Abstract
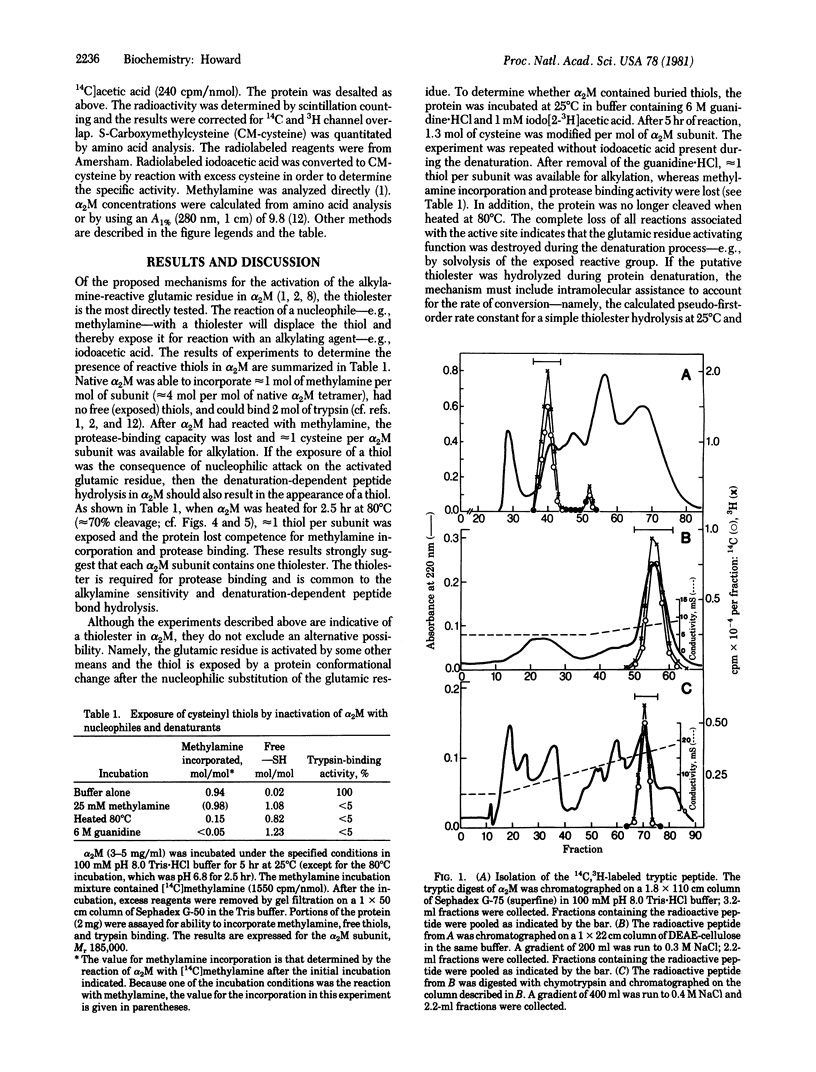
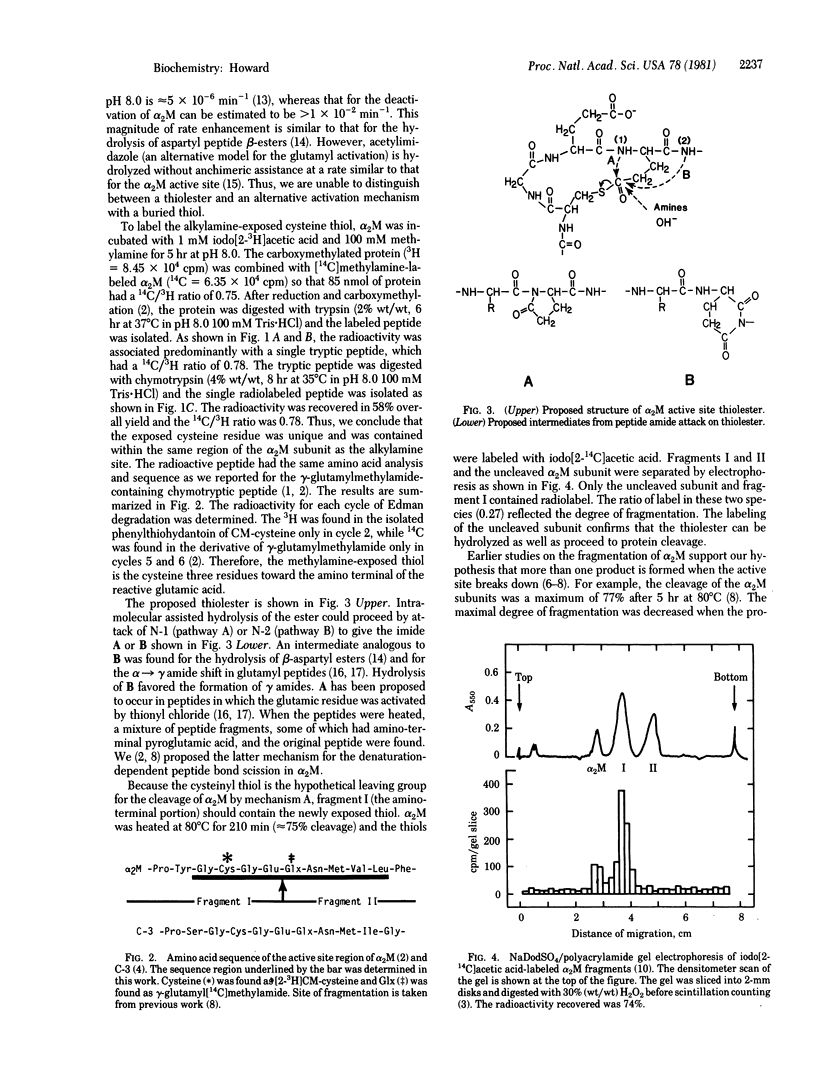
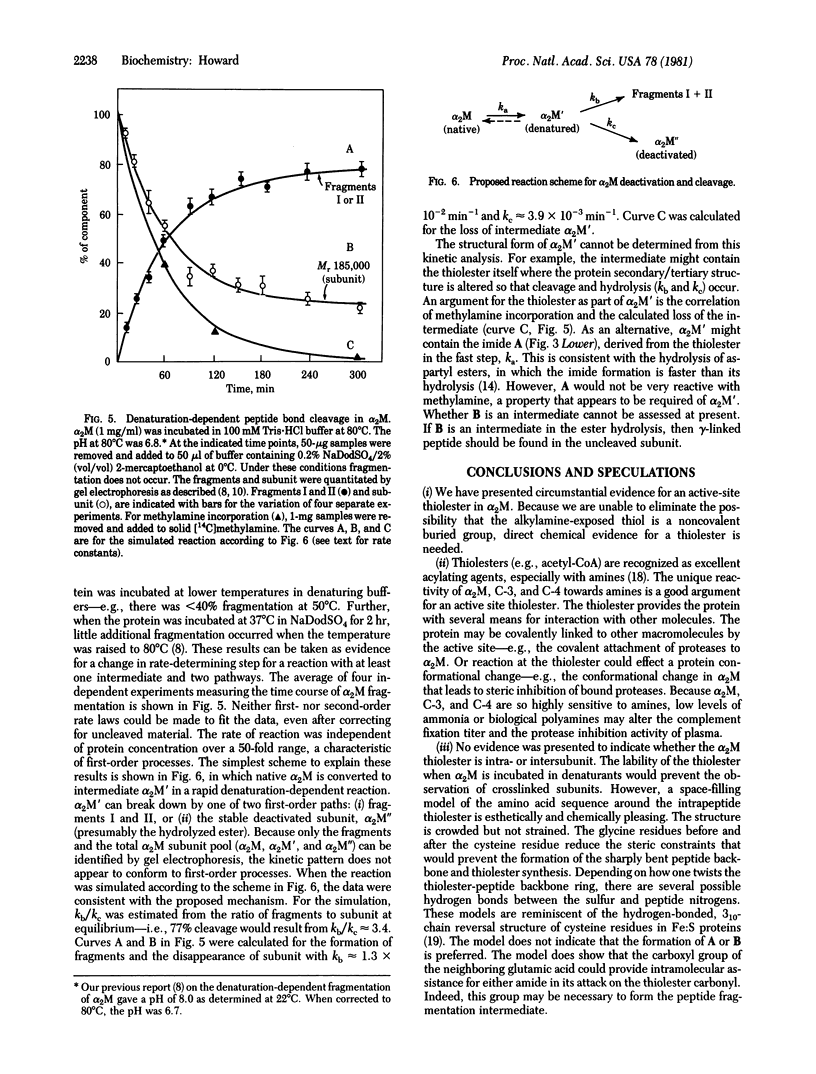
The reaction of methylamine with alpha 2-macroglobulin (alpha 2M) results in the covalent modification of one glutamic residue per subunit as gamma-glutamylmethylamide [Swenson, R. & Howard, J. B. (1979) Proc. Natl. Acad. Sci. USA 76, 4313--4316]. Furthermore, alpha 2M can undergo specific peptide autolysis involving the same reactive glutamic residue [Howard, J. B., Vermeulen, M. & Swenson, R. (1980) J. Biol Chem. 255, 3820--3823]. During both reactions, a cysteinyl thiol is exposed and can be alkylated by iodoacetic acid. After alpha 2M was modified with [14C]methylamine and iodo[2-3H]acetic acid, a tryptic peptide was isolated that contained both labels in the same ratio as in the original protein. From the chymotryptic digest of the tryptic peptide, a single radiolabeled peptide was isolated. The amino acid sequence of the chymotryptic peptide was the same as that previously reported to include gamma-glutamylmethylamide. This is circumstantial evidence for a thiolester between the cysteine and a glutamic acid located three residues away in the primary sequence. A reaction mechanism