Abstract
The vast majority of healthy individuals are left hemisphere dominant for language; however, individuals with left hemisphere epilepsy have a higher likelihood of atypical language organization. The cerebral organization of language in epilepsy has been studied with invasive procedures such as Wada testing and electrical cortical stimulation mapping (ESM), and more recently, with noninvasive neuroimaging techniques such as functional magnetic resonance imaging (fMRI). Investigators have used these techniques to explore the influence of unique clinical features inherent in epilepsy that might contribute to the reorganization of language, such as location of seizure onset, age of seizure onset, and extent of interictal epileptiform activity. In this paper, we review the contribution of these and other clinical variables to the lateralization and localization of language in epilepsy, and how these patient-related variables affect the results from these three different, yet complementary methodologies. Unlike the abrupt language changes that occur following acute brain injury with disruption of established language circuits, converging evidence suggests that the chronic nature of epileptic activity can result in a developmental shift of language from the left to the right hemisphere or re-routing of language pathways from traditional to non-traditional areas within the dominant left hemisphere. Clinical variables have been shown to contribute to cerebral language reorganization in the setting of chronic seizure disorders, yet such factors have not been reliable predictors of altered language networks in individual patients, underscoring the need for language lateralization and localization procedures when definitive identification of language cortex is necessary for clinical care.
Introduction
It is well established that individuals with left hemisphere epilepsy have a higher likelihood of atypical language organization. This phenomenon is clinically relevant, particularly in the context of epilepsy surgery for treatment of pharmacologically refractory seizures, because it is necessary to localize and to spare essential language areas before removing epileptogenic cortex. It is also of theoretical interest, with the potential to shed light on the factors that shape the organization of language mediating cortex. Our understanding of language organization in epilepsy has evolved, first from “disruptive” investigations including the intracarotid amobarbital (Wada) procedure and direct cortical stimulation mapping, and more recently, “activation” methods such as fMRI and other neuroimaging techniques. Unlike the sudden language changes that occur following stroke or traumatic brain injury due to an acute disruption of established language circuits, it has been proposed that ongoing functional or slowly progressive structural disturbances from chronic epileptic activity might shift language from the left to the right hemisphere (Janszky, Mertens, Janszky, Ebner, & Woermann, 2006), or might re-route language pathways during development from traditional to non-traditional sites within the dominant left hemisphere (Duchowny et al., 1996; Liegeois et al., 2004).
Although epilepsy is a heterogeneous disorder, most of the work on language organization in epilepsy has involved temporal lobe epilepsy (TLE) patients, mainly because these patients represent the largest and most homogenous subgroup of patients who undergo surgical treatment for refractory epilepsy, requiring various procedures to assess, lateralize and “localize” language. TLE patients, however, vary widely with respect to numerous factors that could potentially affect language organization, some of which have been more extensively studied than others. Examples include hemisphere of seizure onset (and/or cerebral injury), intra-hemispheric location of seizure onset (i.e., medial temporal versus lateral neocortical), presence/absence of hippocampal sclerosis (HS), age of seizure onset, duration of epilepsy, handedness, seizure frequency, frequency and location of interictal EEG activity, and type, amount and duration of pharmacological treatment. Certainly, investigators have attempted to control for pertinent variables; however, this heterogeneity should be kept in mind, as it likely accounts for some of the variability in findings across studies. Below, we review findings, based primarily on TLE patients, from the three main classes of techniques that have contributed to our understanding of language organization and reorganization in epilepsy.
Wada studies
Wada testing refers to a group of procedures aimed to assess aspects of cognitive functioning during temporary anesthesia of one cerebral hemisphere invoked by injection of sodium amobarbital or similar, short-acting anesthetic agent, into the internal carotid artery (Wada & Rasmussen, 1960). The procedure was originally developed to determine hemispheric language dominance in epilepsy surgery candidates, and was subsequently modified to include assessment of hemispheric memory capacity as well (Milner, Branch, & Rasmussen, 1962). Wada testing enables assessment of hemispheric language ability during temporary anesthesia of brain regions perfused by the internal carotid artery, serving as a gross, reversible surrogate for the effects of surgical resection. The specifics of the procedure vary among surgery centers; however, the hemispheric anesthetic effect is typically verified via scalp EEG (ipsilateral, unilateral slowing) and contralateral hemiplegia/hemiparesis. Hemispheric language assessment typically includes automatic speech (e.g., counting), object naming, repetition, execution of verbal commands and reading. Unilateral language dominance is inferred when all tasks are performed accurately with anesthesia in one hemisphere, and inability to perform tasks with anesthesia of the contralateral cerebral hemisphere. Bilateral language representation is typically inferred when a combination of accurate and inaccurate performances occur following both left and right hemisphere anesthesia. Although developed for clinical purposes, the procedure has facilitated the investigation of language dominance in relation to epilepsy and other forms of brain pathology. Given recent advances in noninvasive neuroimaging techniques, Wada testing could be considered somewhat crude in its approach by today’s standards. However, the procedure is the most reliable means of determining hemispheric language dominance and therefore, remains the gold standard for this purpose (Loring, Meader, Lee, & King, 1992)
Historically, it was assumed that language dominance could be inferred from handedness. However, results from Wada testing with epilepsy patients revealed inconsistencies in the relation between these two variables (Gloning, Gloning, Harb, & Quantember, 1969; Rasmussen & Milner, 1977; Rausch & Walsh, 1984). Research using Wada testing in mixed neurological samples has shown right hemisphere language dominance in approximately 4–37% of right-handers and 25–52 % of non-right-handers (Branch, Milner, & Rasmussen, 1964; Helmstaedter, Kurthen, Linke, & Elger, 1997; Loring et al., 1990; C. A. Mateer & Dodrill, 1983; Powell GE, Polkey CE, & AG, 1987; Rausch & Walsh, 1984; Rey, Dellatolas, Bancaud, & Talairach, 1988; Risse GL, Gates JR, & MC, 1997; Strauss & Wada, 1983; Woods RP, Dodrill CB, & GA, 1988; Zatorre, 1989), whereas an estimate of right language dominance for healthy individuals based on Doppler sonography is 4% (Knecht et al., 2000). In epilepsy, similar to that found in lesion studies showing an increased probability of right hemisphere language with early left hemisphere insult, side of seizure focus has been shown to be the most significant contributor to atypical (i.e., right or bilateral) language representation (Branch, et al., 1964; Loring, et al., 1992). Rausch and Walsh (1984) reported that 15% of their sample of right-handed patients with left TLE were right hemisphere language dominant, and several investigators have reported a significant relation between of age of “injury” (e.g., left cerebral injury, left-sided seizure onset) and atypical language lateralization (Rasmussen & Milner, 1977; Rausch, Boone, & Ary, 1991; Rey, et al., 1988; Satz, Strauss, Wada, & Orsini, 1988; Strauss & Wada, 1983), with earlier age of injury, typically before age 5, more likely resulting in right or mixed language dominance. A recent, large scale retrospective study of 445 epilepsy patients who had undergone bilateral Wada testing found that 46% of left-handers with early left hemisphere lesions were right language dominant, whereas 37% of left-handers with late neocortical left hemisphere lesions were more likely to have bilateral language representation (Möddel, Lneweaver, Schuele, Reinholz, & Loddenkemper, 2009). These results suggest that right dominance might indicate development of functional language areas in the right hemisphere following early insult, whereas bilateral language might indicate compromise to the left language system at a time when left hemisphere dominance has already started to take hold.
