT enzyme. The apoptotic nuclei were labelled with green fluorescence.
2.7. ELISA assay
Cell lysates equivalent to 104 cells and conditioned media after cultivation were subjected to Cell Death Detection ELISAPLUS assay according to the manufacturer’s instructions. This ELISA is a photometric enzyme-immunoassay for the qualitative and quantitative in vitro determination of cytoplasmic histone-associated-DNA-fragments (mono- and oligonucleosomes) generated by apoptotic cell death using mouse monoclonal anti-histone and anti-DNA antibody. Positive and negative controls were included as provided by the manufacturer, the absorbance was measured at 405 nm using Fusion™ universal microplate analyser.
To examine the concentration of IFN-α, the conditioned medium was centrifuged at 10 000×g for 10 min, and the supernatant was subjected to mouse IFN-α ELISA according to the manufacturer’s instructions, the absorbance was measured at 450 nm using Fusion™ universal microplate analyser.
2.8. Measurement of nitrite concentration
To examine the amount of NO produced in the culture, we measured the concentration of nitrite, a stable NO metabolite, using Griess reagent as previously described (Granger et al., 1996). Briefly, 50 μl of the medium supernatant was mixed with 100 μl of Griess reagent, and the absorbance was measured at 550 nm using Fusion™ universal microplate analyser. The amount of nitrite was calculated from a standard curve of sodium nitrite prepared in DMEM+ITS.
2.9. Western blot analysis
Cultured macrophages from plastic or AM (1.5×106) were collected and extracted in cold RIPA buffer [50 mM Tris·Cl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitors including 1 μg ml−1 aprotinin, leupeptin, and pepstatin A, 1 mM PMSF, 50 mM sodium fluoride, and 0.2 mM sodium vanadate], and equal amounts of proteins extracted from lysates were separated on 4–15% sodium dodecyl sulphate-polyacrylamide gels (SDS-PAGE) and then electrophorectically transferred to nitrocellulose membranes. After 1 hr of blocking in 5% nonfat milk, the blots were incubated with primary antibodies to IKK-α, IKK-β, p65 (RelA) subunit of NF-κB, total Akt, phospho-Akt (Ser473), phospho-FKHR (Thr24)/FKHRL1 (Thr32) (all 1:1000), and β-actin (1:3000) as a loading control. The specific binding was detected by anti-mouse or anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies, and visualized by enhanced chemiluminescence method.
2.10. Statistical analysis
All experiments described above were repeated three times, each in triplicate. For Hoechst-33342 staining, five photographs from each well or insert were randomly taken at 400×magnification, apoptotic cells were counted in each photograph. The results were expressed as percentage of apoptotic cells per 100 cells. Data were compiled and analysed by MicroSoft Excel™. Descriptive statistics with respect to the mean and standard deviation was calculated for each group. For studies of the apoptosis, NO and IFN-α concentrations, and group means were compared using the appropriate version of Student’s unpaired t-test. Test results were reported as two-tailed p values, where p<0.05 was considered statistically significant. Summary data are reported as means±S.D.
3. Results
3.1. Morphological changes
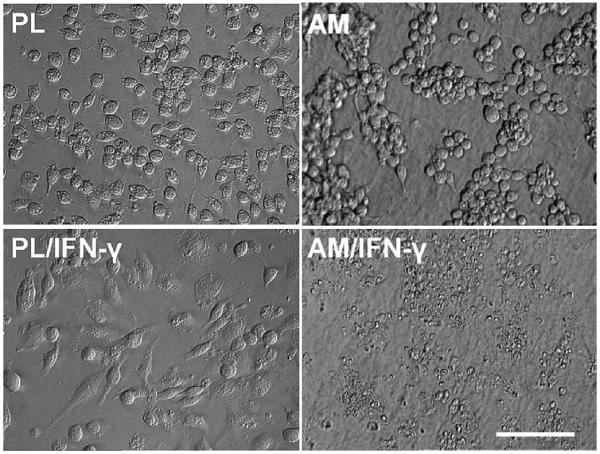
Macrophages attached to both plastic and AM very well within 1 hr after seeding. There was no significant difference in the cell attachment rate between plastic and AM cultures (data not shown). At the end of 48 hr cultivation in DMEM+ITS, macrophages on plastic were evenly distributed, and the majority of them were oval and some were spindle (Fig. 1, PL). After being activated with IFN-γ, cells became larger in general and more of them appeared spindle shape (Fig. 1, PL/IFN-γ), and some fused to form multi-nucleated giant cells (see Hoechst-33342 staining later). In contrast, macrophages cultured on AM became more aggregated, and most of them remained round (Fig. 1, AM). Strikingly, after activated with IFN-γ, most cells shrank, condensed and degenerated into cellular debris (Fig. 1, AM/IFN-γ). This result indicated that macrophages cultured on AM stroma altered their morphology suggestive of cell death after being activated by IFN-γ.
