Abstract
Background. Intravenous (i.v.) iron sucrose similar (ISS) preparations are available but clinical comparisons with the originator iron sucrose (IS) are lacking.
Methods. The impact of switching from IS to ISS on anaemia and iron parameters was assessed in a sequential observational study comparing two periods of 27 weeks each in 75 stable haemodialysis (HD) patients receiving i.v. iron weekly and an i.v. erythropoiesis-stimulating agent (ESA) once every 2 weeks. Patients received IS in the first period (P1) and ISS in the second period (P2).
Results. Mean haemoglobin value was 11.78 ± 0.99 g/dL during P1 and 11.48 ± 0.98 g/dL during P2 (P = 0.01). Mean serum ferritin was similar for both treatment periods (P1, 534 ± 328 μg/L; P2, 495 ± 280 μg/L, P = 0.25) but mean TSAT during P1 (49.3 ± 10.9%) was significantly higher than during P2 (24.5 ± 9.4%, P <0.0001). The mean dose of i.v. iron per patient per week was 45.58 ± 32.55 mg in P1 and 61.36 ± 30.98 mg in P2 (+34.6%), while the mean ESA dose was 0.58 ± 0.52 and 0.66 ± 0.64 μg/kg/week, respectively (+13.8%). Total mean anaemia drug costs increased in P2 by 11.9% compared to P1.
Conclusions. The switch from the originator IS to an ISS preparation led to destabilization of a well-controlled population of HD patients and incurred an increase in total anaemia drug costs. Prospective comparative clinical studies are required to prove that ISS are as efficacious and safe as the originator i.v. IS.
Keywords: anaemia, cost analysis, haemodialysis, intravenous iron, iron sucrose
Introduction
Anaemia is a common comorbidity in chronic kidney disease (CKD). Its prevalence rises as renal function deteriorates, and it is estimated that up to 70% of patients are anaemic at the time of starting dialysis [1, 2]. Iron deficiency is an important contributor to renal anaemia, particularly in haemodialysis (HD) patients as a result of chronic dialysis-related blood loss and increased iron demand due to use of erythropoiesis-stimulating agents (ESA) [2]. Accordingly, intravenous (i.v.) iron supplementation is an important component in the therapeutic armamentarium for the management of anaemia in HD patients [3–5].
There are a number of iron carbohydrate compounds available on the market and among these compounds iron sucrose (IS) complex has a favourable safety profile when administered i.v. [6] as outlined in the European Best Practice Guidelines [3]. Iron carbohydrates such as IS are complex macromolecules, and their physico-chemical and biological properties are closely dependent on the manufacturing process such that subtle structural modifications may affect the stability of the preparation [7, 8]. Stability is of paramount importance, since weakly bound iron may dissociate too rapidly and catalyse the generation of reactive oxygen species [7–10] that in turn induce oxidative stress and inflammation. Potentially, an unstable iron complex could have safety and efficacy implications, especially in chronically ill individuals such as HD patients in whom dialysis and comorbidities already confer an increased burden of oxidative stress and inflammation [11, 12].
Several iron sucrose similar (ISS) preparations have been introduced for the treatment of iron deficiency anaemia in a number of countries worldwide [7, 11, 13] on the basis that they can be considered therapeutically equivalent to the originator i.v. IS (Vifor France SA; manufactured by Vifor International Inc., St. Gallen, Switzerland). However, recent analytical tests and studies in rat models have demonstrated differences between ISS preparations and the originator IS in terms of physico-chemical structure and their effect on inflammatory, haemodynamic and functional markers [7, 13]. To our knowledge, no head-to-head clinical study has yet compared ISS and IS in a patient population.
At the Centre Suzanne Levy, Paris, IS was used to correct iron deficiency anaemia in HD patients for 6 years prior to June 2009 at which point the centre switched to the ISS (Mylan SAS, Saint Priest, France manufactured by Help SA Pharmaceuticals, Athens, Greece) due to economic reasons. An observational retrospective and prospective analysis was undertaken to evaluate the impact of the switch from the originator IS to the ISS on haemoglobin (Hb) levels, iron parameters and dosing requirements for i.v. iron and ESA.