Janszky et al.(2003) used Wada testing to determine language dominance in a more homogenous subgroup of 184 TLE (MTLE) patients due to unilateral hippocampal sclerosis (HS), but without other epileptogenic lesions, thereby eliminating the influence of type of pathology, location of pathology and age of precipitating injury. HS is understood to occur during infancy or early childhood (Engel, Williamson, & Wieser, 1997), and is located distant from classic language areas. The results showed that (24%) of left MTLE-HS patients had atypical language dominance, and most interestingly, atypical language representation in these patients was associated with a significantly higher frequency of interictal discharges and with sensory auras representing seizure propagation to lateral temporal structures. These findings suggest that in addition to structural factors, functional factors such as abnormal EEG activity (i.e., interictal EEG discharges and seizure spread) can influence speech organization.
The advantage of Wada testing is its power as a disruptive technique, enabling assessment of language in one hemisphere, without influence from the other. On the other hand, Wada testing creates a fairly-diffuse unilateral lesion, and therefore provides no specific information regarding intra-hemispheric organization of language. The two techniques discussed below, i.e., electrical stimulation mapping and fMRI, enable detailed examination of intra-hemispheric language organization.
Electrical stimulation mapping
Electrical stimulation mapping (ESM) is an invasive procedure in which electrical stimulation is applied briefly (~2–10 sec) to the cortical surface, effectively creating a reversible functional lesion in a discrete cortical area (Hamberger, 2011). The procedure is used to identify critical sensory, motor or language areas when brain surgery involves the removal or disruption of potentially functional cortex/tissue. Sites with a positive finding, identified via stimulation are typically spared from resection, with the goal of preserving function postoperatively. ESM as a clinical tool for identification of language cortex was pioneered by Wilder Penfield and colleagues, and although initially used for clinical purposes, the procedure now provides a unique opportunity to study structure–function relations with precision within the language dominant hemisphere (Penfield & Roberts, 1959). Our current understanding of stimulation-based, intra-hemispheric patterns of language is based largely on the extensive work of George Ojemann and colleagues (Ojemann, 1983b)
Unlike ESM for identification of sensory or motor cortex, based on stimulation-evoked “positive” responses such as subjective sensation (e.g., tingling) or observable movement (e.g., muscle twitch), stimulation of language cortex does not produce any observable or sensory responses in an inactive patient. Instead, ESM for language relies on “negative” responses, so that the patient must be engaged in a language task and stimulation of language cortex will impair task performance. In this way, the examiner can observe the functional consequences of damage to the cortical site(s) being stimulated. It would seem reasonable that the use of multiple tasks that require different aspects of language would provide the most comprehensive mapping; however, practical constraints such as time limitations, especially during intra-operative mapping, and patient discomfort (i.e., headache, fatigue) restrict the number of tasks typically employed. The vast majority of investigators who conduct ESM for language have relied primarily on visual object naming (“visual naming”) to identify essential language cortex, however, other commonly used language tasks include auditory description naming, reading, and modified forms of the Token Test (for comprehension) (Hamberger, Goodman, Perrine, & Tammy, 2001; Malow et al., 1996), (Boatman, Lesser, & Gordon, 1995; Luders et al., 1986; Ojemann, 1983a, 1990; Schwartz, Devinsky, Doyle, & Perrine, 1999).
The advantages of ESM are its high level of precision, and as a disruptive technique, the ability to identify brain areas that are necessary for language, not merely areas that might participate in ancillary fashion in language functioning. On the other hand, because it is invasive, ESM studies are limited to the areas deemed relevant by the clinical situation, so that only the areas considered likely to be involved in seizure onset and spread will receive electrode coverage. Thus, there are no normative data available for ESM, i.e., it is unknown where ESM based language areas would be found in healthy individuals. Although such information would be interesting heuristically, and might direct the search for language cortex in patients undergoing cortical mapping, this information is not critical to the procedure’s clinical utility. ESM induced language errors are typically sufficient to infer an important functional role of the region stimulated.
Although it is technically unknown where stimulation-induced language errors would be found in healthy individuals, it is tacitly assumed that the pattern would mimic the areas associated with aphasia in stroke patients (i.e., a classic anterior Broca’s and posterior/Wernicke’s pattern). It has been speculated that late-onset (i.e., > 10 years) epilepsy patients might provide a reasonable model of more normative ESM patterns, as it would be expected that by age 10, “normal” language organization would already be established. However, it could also be argued that abnormal EEG discharges or other neurological abnormalities present early in life, yet preceding clinical seizures, could have altered normal language organization (Devinsky et al., 2000). Accordingly, it is sometimes the case that despite preconceived notions regarding the location of critical language cortex, ESM results in individual patients indicate that surgery in those areas can be performed safely. With these advantages and caveats in mind, ESM based findings on language organization in epilepsy are presented below.
ESM findings in epilepsy patients
Studies of ESM in patients with epilepsy have shown stimulation-identified language sites over a wide area of left lateral cortex, extending well beyond traditional Broca’s and Wernicke’s areas that have been identified by more acute injurious lesions such as stroke (Ojemann, 1979). This variability across individuals has been taken as evidence that traditional language landmarks cannot be used to predict localization of essential language cortex, underscoring the need for ESM prior to surgical resection. While this reasoning understandably drives the clinical use of ESM, further studies have sought to determine the influence of the particular tasks used to identify language cortex, and whether and which patient-related variables might underlie the varying patterns of positive language sites among individuals.
Task related patterns
It has been demonstrated repeatedly that at a given cortical site, stimulation can impair one specific language function while causing no disruption to another (e.g., naming but not reading might be impaired with stimulation of an anterior site on the middle temporal gyrus), emphasizing the critical influence of task in the identification of language cortex (Ojemann, 1983a; Schwartz, et al., 1999). From a heuristic perspective, this level of specificity has provided a corpus of data suggesting a functional topography of different aspects of language, to the extent that this can be generalized from preoperative epilepsy patients.
Although ESM studies of task specificity vary considerably in patient sample size, ranging from case reports (Boatman et al., 1998; Hart et al., 1998) to larger scale investigations of over 100 patients (Haglund, Berger, Shamseldin, Lettich, & Ojemann, 1994; Ojemann, 1979), the following patterns have emerged: Cortical sites at which stimulation impairs visual object naming have been reported across virtually all aspects of perisylvian cortex, yet are mainly found in mid- to posterior-temporal cortex and in the basal temporal region (Ojemann, 1979). Auditory description naming is typically disrupted at visual naming sites; however, “pure” auditory naming sites (i.e., where stimulation impairs auditory naming, but not visual naming) have generally been found anterior to visual naming sites in the lateral temporal region, primarily in anterior temporal cortex (Hamberger, et al., 2001; Hamberger, McClelland, et al., 2007). Various aspects of reading have been disrupted by stimulation in middle temporal gyrus and inferior parietal lobule (sentence completion: (Schwartz, et al., 1999)), and the inferior and lateral frontal, parietal and temporal cortex (reading syntax: anterior to visual naming sites, (Ojemann, 1990). Stimulation has been reported to disrupt auditory verbal comprehension primarily in the mid- to posterior-superior temporal gyrus (STG) (Boatman, et al., 1995; Schaffler, H.O., Lesser, & G.J., 1993) and middle portion MTG (Ilmberger, Eisner, U, & Reulen, 2001; Krauss et al., 1996), likely corresponding to classic Wernicke’s area, and in the basal temporal region.