Fig. 1.
Macrophage morphology cultured on plastic (PL) or AM for 48 hr. Cells on plastic distributed evenly, and most were oval. Cells on plastic activated with IFN-γ (PL/IFN-γ) were larger and became spindle. Cells on AM tended to aggregate in clusters and remained round. Cells on AM with IFN-γ activation (AM/IFN-γ) were shrank and degenerated into debris. All photographs are taken at the same magnification; bar represents 100 μm.
3.2. Apoptosis of macrophages cultured on AM after IFN-γ activation
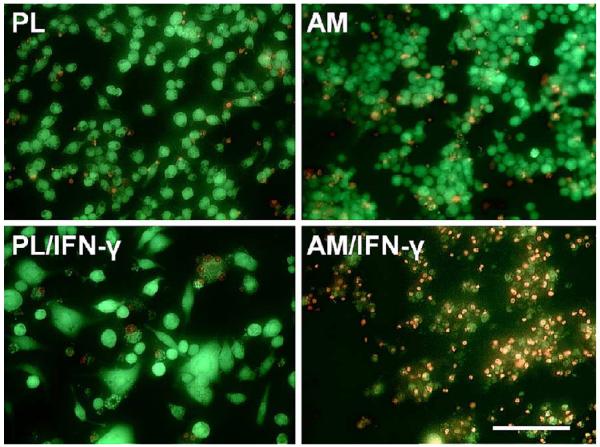
To determine if the aforementioned morphological changes on AM cultures indeed represented cell death, cells in parallel experiments were subjected to LIVE/DEAD assay. As shown in Fig. 2, cells cultured on plastic with or without activation by IFN-γ and cells on AM without IFN-γ for 48 hr were predominantly alive (i.e. yielding green fluorescence) (For web version only). In contrast, a large number of cells on AM after IFN-γ activation were dead (i.e. yielding red fluorescence).
Fig. 2.
LIVE/DEAD staining of macrophages cultured on plastic (PL) or AM with or without IFN-γ for 48 hr. Majority of the cells cultured on PL with or without IFN-γ activation and cultured on AM without IFN-γ activation were live (green fluorescence). In contrast, most cells cultured on AM with IFN-γ were dead (red fluorescence). All photographs are taken at the same magnification; bar represents 100 μm (For interpretation of the reference to colour in this legend, the reader is referred to the web version of this article.).
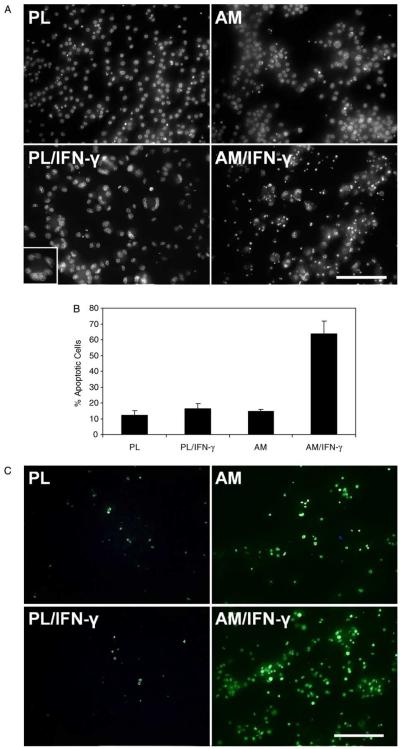
To determine if the aforementioned cell death was mediated by apoptosis, cells in parallel experiments were subjected to Hoechst-33342 staining. As shown in Fig. 3A, fragmented nuclei with strong Hoechst staining were found more in AM/IFN-γ as compared to AM without IFN-γ and to their plastic controls. Percentages of Hoechst-33342-positive apoptotic nuclei were 12.4±2.7% (PL), 16.5±3.1% (PL/IFN-γ), 14.7±1.1% (AM), and 63.8±7.9% (AM/IFN-γ) (Fig. 3B). There was no statistical difference between PL and PL/IFN-γ and between PL and AM (all p>0.05), while there was a statistical difference between AM and AM/IFN-γ and between PL/IFN-γ and AM/IFN-γ (all p<0.001). Furthermore, Hoechst-33342 staining also revealed multinucleated giant cells in plastic cultures when activated by IFN-γ. In contrast, there was no such giant cell in AM/IFN-γ cultures.
Fig. 3.
