Abstract
Persistent expression of certain oncogenes is required for tumor maintenance. This phenotype is referred to as oncogene addiction and has been clinically validated by anticancer therapies that specifically inhibit oncoproteins such as BCR-ABL, c-Kit, HER2, PDGFR, and EGFR. Identifying additional genes that are required for tumor maintenance may lead to new targets for anticancer drugs. Although the role of aberrant Wnt pathway activation in the initiation of colorectal cancer has been clearly established, it remains unclear whether sustained Wnt pathway activation is required for colorectal tumor maintenance. To address this question, we used inducible β-catenin shRNAs to temporally control Wnt pathway activation in vivo. Here, we show that active Wnt/β-catenin signaling is required for maintenance of colorectal tumor xenografts harboring APC mutations. Reduced tumor growth upon β-catenin inhibition was due to cell cycle arrest and differentiation. Upon reactivation of the Wnt/β-catenin pathway colorectal cancer cells resumed proliferation and reacquired a crypt progenitor phenotype. In human colonic adenocarcinomas, high levels of nuclear β-catenin correlated with crypt progenitor but not differentiation markers, suggesting that the Wnt/β-catenin pathway may also control colorectal tumor cell fate during the maintenance phase of tumors in patients. These results support efforts to treat human colorectal cancer by pharmacological inhibition of the Wnt/β-catenin pathway.
Mutational activation of the Wnt pathway occurs in the vast majority of colorectal cancers through truncating mutations in adenomatous polyposis coli (APC) or AXIN2 or mutations in GSK3-target residues in β-catenin (1–5). In normal intestinal cells, APC associates with axin, glycogen synthase kinase-3β (GSK-3β), and casein kinase 1 (CK1) to form a β-catenin destruction complex. This complex phosphorylates β-catenin, resulting in its ubiquitylation and subsequent degradation by the proteasome (6). In contrast, in cells harboring mutations in APC, AXIN2, or β-catenin, β-catenin accumulates and upon its translocation to the nucleus, interacts with the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors to activate specific Wnt target genes (7, 8).
Considerable evidence from human genetic studies and mouse genetic models suggests that mutational activation of the Wnt pathway initiates the process of colon tumorigenesis. Inherited APC mutations cause familial adenomatous polyposis, and acquired APC mutations represent the earliest genetic alteration so far detected in the genesis of sporadic colorectal cancer (9). Rare mutations in AXIN2 or β-catenin can also be present in small neoplastic lesions (5, 10). In experimental mouse models, loss of APC (11, 12) or expression of constitutively active β-catenin (13) is sufficient to drive polyp formation.
Inhibition of the Wnt pathway in colorectal cancer cells in vitro by overexpression of dominant-negative TCF4 or inducible β-catenin siRNA results in rapid cell cycle arrest and blocks a genetic program that is physiologically active in crypt progenitors. Consequently, colorectal cancer cells undergo differentiation (7, 14, 15). By imposing a proliferative crypt progenitor phenotype, aberrant Wnt pathway activation may allow benign tumors (polyps) to persist for many years, providing an opportunity for the acquisition of further mutations (e.g., in KRAS, SMAD-4, and P53 genes) required for the development of malignant colorectal tumors (16).
Although the role of Wnt pathway activation in the initiation of colon tumorigenesis has been well established, it is unclear whether tumors that have acquired additional mutations in oncogenes or tumor suppressor genes remain dependent on Wnt pathway activation. Although β-catenin siRNA inhibits engraftment of colorectal cancer cells (17), a recent study reports that inhibition of Wnt signaling in established colorectal xenografts (mutant for the β-catenin gene) by inducible β-catenin shRNA had no significant effect on tumor growth (18). Human colorectal tumors with mutations in β-catenin are usually less aggressive and smaller than those with APC mutations (19), suggesting that APC and β-catenin mutations are not functionally equivalent. Consistently, in addition to its function in the β-catenin degradation complex, APC can also directly contribute to the regulation of mitosis and apoptosis (20). Such β-catenin–independent APC functions may influence the degree of dependency on Wnt pathway activation for colorectal tumor maintenance. Given the large preponderance of APC mutations in human colorectal cancer, it is crucial to determine whether sustained Wnt pathway activation is required for maintenance of APC-mutant colorectal tumors.
In this study, we provide evidence that Wnt/β-catenin pathway activation is required for maintenance of colorectal tumors harboring APC mutations. We show that β-catenin inhibition in vivo strongly inhibited the growth of established APC-mutant colorectal tumor xenografts. Upon β-catenin inhibition, colorectal cancer cells underwent cell cycle arrest and rapidly started to differentiate into polarized intestinal epithelial cells, producing mucins and displaying microvilli. Such differentiated cancer cells, when relieved from β-catenin inhibition, could dedifferentiate and regain their proliferative potential in vitro. In addition, we report that in human colonic adenocarcinomas high levels of nuclear β-catenin correlated with crypt progenitor but not differentiation markers, suggesting that the Wnt/β-catenin pathway may also control colorectal tumor cell fate during the maintenance phase of tumors in patients.
Results
Sustained Wnt/β-Catenin Pathway Activation Is Required for Maintenance of Colorectal Tumors Harboring APC Mutations.