Perhaps corresponding with traditional anterior, expressive language locations, findings with ESM have been reported to produce speech arrest in posterior inferior frontal cortex (Lesser, Luders, Dinner, Hahn, & L, 1984; Schaffler, et al., 1993), yet also in the posterior STG, inferior parietal lobule and superior aspect of posterior middle temporal gyrus (Schwartz, et al., 1999), and the perisylvian region of frontal, temporal and parietal cortex (Ojemann, 1983b) (More detailed reviews of task related ESM patterns can be found in Ojemann (1983b) and Hamberger, 2007 (Hamberger, 2007)). Thus, the topography of ESM-based language areas in presurgical epilepsy patients appear to overlap with traditional language regions, yet clearly extend beyond the previously-reported, more circumscribed areas.
Patient characteristics
Within the widespread distribution of ESM-based language sites found in epilepsy patients, certain patient-related factors have been reported to account for some of the inter-individual variability. Ojemann and Whitaker (Ojemann & Whitaker, 1978), and later, Devinsky and colleagues (2000) found an effect of intelligence level so that patients with lower IQ scores (Ojemann: IQ < 96; Devinsky IQ < 80) tended to have an overall greater number of naming sites compared to patients with higher IQ scores. Ojemann and Whittaker (1978) also found that patients with lower IQ scores were more likely to have parietal naming sites, whereas patients with higher IQ scores were more likely to have naming sites in the more typical, posterior superior temporal region (Ojemann & Whitaker, 1978). Mateer and colleagues found an effect of gender for naming sites, with men exhibiting more positive sites in general, and a higher proportion of sites in anterior temporal cortex than that observed in women (C. Mateer, Polen, & Ojemann, 1982). Devinsky et al. (2000) and Schwartz et al. (Schwartz, Devinsky, Doyle, & Perrine, 1998) found no gender differences, yet in addition to lower IQ scores, showed that left-handed patients and those with earlier age of seizure onset were more likely to have anterior temporal language sites (i.e., considered “atypical language representation”). These findings have been interpreted to reflect the displacement of classic language areas to adjacent regions due to early injury or epileptic activity (Devinsky, et al., 2000).
Effects of Brain Pathology
As noted above, Wada studies have suggested a greater probability of right hemisphere language among left temporal-lobe seizure patients, particularly in patients with epilepsy onset before age 5 years old (Rausch & Langfitt, 1991; Rausch & Walsh, 1984; Wada & Rasmussen, 1960). By contrast, Duchowny and colleagues (1996) found using ESM that language areas tended to remain within the left hemisphere, adjacent to, or even overlapping with developmental lesions (e.g., dysplasia) and epileptogenic regions in patients with early epilepsy onset (< age 5 years old). Only very large, early lesions acquired before age 5 that destroyed language cortex were associated with right hemisphere language.
In a retrospective study of left hemisphere dominant patients comparing patients with and without lesions, Haglund et al. (1994) found that relative to epilepsy patients without known lesions, individuals with temporal lobe gliomas had proportionally fewer superior temporal gyrus visual naming sites. However, in a more recent study comparing epilepsy patients with space-occupying lesions and epilepsy patients without lesions, Hamberger et al (2007) (Hamberger, McClelland, et al., 2007) found that both groups showed the previously reported auditory naming sites anterior to dual—visual/auditory naming sites (Hamberger, et al., 2001): however, naming sites in the group with lesions were clustered in the superior posterior perisylvian region (i.e., classic posterior language area), whereas naming sites in the non-group without lesions were scattered across the lateral temporal region, a pattern considered more “atypical” for language organization (Devinsky, Perrine, Llinas, Luciano, & Dogali, 1993). Examining the groups more closely, the patients without lesions had lower baseline verbal scores and earlier age of epilepsy onset, thus, it was hypothesized that the lesion-group pattern might have represented more normal language organization than that observed in the non-lesional group (see effect of IQ described above). These authors hypothesized that the more scattered distribution of naming sites in the non-lesion patients might reflect the development of additional naming sites in an attempt to compensate for damage to the original language area caused by chronic abnormal electrophysiological activity. Interestingly, many of the patients without lesions in this study had HS --a relatively common finding on structural MRI (and on postoperative pathological analysis) in TLE patients. In a subsequent study comparing TLE patients with HS and TLE patients without structural pathology, Hamberger and colleagues found proportionally fewer naming sites in anterior temporal cortex in HS patients (consistent with their lower risk of naming decline following anterior temporal resection for seizure control) and that overall, HS patients exhibited a more posterior distribution of auditory naming sites relative to that in TLE patients without structural pathology (Hamberger, Seidel, et al., 2007). Results were interpreted to reflect intra-hemispheric reorganization of language in response to the likely, early development of HS. The posterior displacement of naming sites was also considered consistent with the anterior propagation of epileptiform discharges in TLE (Emerson, Turner, Pedley, Walczak, & Forgione, 1995).
ESM in basal temporal cortex
The basal temporal language area was first identified in a case study using ESM by Luders and colleagues (Luders et al., 1985), and further studied in a series of TLE patients undergoing ESM prior to surgical resection. ESM of basal temporal cortex revealed multiple language functions disrupted during stimulation of primarily the fusiform gyrus, yet no disruption of complex nonverbal tasks (Burnstine et al., 1990; Krauss, et al., 1996; Luders, et al., 1986). Krauss et al. (1996) reported that 20/25 patients exhibited at least one site in the basal temporal region where stimulation impaired either visual naming, Token Test comprehension, reading, spontaneous speech, auditory description naming, or repetition. The most common stimulation evoked impairment involved visual naming (75%), mainly across sites scattered widely along the fusiform gyrus. Comprehension (Token Test performance) was the next most common stimulation-evoked impairment (52%), with considerable overlap between visual naming and comprehension sites. Fewer errors were associated with auditory description naming (24%) and repetition (18%).
Right Hemisphere ESM
Given its highly invasive nature, ESM is generally performed only in the presumed language dominant hemisphere. Theoretically, this creates a strong bias in the data, as it would be reasonable to ask the question regarding the effect of ESM for language conducted in the non-dominant hemisphere. In a single, unique study, Wyllie et al. (1990) reported that 15/15 patients who were left-hemisphere language dominant on Wada testing showed no evidence of right hemisphere language using ESM. Interestingly, 2/7 patients who were shown to be right-sided language dominant on Wada testing had language sites identified in the left hemisphere via ESM, raising the possibility of incomplete transfer of language to the right hemisphere, and highlighting that this appeared undetectable with Wada testing (Wyllie et al., 1990). Using ESM in bilateral language patients, Jabbour et al. (2005) found frontal and/or temporal language areas analogous to the classic essential language areas of the dominant left hemisphere in 4/6 patients identified with left sided ESM (Jabbour, Hempel, Gates, Zhang, & Risse, 2005).