Materials and methods
Study design
This was an observational, non-interventional, single-centre single-cohort study. The study compared two time periods of 27 weeks each: Period 1 (P1; 1 December 2008 to 7 June 2009) during which patients received IS and Period 2 (P2; 29 June 2009 to 3 January 2010) during which patients received the ISS. Data for 3 weeks in June 2009 were excluded as both medications were simultaneously available at the centre. The medical management of the patients did not otherwise change during the study period except for the switch from IS to ISS. The study was initiated in September 2009 in response to concerns about Hb levels following introduction of the ISS. Data up to this point were obtained retrospectively from the patients’ medical records; subsequent data were collected prospectively. As an observational study, it did not require approval of the relevant Ethics Committee according to French regulations.
Objectives
The primary objective was to compare the impact of the switch from IS to the ISS on Hb levels and iron parameters. Secondary objectives were to describe the level of consumption of i.v. iron and ESA drugs and to estimate anaemia drug expenditure for the two treatment periods.
Patients
Patients undergoing chronic HD (three times a week) at the centre were included in the analysis if they underwent at least 60 dialysis sessions during both periods (a lower limit was allowed for patients attending for HD twice a week) and at least one prescription of i.v. iron during the study. IS (5-mL ampoules with 100 mg iron) or ISS (5-mL ampoules with 100 mg iron) were injected i.v. once a week at a dose of 25–100 mg iron. ESA (darbepoetin-α) was injected i.v. once every 2 weeks.
Evaluation
Hb, serum calcium, serum phosphorus, leucocytes, platelets, neutrophils and urea (before the HD session) were assessed every 2 weeks. Transferrin saturation (TSAT), serum ferritin, alkaline phosphatase, parathyroid hormone (PTH), 25-OH vitamin D, albumin, urea (after the HD session), adequacy of dialysis (Kt/V), C-reactive protein (CRP), fibrinogen, gamma-glutamyl transpeptidase (γ-GT), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin were measured every 3 months. All adverse events were reported according to applicable regulations and adverse events resulting in hospitalization were recorded.
Statistical analysis
Between-period comparisons were performed using the paired Student’s t-test and the paired Wilcoxon test for continuous data, and the McNemar test (χ2) for qualitative data. A hierarchical (mixed-effects) model [14] was used to estimate trends in the mean values of Hb for the following three time periods:
(1) baseline, i.e. the period of IS administration (Hb measurements 1–14),
(2) immediately before and after the switch to ISS (between Hb measurements 14 and 15) and
(3) after switch to ISS (measurements 15–28).
To carry out the analysis, splines with three knots at 14, 15 and 28 weeks were used, which defined three slopes corresponding, respectively, to the trends in Hb levels for the three periods defined. Hence, the estimate of the first spline in the mixed-effects model corresponds to change in Hb during the baseline period; similarly, the estimate of the second spline represents the change in Hb values immediately following the introduction of the new product and the estimate of the third spline represents the change in Hb values thereafter (with increases in dose of i.v. iron and ESA).
Both random-intercept and random-coefficients (random intercept and slopes) models were fitted to account for the repeated measures of Hb within each patient.
The cost of i.v. iron medications and ESA over time was calculated for both periods. The unit cost of i.v. iron (public price) was 12.98€/ampoule (100 mg iron) for IS and 10.20€/ampoule (100 mg iron) for ISS. The cost of darbepoetin-α at the centre was 1.47€/μg. Results were extrapolated to obtain a yearly incremental cost per patient by multiplying the daily cost per patient by 365. A national drug expenditure impact was estimated based on the assumption that the total French dialysis population (estimated to be 37 000 patients in January 2009 [15]) would be switched from IS to the ISS. This extrapolation is only indicative as unit cost estimates were centre specific, especially concerning darbepoetin-α.
Results
Patient population
Of 120 patients receiving dialysis at the centre, 75 patients were eligible for inclusion in the analysis. The demographics and clinical profile of the population were typical for adult recipients of chronic HD (Table 1) [16]. The mean number of dialysis sessions per patient performed was similar during P1 (75.1 ± 5.1) and P2 (75.1 ± 6.3, P = 0.97).