Apoptosis assay. (A) Hoechst-33342 staining of macrophages cultured on plastic (PL) or AM with or without IFN-γ activation for 48 hr. Fragmented nuclei with strong Hoechst staining were found more in AM/IFN-γ. Inset showed multinucleated giant cell formed on PL after IFN-γ activation. All photographs are taken at the same magnification; bar represents 100 μm. (B) Percentage of Hoechst 33342-positive apoptotic nuclei. Statistical analysis showed p>0.05 for PL vs. PL/IFN-γ and PL vs. AM, but p<0.01 for AM vs. AM/IFN-γ and PL/IFN-γ vs. AM/IFN-γ. (C) TUNEL staining of fragmented nuclei. TUNEL-positive nuclei were sporadic on PL, PL/IFN-γ, and AM, but dramatically increased in AM/IFN-γ. All photographs are taken at the same magnification; bar represents 100 μm.
To verify that such fragmented nuclei were indeed caused by DNA fragmentation, cells in parallel experiments were subjected to TUNEL assay staining. As shown in Fig. 3C, TUNEL-positive fragmented nuclei were sporadic in PL, PL/IFN-γ, and AM without IFN-γ cultures, but markedly increased in AM/IFN-γ cultures. This result was consistent with that shown by Hoechst-33342 staining (Fig. 3A). It should be noted that the denuded AM without being seeded with any cells did not show any TUNEL-positive cell (data not shown).
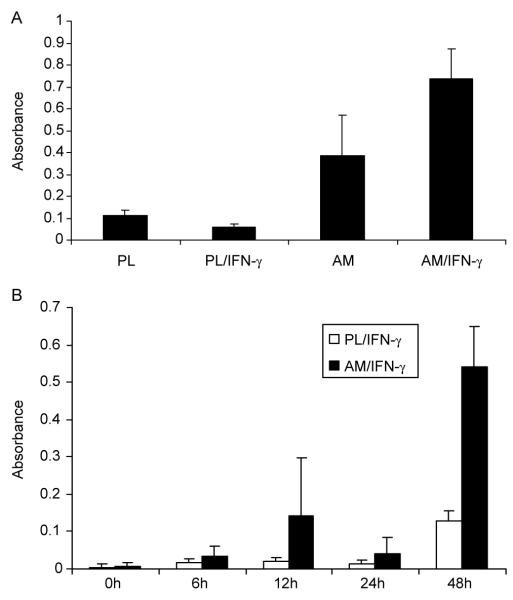
To further prove and quantify the extent of macrophage apoptosis in these cultures, we performed Cell Death Detection ELISA assay to measure cytoplasmic histone-associated DNA mono- and oligo-nucleosome fragments. The results showed that a small amount of apoptosis was detected in plastic cultures after 48 hr in DMEM+ITS, and the apoptosis decreased when macrophages were activated by IFN-γ (Fig. 4A, p<0.05). Macrophages cultured on AM also showed some apoptosis, but there was no significant difference between AM and PL (Fig. 4A, p>0.05). In contrast, macrophages activated by IFN-γ showed a significant increase of apoptosis when cultured on AM (Fig. 4A, p<0.05 for AM vs. AM/IFN-γ and p<0.01 for PL/IFN-γ vs. AM/IFN-γ). When the conditioned medium was used for this assay, the same results as those for the cell lysates were obtained except that the overall absorbance was much less (data not shown), indicating that some histone-associated mono- and oligo-nucleosome fragments were proportionally released by apoptotic cells into the medium. We then used this assay to monitor macrophage apoptosis cultured on plastic or AM under the activation with IFN-γ for different durations during the course of 48 hr. The results showed that the level of apoptosis was at a low level when cells were cultured on AM from 6 to 24 hr without any significant difference between plastic and AM, but became more dramatically different at 48 hr (p<0.01).
Fig. 4.
Cell death detection ELISA assay. (A) Macrophages were cultured on plastic (PL) or AM with or without IFN-γ activation for 48 hr. Statistical analysis showed p>0.05 for PL vs. AM, p<0.05 for PL vs. PL/IFN-γ, and AM vs. AM/IFN-γ, p<0.01 for PL/IFN-γ vs. AM/IFN-γ. (B) Macrophages were cultured on PL or AM with IFN-γ activation for different duration. The statistical analysis showed p<0.01 for PL/IFN-γ vs. AM/IFN-γ at 48 hr, but not the rest of time points.