One shortcoming of ESM is that electrical stimulation can only be applied to the cortical surface, rendering potential language areas in sulcal or deeper regions inaccessible for testing. Additionally, human language comprises multiple, integrated subprocesses, whereas ESM tasks are, by necessity, simplified due to the 10 second time restriction for test trials. As described below, neuroimaging techniques are less constrained in this way, allowing for investigation of more complex, perhaps, more ecologically-valid language tasks.
fMRI and language reorganization
Initially used only for research purposes, functional magnetic resonance imaging (fMRI) has become an increasingly-important clinical tool in the assessment of language areas in epilepsy, especially for use with surgical candidates. fMRI is based on the same general technology as structural MRI, but it extends beyond traditional anatomical imaging by measuring hemodynamic changes that coincide with mental operations; more specifically, it measures increased blood flow to local vasculature which is assumed to accompany neural activity in the brain. This results in a corresponding focal increase in deoxyhemoglobin (associated with the increase in blood flow occurring without a comparable increase in oxygen extraction), which alters the T2-weighted signal because of iron’s paramagnetic properties (Belliveau et al., 1990; Ogawa et al., 1993; Tank, Ogawa, & Ugurbil, 1992; Turner, Bihan, Moonen, Despres, & Frank, 1991). One major advantage of fMRI over other functional imaging techniques is that the deoxyhemoglobin acts as an endogenous contrast agent, thereby excluding the need for potentially harmful radiotracers for enhancement purposes. In a more general sense regarding the study of language and language reorganization, fMRI is advantageous because it is a non-invasive technique which can theoretically be used repeatedly and without time restrictions, it can be used with patients as well as healthy control subjects because it has minimal risk, and it provides detailed information, with high spatial resolution, about radiological changes both within and across cerebral hemispheres at the same time, allowing for the study of both language lateralization and localization. One of the major drawbacks of fMRI, however, is that specific functions might be difficult to isolate (i.e., so-called language tasks might also activate areas associated with auditory processing and sustained attention), so very careful research methods are needed to rule out extraneous influences when interpreting the results. It is also assumed that any underlying pathology has not affected cerebral hemodynamics in the expected regions of activation.
Supporting the use of fMRI as a valid alternative for language lateralization, a number of studies have demonstrated high concordance rates with the Wada procedure (Benke et al., 2006; Binder et al., 1996; Rutten, van Rijen, van Veelen, & Ramsey, 1999; Woermann et al., 2003), although, other research has indicated that the congruence can vary considerably depending on the specific methods that are applied (Balsamo & W.D., 2002; Benson et al., 1999; Lehericy et al., 2000). In a similar way, the use of fMRI for language localization has been supported by several studies which showed good concordance rates with direct ESM (Carpentier et al., 2001; FitzGerald et al., 1997; Pouratian, Bookheimer, Rex, Martin, & Toga, 2002; Ruge et al., 1999), although another study justifiably emphasized caution when making critical surgical decisions because the respective techniques were not perfectly correlated (Roux et al., 2003).
As described above, patients with epilepsy are known to have a higher incidence of atypical language representation than the general population (Rasmussen & Milner, 1977), with rates as high as 23% to 33% in patients with focal left temporal lobe epilepsy (Adcock, Wise, Oxbury, Oxbury, & Matthews, 2003; Brazdil, Zakopcan, Kuba, Fanfrdlova, & Rektor, 2003; Janszky et al., 2003). In one fMRI study (Springer et al., 1999), categorical classification yielded a clear difference between right-handed individuals who were neurologically normal (94% left-hemisphere language dominant, 6% bilateral-symmetric, 0% right-sided language dominant) and right-handed individuals with epilepsy (78% left language dominant, 16% bilateral-symmetric, 6% right language dominant), although the authors emphasized that nearly all of the subjects had some right-sided activation during the language tasks, supporting the notion that language representation is not a purely unilateral phenomenon. These consistent findings have suggested that the pathophysiology underlying seizure disorders can also contribute to a reorganization of language networks in epilepsy. Building on previous evidence from Wada and ESM studies, there is a growing body of fMRI research providing new insights into the mechanisms of neural plasticity for language.
Evidence for inter-hemispheric reorganization
In one study, Janszky et al. (2006) investigated whether the frequency of left-sided interictal activity (i.e., spikes or sharp waves) was associated with atypical speech lateralization between patients with left versus right medial temporal lobe epilepsy (MTLE) (Janszky, et al., 2006). Based on prior research which had shown a transient alteration of normal function caused by isolated interictal discharges (Aarts, Binnie, Smit, & Wilkins, 1984; Pressler, Robinson, Wilson, & Binnie, 2005), it was hypothesized that chronic interictal abnormalities would result in more long-term deficits and therefore induce a shift of language functioning to the right hemisphere. Using a covert word generation task, they showed that left MTLE patients did indeed have a higher incidence of atypical language representation than right MTLE patients. Furthermore, among the left MTLE group only, significant relationships were demonstrated between the frequency of interictal abnormalities and a left-to-right shift of language functions. Importantly, other clinical variables including gender, age, age at epilepsy onset, seizure frequency, IQ, and verbal fluency were not significant contributors. Consistent with their earlier Wada-based study (Janszky, et al., 2003), the authors concluded that chronic frequent interictal epileptic activity can induce language reorganization independently of other factors.
In a different study, Rosenberger et al. (2009) compared language patterns in patients with left hemisphere seizure foci with a normal control group (Rosenberger et al., 2009). Using an auditory-based word decision task to explore activation asymmetries in Broca’s and Wernicke’s areas, they categorized patients into either a left language group or an atypical language group. As expected, the patient groups showed an increased frequency toward right-sided activation overall. Among the patients with atypical lateralization, activation was seen in right-sided homologues of Broca’s and broadly defined Wernicke’s areas, closely mirroring these regions observed in the normal control group. By contrast, the patients with left lateralization showed only a slight trend for differences in the midtemporal gyrus (6mm posterior and 3mm superior) compared to the control group, but there was a considerable degree of variability. According to the authors, the results indicated that there was inter-hemispheric reorganization in the atypical patient group, but there was little evidence for intra-hemispheric reorganization in the left lateralized patient group.
Berl et al. (2005) investigated the degree of language dominance in patients with left- and right-hemisphere seizure foci compared to normal volunteers, using a reading comprehension task (Berl et al., 2005). Regions of interest included the inferior frontal gyrus, midfrontal gyrus, and Wernicke’s area. Not surprisingly, patients with left hemisphere foci had a higher likelihood of atypical language representation than patients with right hemisphere foci. Compared to healthy volunteers, patients in general had more activated voxels and lower asymmetry indices, reflecting an increased involvement of homologous right hemisphere areas, which was interpreted as showing “adaptive efforts at recruiting more widespread language processing networks.”
Using a younger sample, Yuan et al. (2006) performed a retrospective analysis, comparing language lateralization patterns between pediatric epilepsy patients and healthy controls (Yuan et al., 2006). Consistent with the main hypothesis and with previous research on adults, the patient group showed significantly more atypical lateralization patterns. A second purpose of the study was to determine whether age was a critical factor in the observed language reorganization. Previous research had shown that younger, healthy children are more likely to have atypical language lateralization than older healthy children, presumably showing that increased language specialization toward the left hemisphere is a function of normal development (Holland et al., 2001; Schapiro et al., 2004; Szaflarski et al., 2006). Yuan et al. then found a near-significant association between age and language lateralization in the control group, implying a trend that may have been obscured by the small sample size, but no significant association in the patient group, supporting the hypotheses that epilepsy can either disrupt the normal developmental convergence toward preferred language areas in the left hemisphere or, alternatively, either lead to an activation of pre-existing connections or an establishment of new compensatory areas in the right hemisphere.