Table 1.
Baseline demographic and clinical characteristics (n = 75)a
| Age, years | 63.4 ± 15.2 |
| Female/male | 31%/69% |
| Duration of HD, months | 55.0 ± 53.1 |
| Primary renal disease, n (%) | |
| Diabetes | 28 (37.3) |
| Glomerulonephritis | 18 (24.0) |
| Hypertension | 13 (17.3) |
| Other nephropathies | 16 (21.3) |
Continuous variables are shown as mean ± SD.
Efficacy
Haemoglobin.
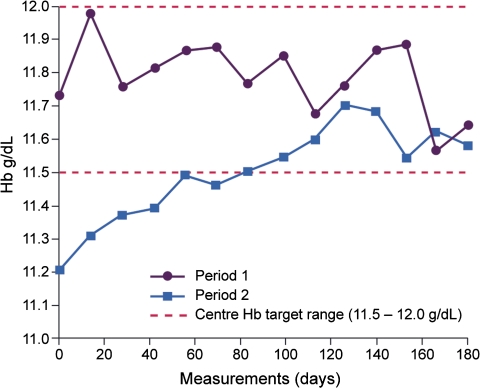
Mean Hb levels throughout the study are presented in Figure 1. Values during IS treatment were statistically significantly higher than during use of the ISS (P1, 11.78 ± 0.99 g/dL; P2, 11.48 ± 0.98 g/dL, P = 0.01). The difference was more pronounced when Hb values during P1 were compared to the first 3 months of P2 (11.78 ± 0.99 g/dL and 11.39 ± 1.12 g/dL, respectively, P = 0.005 versus P1). Mean Hb levels were similar during the first and last 3 months of P2 (11.39 ± 1.12 g/dL and 11.57 ± 1.09 g/dL, respectively, P = 0.15).
Fig. 1.
Mean haemoglobin levels over time by treatment period (grams per deciliter).
The mixed model (random intercept) analysis demonstrated that Hb levels were stable during the IS treatment period as the first slope (Hb measurements 1–14) was not statistically different from 0 (P = 0.105). The second slope (Hb measurements 14–15) was statistically different from 0 (P <0.0001) with a coefficient of −0.4 g/dL per two observations for the period, i.e. switch from IS to the ISS was associated with a significant decrease in mean Hb values of 0.4 g/dL (95% confidence interval −0.55 to −0.23). The third slope (Hb measurements 15–28) was also statistically different from 0 (P = 0.001) with an increase of 0.026 per two observations (0.34 g/dL). Results of the mixed model (random-intercept and slopes) also showed the second slope to be statistically different from 0 (P = 0.002) with a coefficient of −0.40 g/dL, but the third slope did not reach statistical significance (P = 0.167), although the trend was similar (0.020 per two observations).
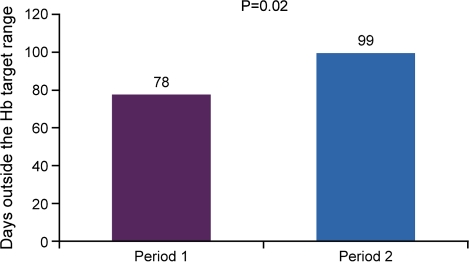
The proportion of patients who had at least one Hb value within the target range (11.5–12.0 g/dL) was 85% during P1 and 79% during P2 (P = 0.25). The mean number of days spent outside the target Hb range was 78 days per patient (cumulative 5880 days) during P1 compared to a mean of 99 days per patient (cumulative 7470 days) during P2 (P = 0.02) (Figure 2).
Fig. 2.
Mean number of days spent outside the haemoglobin target range (11.5–12.0 g/dL) by treatment period.
Iron parameters.
Mean serum ferritin was similar between the treatment periods (P1, 533.8 ± 327.5 μg/L; P2, 494.5 ± 279.9 μg/L, P = 0.25) but was statistically significantly higher in P1 versus the first evaluation performed in P2 (September) (457.7 ± 290.4 μg/L, P = 0.04) (Table 2). Mean TSAT during P1 (49.3 ± 10.9%) was statistically significantly higher than during P2 overall (24.5 ± 9.4%, P < 0.0001) or Month 3 of P2 (23.3 ± 10.2%, P < 0.0001) (Table 2).