3.3. Apoptosis of macrophage cultured on other matrices
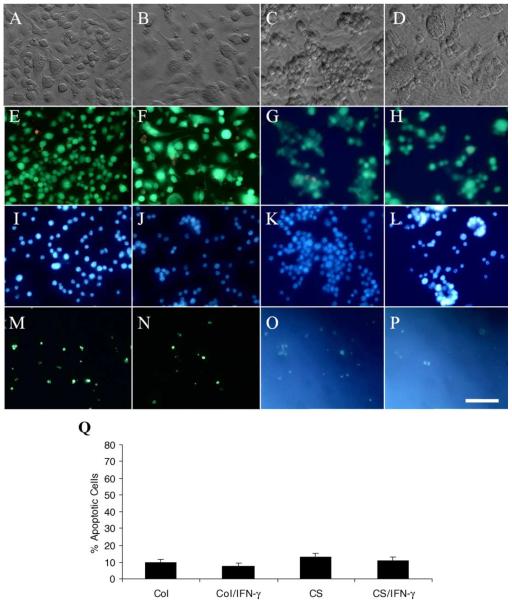
To determine whether macrophage apoptosis can also happen when they are cultured on other matrices, we seeded macrophages on type I collagen coated dish and corneal stroma slice for 48 hr. The results showed that macrophages were evenly distributed on type I collagen (Fig. 5A) similar to that on plastic, while macrophages cultured on corneal stroma became aggregated and remained round (Fig. 5C) similar to that on AM stroma. When activated with IFN-γ, cells became larger and more spindle on type I collagen (Fig. 5B), and cells became more aggregated on corneal stroma (Fig. 5D). Multi-nucleated giant cells formed on both type I collagen and corneal stroma. LIVE/DEAD assay (Fig. 5E–H), Hoechst-33342 staining (Fig. 5I–L) and TUNEL assay (Fig. 5M–P) showed that there were few dead or apoptotic cells on type I collagen and corneal stroma with or without IFN-γ activation, and there was no significant difference of cell apoptosis between IFN-γ treatment and non-treatment on these two matrices (Fig. 5Q) (p>0.05). It should be mentioned that the increased fluorescence in Fig. 5O, P was due to the auto-fluorescence of the tissue when judged by the negative control.
Fig. 5.
Apoptosis of macrophages cultured on type I collagen and corneal stroma. Macrophages were cultured on type I collagen without (A, E, I, M) or with (B, F, J, N) IFN-γ activation, and on corneal stroma without (C, G, K, O) or with (D, H, L, P) IFN-γ activation for 48 hr. Phase contrast image showed macrophages cultured on type I collagen were evenly distributed (A, B), while cells aggregated when cultured on corneal stroma (C, D). LIVE/DEAD assay (E, F, G, H), Hoechst-33342 staining (I, J, K, L) and TUNEL assay (M, N, O, P) showed few dead or apoptotic cells on type I collagen and corneal stroma with or without IFN-γ activation. Percentage of Hoechst 33342-positive apoptotic nuclei (Q) showed no significant difference between IFN-γ treatment and non-treatment on these two matrices (p>0.05) (Col: type I collagen, CS: corneal stroma). All photographs are taken at the same magnification; bar represents 100 μm.
3.4. Role of NO and IFN-α in apoptosis of macrophage cultured on AM
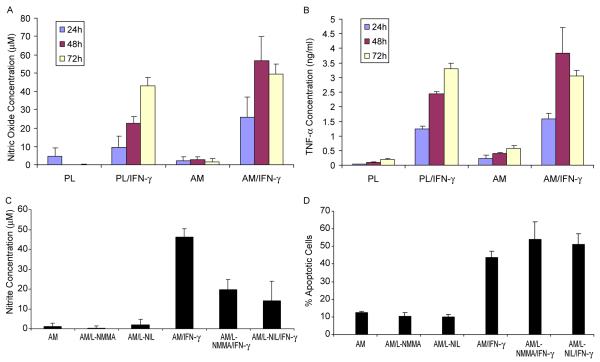
Activated macrophages can produce NO and IFN-α as well as other cytokines. It has been reported that endogenously generated or exogenously applied NO can induce apoptotic cell death in macrophages (Albina et al., 1993). For this reason, we would like to determine whether NO was responsible for the aforementioned apoptosis of macrophages cultured on AM. To do so, we measured nitrite concentrations in the conditioned media collected at the end of different culture duration from 24 to 72 hr, meanwhile we also detected IFN-α production. The results showed that macrophages cultured on PL or AM without IFN-γ activation produced low levels of NO and IFN-α, and there was no difference of NO production between PL and AM through out the culture duration (all p>0.05), while IFN-α concentration was higher on AM than on PL at all time points (all p<0.05). However, when activated with IFN-γ, macrophages cultured on PL continuously produced increasing levels of NO and IFN-α from 24 to 72 hr. In contrast, although macrophages cultured on AM also increased production of NO and IFN-α from 24 to 48 hr, there was no increase in their levels between 48 and 72 hr (p>0.05), indicating that the production of NO and IFN-α had ceased after 48 hr. NO concentration was higher on AM than that on PL at 24 and 48 hr (p<0.01), while IFN-α concentration was higher on AM at 24 hr only (p<0.01) (Fig. 6A, B). The control of incubating AM alone in the culture medium with or without IFN-γ for 48 hr did not reveal any detectable level of NO or IFN-α in the supernatant (data not shown), indicating AM itself did not produce NO and IFN-α. Taken together, these data also indicated that AM acted synergistically with IFN-γ to produce more NO, and AM itself weakly induced IFN-α production by macrophages. The nitrite level promoted by IFN-γ was more pronounced when cells were cultured on AM as compared to plastic at 48 hr (Fig. 6A, p<0.01), a finding correlating well with that of apoptosis (Fig. 3).