Age was also a critical issue addressed in a case report by Hertz-Pannier et al. (2002), but in a different sense (Hertz-Pannier et al., 2002). The critical period for language acquisition has traditionally been thought to end around 6 years of age (Marcotte & Morere, 1990; Woods & Carey, 1979; Woods & Teuber, 1978) although more recent studies have challenged this notion by showing new right hemisphere language functioning in older children following left hemispherectomy (Boatman et al., 1999; Vargha-Khadem et al., 1997). In this case study, Hertz-Pannier et al. (2002) had the unique opportunity to obtain serial fMRI language examinations on a boy who developed intractable epilepsy related to Rasmussen’s syndrome at age 5 years-6 months old and underwent left hemispherotomy (i.e., complete disconnection but not removal of the left hemisphere) at age 9 years old, after previously normal and complete language acquisition. Pre-operative fMRI (obtained at age 6 years-10 months) reportedly showed left lateralization during a covert semantic word-generation task. By contrast, post-operative fMRI (obtained at age 10 years-6 months) reportedly showed right-sided activation during word generation, sentence generation, and story listening tasks. Most importantly, the authors stated that the latter activation was “seen mainly in regions that could not be detected preoperatively, but mirrored those previously found in the left hemisphere (inferior frontal, temporal, and parietal cortex), suggesting reorganization in a pre-existing bilateral network.” This de novo activation, it was suggested, provides direct longitudinal evidence that the boy’s previously non-dominant hemisphere was capable of sustaining late plasticity changes for language, a compelling argument which could formerly only be inferred from cross-sectional research.
Evidence for both inter- and intra-hemispheric reorganization
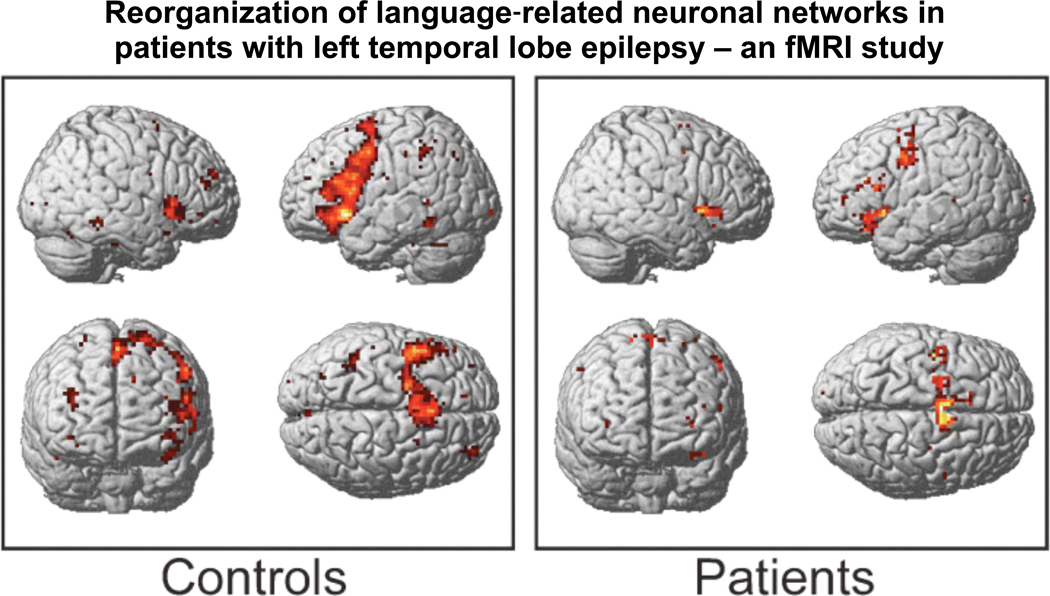
Brazdil et al. (2005) studied right-handed patients with unilateral left TLE (including suspected mesial onset zones and documented hippocampal sclerosis) versus a healthy control sample (Brazdil et al., 2005) (see Figure 1). As anticipated, the patient group showed a significantly more bi-hemispheric representation of language, based on a silent word generation task. Rather than simply looking at inter-hemispheric differences, however, the authors went one step further by looking for possible intra-hemispheric differences between the groups. Activation patterns among the healthy controls generally corresponded with known language circuits. Findings among the epilepsy patients were less consistent, with the most extensive cortical activation seen in the right inferior frontal gyrus, as well as significant activation in the left medial frontal gyrus extending into the right anterior cingulate gyrus. Perhaps most importantly, however, the patient group had a relative absence of activation in the traditional Broca’s area. Other clear differences were noted in the anterior cingulate, basal ganglia, and cerebellum, but these did not reach the level of statistical significance. Based on their findings, the authors argued that language does not simply shift from one hemisphere to the other, but that there is probably a very complex and individualized pattern of reorganization which involves both inter- and intra-hemispheric changes in neuronal networks.
Figure 1. With permission from European Journal of Neurology (Brazdil, et al., 2005).
The 3D views of average language activations for 13 epileptic patients and for 13 healthy controls. All activated voxels meet a significance threshold of P < 0.001 (uncorrected at a voxel level).
In related research, Mbwana et al. (2009) examined the location of language processing regions among a group of patients with left hemisphere seizure foci and normal control subjects (Mbwana et al., 2009). Although the patient sample was quite heterogeneous, the main purpose was to determine if left-sided epilepsy was associated with inter-hemispheric reorganization to right-sided homologues of Broca’s and Wernicke’s areas and/or intra-hemispheric reorganization in brain regions adjacent to these traditional language sites. As expected, the control subjects showed primary activation in known language areas. Findings among the patients were much less consistent, however, and several subgroups were identified. Group 1a had predominant left-sided activation with a significant cluster in the left posterior superior temporal sulcus. Group 1b had predominant left-sided activation with no significant differences from the control group. Group 2a had predominant right-sided activation with significant clusters in the in the right inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus, middle temporal gyrus, right cingulate, and left cerebellum. Group 2b had predominant right-sided activation with significant clusters in the right inferior gyrus, middle frontal gyrus, superior frontal gyrus, right angular gyrus, and left cerebellum. Taken together, the results were interpreted as showing tendencies for both intra-hemispheric reorganization (i.e., compensation by ipsilateral adjacent regions as shown in Group 1a) and inter-hemispheric reorganization (i.e., recruitment of contralateral homologous regions as shown in Groups 2a and 2b) in left-sided seizure disorders. With the exceptions of handedness and MRI-detected structural pathology, other factors such as gender, age, age of onset, indications of early insult, seizure duration, and location of seizure focus were not significantly different between Group 1 (left dominant) and Group 2 (right dominant), further suggesting that patterns of language reorganization must be formally tested on an individual basis, as they cannot be predicted reliably by these descriptive clinical variables.