Table 2.
Serum ferritin and TSAT during Period 1 and at Month 3 of Period 2 (September 2009)
| Period 1 (n = 75) | Month 3, Period 2 (n = 75) | P-value | |
| Serum ferritin (μg/L) | |||
| Mean ± SD | 533.8 ± 327.5 | 457.7 ± 290.4 | 0.04 |
| Median (range) | 551.2 (38.3–1051.5) | 432.1 (13.7–1541.1) | |
| TSAT (%) | |||
| Mean ± SD | 49.3 ± 10.9 | 23.3 ± 10.2 | <0.0001 |
| Median (range) | 47.7 (32.0–80.0) | 23.0 (5.0–44.0) |
Intravenous iron consumption.
The mean dose of i.v. iron per patient was 1231 ± 879 mg in P1 and 1657 ± 866 mg in P2 (P = 0.001), representing a 34.6% increase in i.v. iron during P2 versus P1. Corresponding values for i.v. iron per patient per week were 61.36 ± 30.98 mg in P2 and 45.58 ± 32.55 mg in P1 (P = 0.001), and the cumulative dose for the total study population was 92 300 mg in P1 versus 124 250 mg in P2.
ESA consumption.
The mean dose of ESA per patient was 980 ± 756 and 1103 ± 908 μg, respectively, during P1 and P2 (P = 0.12) representing a 12.6% increase. The mean dose according to body weight was 0.58 ± 0.52 μg/kg/week in P1 and 0.66 ± 0.64 μg/kg/week during P2 (P = 0.13) representing a 13.8% increase. The cumulative dose of ESA in P1 was 73 510 μg for the total study population and 82 750 μg in P2 (+12.6%).
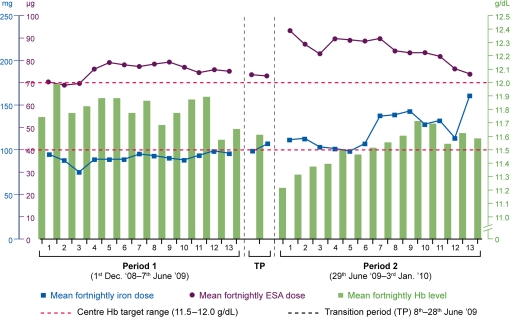
The mean fortnightly i.v. iron dose and concomitant ESA dose throughout the study versus achieved Hb levels are shown in Figure 3.
Fig. 3.
Mean fortnightly dose of i.v. iron (milligrams) and ESA [darbepoetin-α (micrograms)] versus achieved haemoglobin levels (grams per deciliter).
Economic impact.
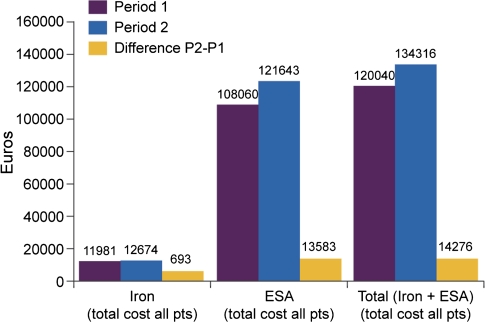
The cumulative cost of i.v. iron was 11 981€ during P1 and 12 674€ during P2. For ESA, the cumulative cost was 108 060€ during P1 and 121 643€ during P2. The total cost from the health care provider’s perspective (including both i.v. iron and ESA) was 120 040€ and 134 316€ during P1 and P2, respectively, a difference of 14 276€ (+11.9% increase) (Figure 4). The incremental cost of switching from IS to the ISS for 1 year was estimated to be about 368€ per patient.
Fig. 4.
Anaemia drug expenditure.