Fig. 6.
NO and IFN-α production and cell apoptosis. (A) Concentration of nitrite in the conditioned medium of macrophages cultured on plastic (PL) or AM for different durations. The differences were significant between AM/IFN-γ and PL/IFN-γ at 24 and 48 hr (each p<0.01). (B) Concentration of IFN-α in the conditioned medium of macrophages cultured on plastic (PL) or AM for different durations. The differences were significant between AM and PL at all time points (each p<0.01), and between AM/IFN-γ and PL/IFN-γ at 24 hr (p<0.01). (C) Concentration of nitrite in the conditioned medium of macrophages cultured on AM activated with IFN-γ with or without L-NMMA or L-NIL for 48 hr. The differences were significant between AM/IFN-γ and the other five groups (all p<0.01). (D) Percentages of Hoechst 33342-positive apoptotic nuclei cultured on AM activated with IFN-γ with or without L-NMMA or L-NIL for 48 hr. The differences were not significant between AM and AM/L-NMMA or AM/L-NIL, and between AM/IFN-γ and AM/L-NMMA+IFN-γ or AM/L-NIL+IFN-γ (all p>0.05).
To determine whether a higher level of NO and apoptosis was causatively related, we blocked NO production by adding NG-monomethyl-L-arginine acetate (L-NMMA) or L-N6-(1-iminoethyl) lysine hydrochloride (L-NIL), i.e. inhibitors of NO synthase. The results showed that 500 μM L-NMMA or L-NIL significantly attenuated IFN-γ induced nitrite levels more than 50% for cells cultured on AM, i.e. from the baseline of 46.2±4.3 to 19.8±4.9 and 14.0±9.8 μM for L-NMMA and L-NIL, respectively (Fig. 6C, both p<0.01). Nevertheless, the extent of macrophage apoptosis as determined by Hoechst-33342 staining was not significantly attenuated accordingly (Fig. 6D). These results collectively indicated that macrophage apoptosis on AM under the activation of IFN-γ was not mediated by overproduction of NO.
3.5. Cell signalling pathways involved in macrophage apoptosis on AM
To further investigate the mechanism of macrophage apoptosis on AM, we studied two major cell survival signalling pathways, NF-κB and Akt-FKHR signalling pathways, by determining the expression of total IKK-α, IKK-β, p65 (RelA) subunit of NF-κB, Akt, phospho-Akt (Ser473), and phospho-FKHR (Thr24)/FKHRL1 (Thr32) from macrophages cultured on plastic or AM with or without IFN-γ for 24 hr, when quantitative ELISA for apoptosis was not apparent (Fig. 4B). Western blot analyses showed that p65 (RelA) subunit of NF-κB and total Akt were slightly up-regulated in macrophages cultured on plastic with IFN-γ activation. The levels of IKK-α, IKK-β, total Akt, and phosphor-Akt (Ser473) were only down-regulated in macrophages cultured on AM with IFN-γ activation, while p65 (RelA) subunit of NF-κB and phospho-FKHR (Thr24)/FKHRL1 (Thr32) were down-regulated in macrophages cultured on AM without IFN-γ activation, and further down-regulated when activated with IFN-γ (Fig. 7). These results indicated that these two signalling pathways were both involved in the apoptosis of macrophages cultured on AM.
Fig. 7.