Looking at a younger sample, Liegeois and colleagues studied language lateralization in children/ adolescents with epilepsy secondary to known structural lesions and a matched control group. Among the patients, half had lesions that were adjacent to or within anterior language cortex (i.e., Broca’s area) and half had lesions that were remote from traditional language sites (i.e., hippocampus, parahippocampus, temporal pole) (Liegeois, et al., 2004). Consistent with expectations, all of the control subjects showed predominant left-hemisphere lateralization. Among the patients, however, 50% were left-lateralized, 10% had bilateral language representation, and 40% were right-lateralized, reflecting a significant group difference. Of most significance, though, when the effect of lesion location was taken into consideration, the results contradicted previous findings reported in the literature (Devinsky et al., 1993; Isaacs, Christie, Vargha-Khadem, & Mishkin, 1996; Lazar et al., 2000; Rasmussen & Milner, 1977). Among the patients with lesions near Broca’s area, 80% still showed left-sided language dominance and, inversely, among the patients with lesions away from traditional language sites, 80% showed bilateral or right-sided language dominance. Clinical factors such as handedness, EEG abnormalities in the left frontal lobe or right hemisphere, early onset of chronic epilepsy, and age at first seizure did not appear to have any bearing on language lateralization. Interestingly, language reorganization appeared more likely when lesions involved the hippocampal region rather than classic language regions such as Broca’s or Wernicke’s areas. Similarly, an fMRI study in adults with varied lesion locations found that patients with left hippocampal sclerosis were more likely to have atypical language lateralization compared to patients with lesions in other locations (Weber et al., 2006). These results, together with the well established role of the left hippocampus in verbal learning, suggest a role of the left hippocampus in determining language lateralization. As in the other studies reviewed above, these findings provided similar evidence that language functioning can be re-routed both inter- and intra-hemispherically in patients with epilepsy, but the precise pattern of reorganization cannot always be predicted on the basis of lesion location and/or clinical factors alone.
The studies reviewed here are a brief, yet representative sampling of the still-growing fMRI literature on the subject of language and language reorganization in epilepsy. The inconsistent findings may be attributable, to some extent, to differential methodologies employed between studies. However, echoing the common sentiments of the researchers, it seems clear that language reorganization in epilepsy is a very complex and individualistic process which requires ongoing exploration to better our understanding of this phenomenon.
Closing comments
Without question, epilepsy has made significant contributions to the neuroscience of brain and language (Loring, 2010). Wada testing first challenged the notion of handedness as a reliable predictor of language dominance, allowing investigators to begin to elucidate the more complex relations between the nature and timing of left hemisphere brain abnormalities and language organization. Within a cerebral hemisphere, ESM provides investigators with a practical application of the lesion method, yet with a level of precision and control not afforded by naturally occurring lesions. As a neuroimaging technique, fMRI serves as a noninvasive activation method that enables detailed investigation of both hemispheres simultaneously, and allows for comparison with healthy controls, potentially creating a more comprehensive and more realistic picture of brain-language relations. Importantly, these distinct approaches have demonstrated a good degree of concordance on a theoretical level, yet what also emerged is a considerable degree of individual variability, attesting to the clinical importance of these methods to establish empirically language localization in individual patients.
Whereas traditional lesion studies have provided the foundation for our understanding of brain-language relations, epilepsy uniquely contributes the effects of both structural and functional disturbances within, adjacent and remote from classic language areas, at varying points during development, offering a rich resource of information regarding developmental aspects of brain-language relations. Over the years, efforts to improve patient care have also resulted in more sophisticated means of examining language organization in this population Moreover, as our understanding of factors that influence brain organization has advanced, creative use of the older techniques such as Wada testing and ESM have provided new insights as well. The unique features of epilepsy together with ongoing advances in technology and in our understanding of brain functioning, promise further advances in the neuroscience of organization and reorganization of language in individuals with epilepsy.
Acknowledgements
This work was supported by the National Institutes of Health: The National Institute of Neurological Disorders and Stroke [grant NIH R01 NS35140 to M.H.]
Acronym key
- EEG
Electroencephalogram
- ESM
Electrical Stimulation Mapping
- fMRI
functional Magnetic Resonance Imaging
- HS
Hippocampal Sclerosis
- MRI
Magnetic Resonance Imaging
- MTLE
Medial temporal lobe epilepsy
- TLE
Temporal lobe epilepsy
Footnotes
Disclosure: The authors report no conflicts of interest
References
- Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984;107:293–308. doi: 10.1093/brain/107.1.293. [DOI] [PubMed] [Google Scholar]
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, W.D G. The utility of functional mangnetic resonance imaging in epilepsy and language. Current Neurology and Neuroscience Reports. 2002;2:141–149. doi: 10.1007/s11910-002-0023-4. [DOI] [PubMed] [Google Scholar]
- Belliveau J, Rosen B, Kantor H, Rzedzian RKD, McKinstry R, Vevea J, et al. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med. 1990;14:538–546. doi: 10.1002/mrm.1910140311. [DOI] [PubMed] [Google Scholar]
- Benke T, Koylu B, Visani P, Karner E, Brenneis C, Bartha L, et al. Language lateralization in temporal lobe epilepsy: A comparison between fMRI and the Wada test. Epilepsia. 2006;47(8):1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LaSueur LL, Kennedy DN, Kwong KK, Buchbinder BR, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- Berl MM, Balsamo LM, Xu B, Moore EN, Weinstein SL, Conry JA, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65(10):1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Boatman D, Freeman J, Vining E, Pulsifer M, Miglioretti D, Minahan R, et al. Language recovery after left hemispherectomy in children with late-onset seizures. Annals of Neurology. 1999;46:579–586. doi: 10.1002/1531-8249(199910)46:4<579::aid-ana5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Boatman D, Hart J, Lesser RP, Honneycut N, Anderson NB, Miglioretti D, et al. Right hemisphere speech perception revealed by amobarbital injection and electrical interference. Neruology. 1998;51(2):458–464. doi: 10.1212/wnl.51.2.458. [DOI] [PubMed] [Google Scholar]
- Boatman D, Lesser RP, Gordon B. Auditory speech processing in the left temporal lobe: an electrical interference study. Brain and Language. 1995;51:269–290. doi: 10.1006/brln.1995.1061. [DOI] [PubMed] [Google Scholar]