Safety
Mean values for serum phosphorus, Ca × P, 25-OH vitamin D and urea decreased significantly, and total bilirubin increased significantly, during P2 compared to P1 (Table 3). There were no significant differences between the two treatment periods regarding serum calcium, PTH, CRP, albumin, fibrinogen, γ-GT, alkaline phosphatase, ALT, AST, leucocytes, platelets, neutrophils or Kt/V. Mean CRP showed a non-significant increase from P1 [6.3 ± 6.9 mg/L (median 3.3 mg/L)] to the last 3 months of P2 [9.1 ± 14.6 (median 3.0 mg/L), P = 0.09] in 70 patients. A further CRP measurement was performed in January 2010 on the last day of use of ISS and the mean value was 13.7 mg/L.
Table 3.
Laboratory values with statistically significant differences between Periods 1 and 2 (n = 75)
| Period 1 | Period 2 | P-value | |
| Serum phosphorus (mmol/L) | |||
| Mean ± SD | 1.8 ± 0.5 | 1.6 ± 0.4 | 0.0002 |
| Median (range) | 1.7 (0.6–3.1) | 1.6 (0.8–2.3) | |
| Ca × P | |||
| Mean ± SD | 4.0 ± 1.1 | 3.7 ± 0.8 | <0.0001 |
| Median (range) | 4.0 (1.4–6.4) | 3.7 (1.8–5.1) | |
| 25-OH vitamin D (ng/mL) | |||
| Mean ± SD | 20.0 ± 9.2 | 16.7 ± 9.39 | 0.008 |
| Median (range) | 18.0 (5–45.5) | 16.0 (0–43) | |
| Urea before (mmol/L) | |||
| Mean ± SD | 22.6 ± 6.1 | 20.6 ± 4.6 | <0.0001 |
| Median (range) | 21.8 (11.9–42.7) | 20.7 (12.2–37.7) | |
| Urea after (mmol/L) | |||
| Mean ± SD | 6.6 ± 2.7 | 5.8 ± 1.9 | 0.002 |
| Median (range) | 6.3 (2.2–18.1) | 5.6 (2.1–11.1) | |
| Total bilirubin (μmol/L) | |||
| Mean ± SD | 7.7 ± 1.8 | 8.8 ± 2.3 | 0.001 |
| Median (range) | 7.7 (4.3–13.3) | 8.5 (5.0–18.0) |
No adverse events were observed in relation to the study drugs during the study and no hospitalization related to i.v. iron supplementation occurred. Adverse events that resulted in hospitalization were considered to be related to the pre-existing conditions including CKD. There was no significant difference concerning the proportion of hospitalized patients between P1 [12/75 (16.0%)] and P2 [11/75 (14.7%), P = 0.78]. Five patients were hospitalized twice during P1 and P2. Seven patients were hospitalized during P1 only and six patients were hospitalized during P2 only. The cumulative length of hospital stay was 191 days during P1 and 107 days during P2. The mean length of stay per hospitalized patient was similar during P1 and P2 (10.6 days versus 5.9 days, respectively, P = 0.461, Wilcoxon test on paired data).
Discussion
This is the first study to evaluate the clinical impact of switching from the originator i.v. IS to an ISS. In this population of stable HD patients, switch to an ISS was associated with a significant reduction in Hb level and reduced iron indices despite an increase in i.v. iron dose, ESA dose and an overall increase in drug expenditure.
The decrease in Hb level during ISS therapy coupled with a shorter proportion of time spent within the Hb target range may have clinical implications for this high-risk population. Cardiac complications and impaired quality of life are well-documented consequences of poor anaemia control in the CKD and HD populations [4, 5, 17, 18]. Moreover, there was a marked and significant increase in the dose of iron that was required after the switch, accompanied by a numerical increase in ESA dose. Long-term exposure to higher doses of drugs such as i.v. iron and ESA increase the risk of toxicity [19] and are associated with increased oxidative stress and inflammation [9] that can exacerbate the existing pro-oxidant effects of co-morbidities, uraemia and the dialysis procedure [20].