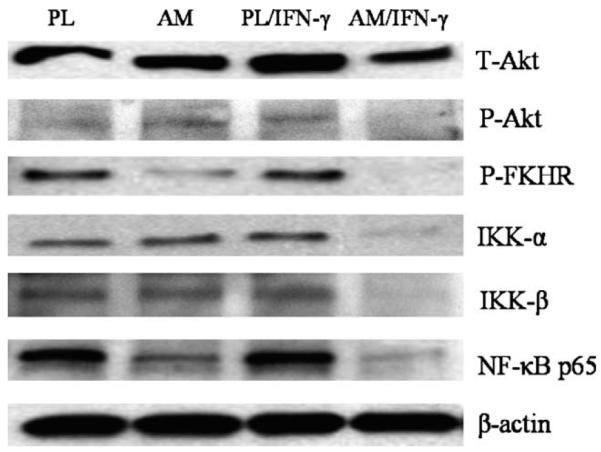
Western blot analyses. Macrophages were cultured on plastic (PL) or AM with or without IFN-γ activation for 24 hr. Western blot showed total Akt (T-Akt), phospho-Akt (Ser473) (P-Akt), IKK-α, and IKK-β were down-regulated in macrophages cultured on AM with IFN-γ activation. However, phospho-FKHR (Thr24)/FKHRL1 (Thr32) (P-FKHR) and p65 (RelA) subunit of NF-κB (NF-κB p65) were already down-regulated in macrophages cultured on AM, but became further down-regulated when activated with IFN-γ. One representative experiment of three is shown.
4. Discussion
Macrophages are derived from hematopoietic stem cells in the bone marrow. Once leaving the circulation and settling in their target tissues, they differentiate into mature tissue macrophages which are robust, long-lived, and resistant to many apoptotic stimuli including Fas and IFN-α death receptor ligation, ionising radiation, and multiple antineoplastic or cytotoxic agents (Perlman et al., 1999). To carry out their functional activities, macrophages must become activated. IFN-γ, a cytokine mainly released by activated T lymphocytes and NK cells, is one of the most potent activating stimuli for macrophages (Celada and Nathan, 1994). Sustained macrophage activation and excessive production of inflammatory mediators can perpetuate numerous pathological processes and contribute to induction of stress responses to tissue injuries (Huitinga et al., 1995; Bottino et al., 1998). Therefore, one important strategy to control tissue inflammation is to limit the life span of activated macrophages by facilitating their apoptosis, a new strategy recognized to facilitate resolution of inflammation in vivo (Savill, 1997).
Mature macrophages can survive and remain functionally active without growth factors (Munn et al., 1995; Liu et al., 2001). On plastic and in serum-free DMEM+ITS, about 90% of macrophages remained alive for at least 48 hr based on LIVE/DEAD assay. Addition of IFN-γ further increased their survival by decreasing apoptosis based on Cell Death Detection ELISA assay. This finding might be explained by a prior report showing that macrophages treated with IFN-γ induce p21Waf1, arrest the cell cycle at the G1/S boundary, but do not undergo apoptosis. Moreover, pretreatment of macrophages with IFN-γ can protect cells from apoptosis induced by lipopolysaccharide (LPS) or dexamethasone (Xaus et al., 1999).
But, strikingly, once adhering onto AM stroma matrix, IFN-γ-activated macrophages underwent rapid apoptosis. The evidence of apoptosis was supported by morphological changes and by four different assays. We also noted that apoptosis of IFN-γ-activated macrophages was a rapid event taking place between 24 and 48 hr. There was no difference in apoptosis of macrophages when seeded on type I collagen coated dishes and on corneal stroma slices, further supporting that the increased apoptosis by AM stromal matrix under IFN-γ stimulation is unique.
To investigate the mechanism of macrophage apoptosis on AM, we first measured NO production by macrophages because endogenously generated or exogenously applied NO could induce apoptotic cell death in macrophages (Albina et al., 1993). Our results showed that macrophages cultured on AM without IFN-γ activation produced negligible NO, indicating there was no endotoxin such as LPS contamination in the culture system. After IFN-γ activation, macrophages on AM produced more NO than on PL, but addition of NO inhibitors, which effectively attenuated NO production, still could not prevent macrophage apoptosis, indicating that overproduction of NO is not responsible for inducing macrophage apoptosis by AM.
We noted that macrophages cultured on AM without IFN-γ produced a low level of IFN-α which was higher than on plastic. This result might be explained by the finding that such an extracellular matrix component as decorin, which is presented in AM stroma (Meinert et al., 2001), induces mRNA expression of IFN-α and enhances IFN-γ dependent macrophage activation, meanwhile increasing the production of IFN-α and iNOS (Comalada et al., 2003). This interpretation also partially explains the synergistic action of AM and IFN-γ in producing more NO and IFN-α. It has been known that as the primary producer of IFN-α, macrophage has evolved to survive the cytotoxic effects of IFN-α (Kikuchi et al., 1996). In our culture system, IFN-α concentration was higher on AM only at 24 hr after IFN-γ activation, at which time, macrophage apoptosis was not obvious, indicating that the IFN-α concentration was not correlated well with macrophage apoptosis.