- Branch C, Milner B, Rasmussen T. Intracarotid sodium amytal for the lateralization of cerebral speech dominance. Journal of Neurosurgery. 1964;21:399–405. doi: 10.3171/jns.1964.21.5.0399. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Chlebus P, Mikl M, Pazourkova M, Krupa P, Rektor I. Reorganization of language-related neuronal networks in patients with left temporal lobe epilepsy – an fMRI study. European Journal of Neurology. 2005;12:268–275. doi: 10.1111/j.1468-1331.2004.01127.x. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Zakopcan J, Kuba R, Fanfrdlova Z, Rektor I. Atypical hemispheric language dominance in left temporal lobe epilepsy as a result of the reorganization of language functions. Epilepsy & Behavior. 2003;4:414–419. doi: 10.1016/s1525-5050(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Burnstine TH, Lesser R, Hart J, Uematsu S, Zinreich SJ, Krauss GL, et al. Characterization of the basal temporal language area in patients with left temporal lobe epilepsy. Neurology. 1990;40:966–970. doi: 10.1212/wnl.40.6.966. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Pugh KR, Westerveld M, Studholme C, Skrinjar O, Thompson JL, et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42(10):1241–1254. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Perrine K, Hirsch J, McMullen W, Pacia S, Doyle W. Relation of cortical language distribution and cognitive function in surgical epilepsy patients. Epilepsia. 2000;41(4):400–404. doi: 10.1111/j.1528-1157.2000.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Perrine K, Llinas R, Luciano DJ, Dogali M. Anterior temporal language areas in patients with early onset of TLE. Annals of Neurology. 1993;34:727–732. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Perrine K, Llinas R, Luciano DJ, Dogali M, Devinsky O, et al. Anterior temporal language areas in patients with early onset of temporal lobe epilepsy. Annals of Neurology. 1993;34(5):727–732. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- Duchowny M, Jayakar P, Harvey AS, Resnick T, Alvarez L, Dean P, et al. Language cortex representation: Effects of developmental versus acquired pathology. Ann Neurol. 1996;40:31–38. doi: 10.1002/ana.410400108. [DOI] [PubMed] [Google Scholar]
- Emerson RG, Turner CA, Pedley TA, Walczak TS, Forgione M. Propagation patterns of temporal spikes. Electroencephalography and Clinical Neurophysiology. 1995;94:338–348. doi: 10.1016/0013-4694(94)00316-d. [DOI] [PubMed] [Google Scholar]
- Engel JJ, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy. In: J E Jr, Pedley TA, editors. Epilepsy: A comprehensive textbook. Lippincott-Raven: New York; 1997. pp. 2417–2426. [Google Scholar]
- FitzGerald DB, Cosgrove GR, Ronner S, Jiang H, Buchbinder BR, Belliveau JW, et al. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. American Journal of Neuroradiology. 1997;18:1529–1539. [PMC free article] [PubMed] [Google Scholar]
- Gloning I, Gloning K, Harb G, Quantember R. Comparison of verbal behavior in right-handed and nonright-handed patients with anatomically verified lesions in one hemisphere. Cortex. 1969;5 doi: 10.1016/s0010-9452(69)80006-7. [DOI] [PubMed] [Google Scholar]
- Haglund M, Berger M, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34(4):567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychology Review. 2007;4:477–489. doi: 10.1007/s11065-007-9046-6. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ. Cortical Mapping. In: Caplan KaJDB., editor. Encyclopedia of Clinical Neuropsychology. Springer Science: New York; 2011. [Google Scholar]
- Hamberger MJ, Goodman RR, Perrine K, Tammy T. Anatomical dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56:56–61. doi: 10.1212/wnl.56.1.56. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S, 3rd, McKhann GM, 2nd, Williams AC, Goodman RR, Hamberger MJ, et al. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia. 2007;48(3):531–538. doi: 10.1111/j.1528-1167.2006.00955.x. [Comparative Study Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, Williams AC, Perrine K, Devinsky O, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain. 2007;130:2942–2950. doi: 10.1093/brain/awm187. [Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- Hart J, Crone NE, Lesser RP, J S, Miglioretti D, Hall C, et al. Temporal dynamics of verbal object comprehension. Proc. Natl. Acad. Sci. USA. 1998 May;95:6498–6503. doi: 10.1073/pnas.95.11.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain and Cognition. 1997;33:135–150. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaque I, Renaux-Kieffer V, Van de Moortele P-F, Delalande O, et al. Late plasticity for language in a child’s non-dominant hemisphere: a pre- and post-surgery fMRI study. Brain. 2002;25:361–372. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball JWS. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Ilmberger J, Eisner W, U S, Reulen H. Performance in picture naming and word comprehension: Evidence for common neural substrates from intraoperative language mapping. Brain and Language. 2001;76:111–118. doi: 10.1006/brln.2000.2415. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Christie D, Vargha-Khadem F, Mishkin M. Effects of hemispheric side of injury, age at injury, and presence of seizure disorder on functional ear and hand asymmetries in hemiplegic children. Neuropsychologia. 1996;34:127–137. doi: 10.1016/0028-3932(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Jabbour RA, Hempel A, Gates JR, Zhang W, Risse GL. Right hemisphere language mapping in patients with bilateral language. Epilepsy & Behavior. 2005;6(4):587–592. doi: 10.1016/j.yebeh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Heinemann D, Schultz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126(9):2043–2051. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: An fMRI study. Epilepsia. 2006;47(5):921–927. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Krauss GL, Fisher RS, Plate C, Hart J, Uematsu S, Gordon B, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37(5):476–483. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Lazar RM, Marshall RS, Pile-Spellman J, Duong HC, Mohr JP, Young WL, et al. Interhemispheric transfer of language in patients with left frontal cerebral arteriovenous malformation. Neuropsychologia. 2000;38:325–1332. doi: 10.1016/s0028-3932(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Luders H, Dinner DS, Hahn J, L C. The location of speech and writing functions in the frontal language area. Brain. 1984;107:275–291. doi: 10.1093/brain/107.1.275. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SF, Gadian DG, Vergha-Khadem F, et al. Language reorganization in children with early-onset lseions of the left hemisphere: An fMRI study. Brain. 2004;127:1229–1236. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- Loring DW. History of neuropsychology through epilepsy eyes. Archives of Clinical Neuropsychology. 2010;25(4):259–273. doi: 10.1093/arclin/acq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring DW, Meader KJ, Lee GP, King DW. Amobarbital effects and lateralized brain function. Springer-Verlag: New York, Inc; 1992. [Google Scholar]
- Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, et al. Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28:831–838. doi: 10.1016/0028-3932(90)90007-b. [DOI] [PubMed] [Google Scholar]
- Luders H, Lesser R, Hahn J, Dinner DS, Morris HH, Resor SR, et al. Basal temporal language area demonstrated by electrical stimulation. Neurology. 1986;36:505–510. doi: 10.1212/wnl.36.4.505. [DOI] [PubMed] [Google Scholar]
- Luders H, Lesser RP, Hahn JF, Dinner DS, Morris HH, Harrison M. Language disturbances produced by electrical stimulation of the basal temporal region. Annals of Neurology. 1985;18:151. [Google Scholar]
- Malow BA, Blaxton TA, Susumu S, Bookheimer S, Kufta CV, Figlozzi CM, et al. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37(3):245–252. doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Marcotte AC, Morere DA. Speech lateralization in deaf populations: evidence for a developmental critical period. Brain and Language. 1990;39:34–152. doi: 10.1016/0093-934x(90)90008-5. [DOI] [PubMed] [Google Scholar]
- Mateer C, Polen S, Ojemann GA. Sexual variation in cortical localization of naming as determined by stimulation mapping. Commentary on McGlone 1980. Behavioral and Brain Sciences. 1982;5:310–311. [Google Scholar]
- Mateer CA, Dodrill CB. Neuropsychological and linguistic correlates of atypical language lateralization: evidence from sodium amytal studies. Human Neurobiology. 1983;2(3):135–142. [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132:347–356. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Branch C, Rasmussen T. Study of short term memory after intracarotid injection of sodium amobarbital. Transactions of the American Neurological Association. 1962;91:306–308. [Google Scholar]