The marked reduction in Hb that was already apparent at the start of the ISS treatment period reflects the immediate effect of switching i.v. iron preparation. Towards the end of the 20-day interval in June 2009 when both the IS and ISS preparations were used, stock levels available at the centre suggest that two-thirds of patients were likely to have received ISS. Intravenous iron treatment exerts a rapid effect, with the administered iron being incorporated into red blood cells within days [21], such that the switch to ISS appears to have quickly led to lower Hb levels. We are not aware of any other factors that could have accounted for the change in Hb level from the end of Period 1 to the start of Period 2 in this single cohort of patients for whom management was otherwise consistent throughout the study.
The discrepancy in Hb levels seen during administration of IS and ISS may be due to variations in the stability of the complexes and the bioavailability of iron from the two preparations. It can be hypothesized that the kinetics of iron dissociation differ between the two complexes, affecting the pattern of iron distribution and storage. This is supported by the halving of mean TSAT level during ISS therapy compared to the original IS, which occurred despite regular application of i.v. iron and an increased iron dose. The reduction in TSAT indicates that a lower proportion of iron was available for erythropoiesis. If iron is not utilized for erythropoiesis, it may be sequestered in other compartments in the body from which it is not available for erythropoiesis and could potentially cause harmful effects, which would be consistent with the increased levels of bilirubin and CRP observed during the ISS treatment period. Two recent studies found significant iron deposits in the liver, kidney and heart tissue of rats following administration of ISS preparations compared to the originator i.v. IS [7, 13]. Moreover, proinflammatory cytokine levels such as interleukin-6 and tumor necrosis factor-α were higher in the liver, heart and kidney tissues of rats treated with ISS [7, 13], possibly due to iron accumulation in the wrong compartment. These results may indicate reduced stability of ISS complexes and increased oxidative stress and inflammation secondary to the presence of weakly bound iron [7, 8, 13]. Further studies are required to examine this issue in more detail.
Both preparations showed a good safety profile, with no adverse events related to either study drug being observed. A longer term prospective controlled study is required to evaluate the impact of the ISS on clinical safety parameters and to investigate the interchangeability with the originator IS.
The lower levels of TSAT and serum ferritin during ISS therapy, particularly during the first 3 months after switch, are likely to have contributed to increased ESA dosing, with economic implications. Besarab et al. [22] demonstrated that maintenance of TSAT between 30 and 50% through maintenance i.v. iron administration improves anaemia and reduces ESA dose requirements. Economically, in contrast to the expected reduction in drug cost due to the purchase of the less expensive ISS (−21%), total anaemia drug expenditure increased by 11.9% due to higher dosing of ESA and i.v. iron. This resulted in an absolute drug expenditure increase of ∼368€/year per patient, equivalent to ∼13.6 million €/year if extrapolated to the French HD population.
In conclusion, the switch from the originator IS to an ISS preparation led to destabilization of a well-controlled population of HD patients, with a significant decrease of Hb levels and iron indices coupled with a need to increase anaemia drug consumption. The economic rationale for switch to a less expensive iron preparation was negated by the increase in total drug costs. Prospective comparative studies are required to confirm the efficacy and safety of ISS and their interchangeability with the originator i.v. IS.
Acknowledgments
Data were recorded from Hemodial (PHP development). Independent statistical analysis was performed by Cemka Eval. Vifor Pharma Ltd provided financial support for the third party independent statistical analysis and did not contribute to the study design. Editorial support was provided by Denise Bumford of Denise Bumford Ltd, Northants, UK. The publication of this article was supported by Vifor Pharma Ltd. The authors wish to thank Dr Alessandra Moglia, PharmD, MSc, MPharmacol (Vifor Pharma Ltd, Zurich, Switzerland) for her contributions and critical review.
Conflict of interest statement. J.R. has received a consultancy fee from Vifor Pharma Ltd following the conclusion of this study. A.L. is currently conducting research for Vifor Pharma Ltd. A.K., E.L. and A.D. do not have any conflict of interest.