involving a pyroglutamyl intermediate derived from the thiolester is suggested to explain the autolysis. Kinetic analysis of the autolysis reaction is consistent with this intermediate and mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E., Watenpaugh K. D., Jensen L. H. NH---S hydrogen bonds in Peptococcus aerogenes ferredoxin, Clostridium pasteurianum rubredoxin, and Chromatium high potential iron protein. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4854–4858. doi: 10.1073/pnas.72.12.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J. P., Howard J. B. Effect of methylamine on the structure and function of the fourth component of human complement, C4. J Biol Chem. 1980 Nov 10;255(21):10025–10028. [PubMed] [Google Scholar]
- Harpel P. C., Hayes M. B., Hugli T. E. Heat-induced fragmentation of human alpha 2-macroglobulin. J Biol Chem. 1979 Sep 10;254(17):8669–8678. [PubMed] [Google Scholar]
- Howard J. B. Methylamine reaction and denaturation-dependent fragmentation of complement component 3. Comparison with alpha2-macroglobulin. J Biol Chem. 1980 Aug 10;255(15):7082–7084. [PubMed] [Google Scholar]
- Howard J. B., Vermeulen M., Swenson R. P. The temperature-sensitive bond in human alpha 2-macroglobulin is the alkylamine-reactive site. J Biol Chem. 1980 May 10;255(9):3820–3823. [PubMed] [Google Scholar]
- JENCKS W. P., CARRIUOLO J. Imidazole catalysis. III. General base catalysis and the reactions of acetyl imidazole with thiols and amines. J Biol Chem. 1959 May;234(5):1280–1285. [PubMed] [Google Scholar]
- Janatova J., Lorenz P. E., Schechter A. N., Prahl J. W., Tack B. F. Third component of human complement: appearance of a sulfhydryl group following chemical or enzymatic inactivation. Biochemistry. 1980 Sep 16;19(19):4471–4478. [PubMed] [Google Scholar]
- Janatova J., Tack B. F., Prahl J. W. Third component of human complement: structural requirements for its function. Biochemistry. 1980 Sep 16;19(19):4479–4485. doi: 10.1021/bi00560a015. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Amino acid sequence of the tryptic peptide containing the alkylamine-reactive site from human alpha 2-macroglobulin. Identification of gamma-glutamylmethylamide. J Biol Chem. 1980 Sep 10;255(17):8087–8091. [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Structural characterization of human alpha2-macroglobulin subunits. J Biol Chem. 1979 Jun 10;254(11):4452–4456. [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]