- Möddel G, Lneweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50(6):1505–1016. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim S-G, Merkle H, Ellermann JM, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. Journal of Biophysics. 1993;64(3):803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA. Individual variability in the cortical localization of language. Journal of Neurosurgery. 1979;50:164–169. doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Brain organization for language from the perspective of electrical stimulation mapping. Behavioral Brain Research. 1983a;6:189–230. [Google Scholar]
- Ojemann GA. Electrical stimulation and the neurobiology of language. The Behavioral and Brain Sciences. 1983b;2:221–230. [Google Scholar]
- Ojemann GA. Organization of language cortex derived from investigations during neurosurgery. Seminars in Neuroscience. 1990;2:297–305. [Google Scholar]
- Ojemann GA, Whitaker H. Language localization and variability. Brain and Language. 1978;6(239–260) doi: 10.1016/0093-934x(78)90061-5. [DOI] [PubMed] [Google Scholar]
- Penfield W, Roberts L. Evidence from cortical mapping. In: Penfield LRW, editor. Speech and Brain Mechanisms. Princeton, New Jersey: Princeton University Press; 1959. pp. 119–137. [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. Journal of neurosurgery. 2002;97(1):21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Powell GE, Polkey CE, AG C. Lateralisation of memory functions in epileptic patients by use of the sodium amytal (Wada) technique. Journal of Neurology, Neurosurgery and Psychiatry. 1987;50(6):665–672. doi: 10.1136/jnnp.50.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RM, Robinson WO, Wilson GA, Binnie CD. Treatment of interictal epileptiform discharges can improve behavior in children with behavioural problems and epilepsy. Journal of Pediatrics. 2005;146:112–117. doi: 10.1016/j.jpeds.2004.08.084. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. New York Academy of Science. 1977;299:355–379. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Rausch R, Boone K, Ary CM. Right-hemisphere language dominance in temporal lobe epilepsy: Clinical and neuropsychological correlates. Journal of Clinical and Experimental Neuropsychology. 1991;13:217–231. doi: 10.1080/01688639108401039. [DOI] [PubMed] [Google Scholar]
- Rausch R, Langfitt J. Memory evaluation during the intracarotid sodium amobarbital procedure. Epilepsy Surgery. 1991:507–514. [Google Scholar]
- Rausch R, Walsh GO. Right-hemisphere language dominance in right-handed epileptic patients. Archives of Neurology. 1984;41(10):1077–1080. doi: 10.1001/archneur.1984.04050210075018. [DOI] [PubMed] [Google Scholar]
- Rey M, Dellatolas GJ, Bancaud J, Talairach J. Hemispheric lateralization of motor and speech functions after early brain lesion: study of 73 epileptic patients with intracarotid amytal test. Neuropsychologia. 1988;26:167–172. doi: 10.1016/0028-3932(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Risse GL, Gates JR, MC F. A reconsideration of bilateral language representation based on the intracarotid amobarbital procedure. Brain and Cognition. 1997;33(1):118–132. doi: 10.1006/brcg.1997.0887. [DOI] [PubMed] [Google Scholar]
- Rosenberger LR, Zeck J, Berl MM, Moore EN, Ritzl EK, Shamim S, et al. Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology. 2009;72(21):1830–1836. doi: 10.1212/WNL.0b013e3181a7114b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Boulanouar K, Lotterie J, Mejdoubi M, LeSage JP, Berry I. Language functional magnetic resonance imaging in preoperative assessment of language areas: Correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335–1347. doi: 10.1227/01.neu.0000064803.05077.40. [DOI] [PubMed] [Google Scholar]
- Ruge MI, Victor J, Hosain S, Correa DD, Relkin NR, Tabar V, et al. Concordance between functional MRI and intraoperative language mapping. Stereotactic and Functional Neurosurgery. 1999;72:95–102. doi: 10.1159/000029706. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, van Rijen PC, van Veelen CW, Ramsey NF. Language area localization with three-dimensional functional magnetic resonance imaging matches intrasulcal electrostimulation in Broca's area. Annals of Neurology. 1999;46(3):405–408. doi: 10.1002/1531-8249(199909)46:3<405::aid-ana17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Satz P, Strauss E, Wada J, Orsini DL. Some correlates of intra- and interhemispheric speech organization after left focal brain injury. Neuropsychologia. 1988;26(2):345–350. doi: 10.1016/0028-3932(88)90087-5. [DOI] [PubMed] [Google Scholar]
- Schaffler L, H.O L, Lesser RP, G.J C. Comprehension deficits elicited by electrical stimulation of Broca's area. Brain. 1993;116:695–715. doi: 10.1093/brain/116.3.695. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TH, Devinsky O, Doyle W, Perrine K. Preoperative predictors of anterior temporal language areas. J Neurosurgery. 1998;89:962–970. doi: 10.3171/jns.1998.89.6.0962. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Devinsky O, Doyle W, Perrine K. Function-specific high-probability "nodes" identified in posterior language cortex. Epilepsia. 1999;40(5):575–583. doi: 10.1111/j.1528-1157.1999.tb05559.x. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PSF, et al. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain. 1999;122:2033–2045. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Strauss E, Wada J. Lateral preferences and cerebral speech dominance. Cortex. 1983;19:165–177. doi: 10.1016/s0010-9452(83)80012-4. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW, Szaflarski JP, Holland SK, et al. fMRI study of language lateralization in children and adults. Human Brain Mapping. 2006;27(3):202–212. doi: 10.1002/hbm.20177. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank DW, Ogawa S, Ugurbil K. Mapping the brain with MRI. Brain Imaging. 1992;2(10):525–528. doi: 10.1016/0960-9822(92)90011-x. [DOI] [PubMed] [Google Scholar]
- Turner R, Bihan DL, Moonen CTW, Despres D, Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magnetic Resonance in Medicine. 1991;22:156–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Carr LJ, Isaacs E, Brett E, Adams C, Mishkin M. Onset of speech after left hemispherectomy in a nine-year-old boy. Brain. 1997;120:159–182. doi: 10.1093/brain/120.1.159. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance: experimental and clinical observations. Journal of Neurosurgery. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, et al. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;29(2):346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Woods BT, Carey S. Language deficits after apparent clinical recovery from childhood aphasia. Annals of Neurology. 1979;6:405–409. doi: 10.1002/ana.410060505. [DOI] [PubMed] [Google Scholar]
- Woods BT, Teuber HL. Changing patterns of childhood aphasia. Annals of Neurology. 1978;3:273–280. doi: 10.1002/ana.410030315. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dodrill CB, GA O. Brain injury, handedness, and speech lateralization in a series of amobarbital studies. Annals of Neurology. 1988;23(5):510–518. doi: 10.1002/ana.410230514. [DOI] [PubMed] [Google Scholar]
- Wyllie E, Luders H, Murphy D, Morris Hr, Dinner DS, Lesser R, et al. Intracarotid amobarbital (Wada) test for language dominance: correlation with results of cortical stimulation. Epilepsia. 1990;31(2):156–161. doi: 10.1111/j.1528-1167.1990.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Yuan W, Szaflarski JP, Schmithorst VJ, Schapiro M, Byars AW, Strawsburg RH, et al. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47(3):593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [Comparative Study Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ. Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium amytal test. Neuropsychologia. 1989;27(10):1207–1219. doi: 10.1016/0028-3932(89)90033-x. [DOI] [PubMed] [Google Scholar]