References
- 1.Valderrábano F, Hörl WH, Macdougall IC, et al. PRE-dialysis survey on anaemia management. Nephrol Dial Transplant. 2003;18:89–100. doi: 10.1093/ndt/18.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Macdougall IC. Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: oral or intravenous? Curr Med Res Opin. 2010;26:473–482. doi: 10.1185/03007990903512461. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19(Suppl 2):1–47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 4.National Collaborating Centre for Chronic Conditions. Anaemia Management in Chronic Kidney Disease: National Clinical Guideline for Management in Adults and Children. London: Royal College of Physicians; 2006. pp. 1–172. [PubMed] [Google Scholar]
- 5.National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Bailie GR, Clark JA, Lane CE, et al. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20:1443–1449. doi: 10.1093/ndt/gfh820. [DOI] [PubMed] [Google Scholar]
- 7.Toblli JE, Cao G, Oliveri L, et al. Differences between the original iron sucrose complex Venofer® and the iron sucrose similar Generis®, and potential implications. Port J Nephrol Hypert. 2009;23:53–63. [Google Scholar]
- 8.Schellekens U, Klinger E, Muhlebach S, et al. The therapeutic equivalence of complex drugs. Regul Toxicol Pharmacol. 2011;59:176–183. doi: 10.1016/j.yrtph.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Geisser P, Baer M, Schaub E. Structure/histotoxicity relationship of parenteral iron preparations. Drug Res. 1992;42:1439–1452. [PubMed] [Google Scholar]
- 10.Piga A, Roggero S, Longo F, et al. Evaluation and treatment of secondary iron overload. In: Beaumont C, Beris P, Beuzard Y, et al., editors. Disorders of Erythropoiesis, Erythrocytes and Iron Metabolism. Marseille, France: European Society of Haematology Handbook: Club de Globule et du Fer; 2009. pp. 585–604. [Google Scholar]
- 11.Goldberg LA. Iron sucrose similars. Hosp Pharm Eur. 2010;48:21–22. [Google Scholar]
- 12.Garcia-Fernandez N, Echeverria A, Sanchez-Ibarrola A, et al. Randomized clinical trial on acute effects of i.v. iron sucrose during haemodialysis. Nephrology. 2010;15:178–183. doi: 10.1111/j.1440-1797.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 13.Toblli JE, Cao G, Oliveri L, et al. Differences between original intravenous iron sucrose and iron sucrose similar preparations. Drug Res. 2009;59:176–190. doi: 10.1055/s-0031-1296383. [DOI] [PubMed] [Google Scholar]
- 14.Raudenbush SW, Bryk AS. Hierarchical Linear Models. Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 15.Couchoud C. The Renal Epidemiology and Information Network Registry (REIN) BEH. :75–77. http://www.invs.sante.fr/beh/2010/09_10/index.htm (4 October 2010, date last accessed) [Google Scholar]
- 16.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the dialysis outcomes and practice patterns study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 17.Locatelli F, Covic A, Eckardt K-U, et al. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP) Nephrol Dial Transplant. 2009;24:348–354. doi: 10.1093/ndt/gfn653. [DOI] [PubMed] [Google Scholar]
- 18.Locatelli F, Aljama P, Canaud B, et al. Target haemoglobin to aim for with erythropoiesis-stimulating agents: a position statement by ERBP following publication of the Trial to Reduce Cardiovascular Events with Aranesp(R) Therapy (TREAT) Study. Nephrol Dial Transplant. 2010;25:2846–2850. doi: 10.1093/ndt/gfq336. [DOI] [PubMed] [Google Scholar]
- 19.Singh AK. What is causing the mortality in treating the anemia of chronic kidney disease: erythropoietin dose or haemoglobin level? Curr Opin Nephrol Hypertens. 2010;19:420–424. doi: 10.1097/MNH.0b013e32833cf1d6. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli F, Canaud C, Eckardt KU, et al. Oxidative stress in end-stage renal disease. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 21.Beshara S, Lundqvist H, Sundin J, et al. Pharmacokinetics and red cell utilization of iron(III) hydroxide-sucrose complex in anaemic patients: a study using positron emission tomography. Br J Haematol. 1999;104:296–302. doi: 10.1046/j.1365-2141.1999.01179.x. [DOI] [PubMed] [Google Scholar]
- 22.Besarab A, Dalton CL. Maintaining higher TSATs and other iron indices is beneficial in management of anemic hemodialysis patients. Nephrol Nurs. 2001;28:429–434. [PubMed] [Google Scholar]