Instead, suppression of both NF-κB and Akt-FKHR pathways took place before macrophage apoptosis facilitated by AM after IFN-γ activation. NF-κB signalling pathway is a critical player in the control of apoptosis (for reviews see(Karin and Ben Neriah, 2000)). In most cells, Rel/NF-κB subunits are sequestered in the cytoplasm as inactive homo- or heterodimers because of their binding with IκB factors. In response to a wide variety of stimuli, NF-κB is activated by removing IκB through phosphorylation by the IκB kinase complex (IKK), which contains the catalytic subunits of IKKα and IKKβ and the regulatory subunit of IKKγ/NEMO protein. As a result, NF-κB dimers are translocated to the nucleus, bind to DNA, and transcriptionally activate genes involved in immune responses, inflammation, viral infection, cell proliferation and survival. NF-κB was shown to be essential for the survival of macrophages by maintaining mitochondrial integrity (Pagliari et al., 2000). Akt is another survival factor which can block apoptosis induced by apoptotic stimuli (for reviews see (Datta et al., 1999)). The canonical way that Akt promotes survival is mediated by phosphorylating and sequestrating Forkhead transcription factors in the cytoplasm, thereby preventing them from inducing the transcription of critical death genes (Biggs et al., 1999; Tang et al., 1999; Brunet et al., 1999). Akt has also been shown to be constitutively activated in human monocyte-differentiated macrophages, and is vital for their survival (Liu et al., 2001).
In our experiments, p65 (RelA) subunit of NF-κB and phosphorylation of FKHR/FKHRL1 was already down-regulated in macrophages cultured on AM without IFN-γ activation, while there was no apparent apoptosis. However, total and phosphorylated Akt, IKKα and IKKβ, and p65 (RelA) subunit of NF-κB and phosphorylation of FKHR/FKHRL1 were further down-regulated after IFN-γ activation. This result indicated that IFN-γ may synergistically act with AM to suppress both p65 (RelA) and FKHR/FKHRL1 to a critical level to elicit apoptosis. A similar phenomenon was reported by Beppu et al. (Beppu et al., 2003), who found that a combination of IFN-γ and cyclosporin A synergistically induces apoptosis of gastric carcinoma cells through the inhibition of IFN-γ-activated NF-κB by cyclosporine A (Morisaki et al., 2000).
It is well known that IFN-α can activate apoptosis through caspase pathway, but it also can exert anti-apoptotic action via NF-κB, PI-3K/Akt, Erk/MAPK, and other mediators. NF-κB activation usually precedes the apoptotic effects of IFN-α, that is why IFN-α activates NF-κB in all cell types, while it very rarely induces apoptosis (for reviews see (Gaur and Aggarwal, 2003). However, if NF-κB pathway is inhibited beforehand, macrophages become sensitive to IFN-α-induced apoptosis (Liu et al., 2004). Our experiment showed that the NF-κB pathway had already been down-regulated when IFN-α concentration was elevated on AM cultures, further explaining why apoptosis of macrophages was further facilitated in the event of a high IFN-α concentration.
Our data collectively indicate that the apoptosis promoted by AM is not mediated directly by NO and IFN-α, but rather caused by interruption of survival pathways mediated by both NF-κB and Akt-FKHR. Because production of both NO and IFN-α was also down-regulated after macrophage apoptosis, this finding also helps explain how AM graft on the ocular surface exerts its anti-inflammatory action. Future investigation into the factor(s) in AM that is responsible for suppressing NF-κB and Akt-FKHR pathways during the crosstalking with IFN-γ signalling pathway may help unravel new therapeutics to combat unwanted inflammation mediated by macrophages.
Acknowledgements
Supported in part by RO1 EY06Sig from NIH, nEI, in part by a research grant from TissueTech, Inc., and in part by an unrestricted grant from Ocular Surface Research & Education Foundation, Miami, FL.
References
- Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J. Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- Beppu K, Morisaki T, Matsunaga H, Uchiyama A, Ihara E, Hirano K, Kanaide H, Tanaka M, Katano M. Inhibition of interferon-gamma-activated nuclear factor-kappa B by cyclosporin A: a possible mechanism for synergistic induction of apoptosis by interferon-gamma and cyclosporin A in gastric carcinoma cells. Biochem. Biophys. Res. Commun. 2003;305:797–805. doi: 10.1016/s0006-291x(03)00853-2. [DOI] [PubMed] [Google Scholar]
- Biggs WH, III., Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl Acad. Sci. USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316–323. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Celada A, Nathan C. Macrophage activation revisited. Immunol. Today. 1994;15:100–102. doi: 10.1016/0167-5699(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Comalada M, Cardo M, Xaus J, Valledor AF, Lloberas J, Ventura F, Celada A. Decorin reverses the repressive effect of autocrine-produced TGF-beta on mouse macrophage activation. J. Immunol. 2003;170:4450–4456. doi: 10.4049/jimmunol.170.9.4450. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv. Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin. Sci. (London) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem. Pharmacol. 2003;66:1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- Granger DL, Taintor RR, Boockvar KS, Hibbs JB., Jr. Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods Enzymol. 1996;268:142–151. doi: 10.1016/s0076-6879(96)68016-1. [DOI] [PubMed] [Google Scholar]
- Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest. Ophthalmol. Vis. Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- Heiligenhaus A, Meller D, Meller D, Steuhl K-P, Tseng SCG. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest. Ophthalmol. Vis. Sci. 2001;42:1969–1974. [PubMed] [Google Scholar]
- Huitinga I, Ruuls SR, Jung S, Van Rooijen N, Hartung HP, Dijkstra CD. Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats. Clin. Exp. Immunol. 1995;100:344–351. doi: 10.1111/j.1365-2249.1995.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Neriah Y. Ben. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Iizuka R, Sugiyama S, Gon G, Mori H, Arai M, Mizumoto K, Imajoh-Ohmi S. Monocytic differentiation modulates apoptotic response to cytotoxic anti-Fas antibody and tumor necrosis factor alpha in human monoblast U937 cells. J. Leukoc. Biol. 1996;60:778–783. doi: 10.1002/jlb.60.6.778. [DOI] [PubMed] [Google Scholar]
- Liu H, Ma Y, Pagliari LJ, Perlman H, Yu C, Lin A, Pope RM. TNF-alpha-induced apoptosis of macrophages following inhibition of NF-kappa B: a central role for disruption of mitochondria. J. Immunol. 2004;172:1907–1915. doi: 10.4049/jimmunol.172.3.1907. [DOI] [PubMed] [Google Scholar]
- Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J. Exp. Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert M, Eriksen GV, Petersen AC, Helmig RB, Laurent C, Uldbjerg N, Malmstrom A. Proteoglycans and hyaluronan in human fetal membranes. Am. J. Obstet. Gynecol. 2001;184:679–685. doi: 10.1067/mob.2001.110294. [DOI] [PubMed] [Google Scholar]
- Meller D, Tseng SCG. Conjunctival epithelial cell differentiation on amniotic membrane. Invest. Ophthalmol. Vis. Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- Morisaki T, Matsunaga H, Beppu K, Ihara E, Hirano K, Kanaide H, Mori M, Katano M. A combination of cyclosporin-A (CsA) and interferon-gamma (INF-gamma) induces apoptosis in human gastric carcinoma cells. Anticancer Res. 2000;20:3363–3373. [PubMed] [Google Scholar]
- Munn DH, Beall AC, Song D, Wrenn RW, Throckmorton DC. Activation-induced apoptosis in human macrophages: developmental regulation of a novel cell death pathway by macrophage colony-stimulating factor and interferon gamma. J. Exp. Med. 1995;181:127–136. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TP, Li Q, Ashraf MF, Matteson DM, Stark WJ, Chan CC. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch. Ophthalmol. 1998;116:1470–1474. doi: 10.1001/archopht.116.11.1470. [DOI] [PubMed] [Google Scholar]
- Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol. Cell Biol. 2000;20:8855–8865. doi: 10.1128/mcb.20.23.8855-8865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WC, Tseng SCG. Modulation of acute inflammation and keratocyte death by suturing, blood and amniotic membrane in PRK. Invest. Ophthalmol. Vis. Sci. 2000;41:2906–2914. [PubMed] [Google Scholar]
- Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J. Exp. Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. Apoptosis in resolution of inflammation. J. Leukoc. Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiin-flammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408–413. doi: 10.1097/00003226-200105000-00015. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sonoda K, Sakamoto T, Yoshikawa H, Ashizuka S, Ohshima Y, Kishihara K, Nomoto K, Ishibashi T, Inomata H. Inhibition of corneal inflammation by the topical use of Ras farnesyltransferase inhibitors: selective inhibition of macrophage localization. Invest. Ophthalmol. Vis. Sci. 1998;39:2245–2251. [PubMed] [Google Scholar]
- Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- Tseng SCG. Amniotic membrane transplantation for ocular surface reconstruction. Biosci. Rep. 2002;21:481–489. doi: 10.1023/a:1017995810755. [DOI] [PubMed] [Google Scholar]
- Wang MX, Gray TB, Parks WC, Prabhasawat P, Culbertson WW, Forster RK, Hanna K, Tseng SCG. Corneal haze and apoptosis is reduced by amniotic membrane matrix in excimer laser photoablation in rabbits. J. Cat. Refract. Surg. 2001;27:310–319. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- Xaus J, Cardo M, Valledor AF, Soler C, Lloberas J, Celada A. Interferon gamma induces the expression of p21waf-1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity. 1999;11:103–113. doi: 10.1016/s1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]