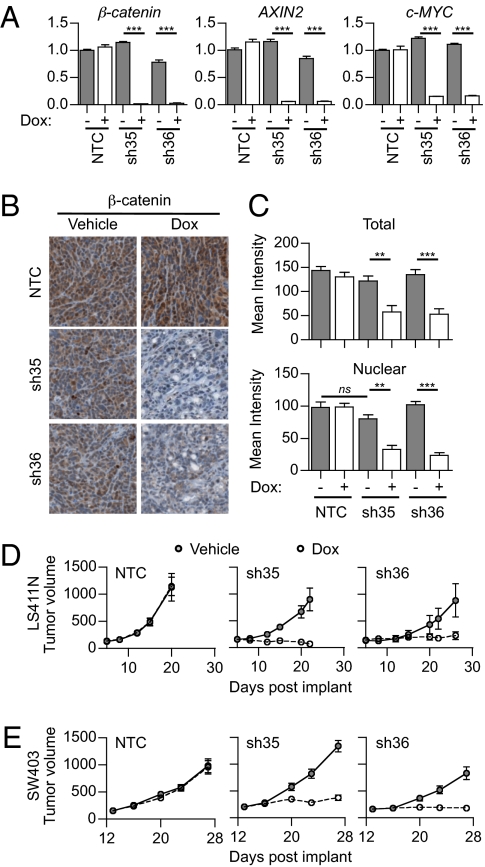
To analyze the role of sustained Wnt pathway activation in maintenance of colorectal tumors, we used doxycycline-inducible β-catenin shRNAs to temporally control Wnt pathway activation in vivo. We infected LS411N and SW403 colorectal cancer cells (both APC mutant) with a robust inducible single-lentiviral vector pLKO-Tet-On (21), containing either control nontargeting (NTC) shRNA or two distinct β-catenin–targeting shRNA sequences (shRNA-35 or shRNA-36). Polyclonal stable lines were obtained after puromycin selection and inoculated s.c. in Nu/Nu mice. To confirm that the doxycycline treatment led to inhibition of the Wnt/β-catenin pathway, we treated mice bearing established tumors (100–300 mm3) with doxycycline for 3 d and analyzed various molecular components of the Wnt/β-catenin pathway. Upon doxycycline treatment, β-catenin expression was dramatically reduced at the level of both mRNA (knockdown efficiency >95%, Fig. 1A) and protein in the β-catenin shRNA tumors but not in the NTC shRNA tumors (Fig. 1 B and C and Fig. S1 A and B). As expected, silencing of β-catenin caused a concomitant reduction of β-catenin target genes AXIN2 and c-MYC at the mRNA and protein levels (Fig. 1A and Fig. S1 E–H). We noted that β-catenin inhibition had a more dramatic effect on c-MYC expression in SW403 versus LS411N cells (up to 99% and 50% reduction of nuclear c-MYC staining intensity, respectively). Specificity of the β-catenin shRNAs was confirmed in vitro: Decreased cell viability was noted only in LS411N and SW403 colorectal cancer cell lines, not in RKO colorectal cancer cells that are wild type for β-catenin and APC (Fig. S1 I–N). Taken together, these results indicate that the β-catenin shRNAs efficiently and specifically inhibit the Wnt/β-catenin pathway.
Fig. 1.
Tumor growth is inhibited by β-catenin shRNA in vivo. (A–C) LS411N cancer cells stably expressing dox-inducible NTC, sh35, or sh36 β-catenin shRNA were inoculated into mice. Tumor-bearing mice were treated for 3 d with either vehicle or doxycycline (n = 3). (A) Quantitative RT-qPCR of tumor β-catenin, AXIN2, and c-MYC after 3 d of treatment. Graphs represent mean ± SEM values. Arbitrary units are shown. (B) Representative images of β-catenin staining by IHC after 3 d of treatment. (C) Mean signal intensity of total β-catenin (Upper) and nuclear β-catenin (Lower) after 3 d of treatment. Graphs represent mean ± SEM values. (D and E) LS411N (D) or SW403 (E) cancer cells stably expressing dox-inducible NTC, sh35, or sh36 β-catenin shRNA were inoculated into mice. When tumor volume reached 100–300 mm3, mice were treated continuously with either vehicle (gray circles) or doxycycline (white circles) and tumor growth was monitored. Graphs represent mean ± SEM values. Two independent experiments are represented (n = 6–8 per treatment group).
We next investigated the effect of β-catenin shRNAs on tumor growth and treated mice bearing established tumors with doxycycline for 14 d. Such treatment induced long-term silencing of β-catenin as demonstrated by down-regulation of β-catenin at day 14 (Fig. S1 C and D). Both LS411N and SW403 β-catenin shRNA tumors showed a significant inhibition of tumor growth following doxycycline treatment (Fig. 1 D and E). This effect was due to inhibition of the Wnt/β-catenin pathway rather than doxycycline treatment alone, as NTC shRNA tumors progressed rapidly despite treatment. Interestingly, nuclear β-catenin depletion by β-catenin shRNAs was more complete after short doxycycline treatment in LS411N tumors (60–75% and 45% reduction of nuclear β-catenin at days 3 and 14, respectively). This observation suggests that there might be some selective pressure favoring cancer cells that either had mild initial β-catenin depletion or managed to escape silencing by β-catenin shRNAs. We conclude that sustained Wnt/β-catenin pathway activation is required for the maintenance of colorectal tumor xenografts harboring APC mutations.
In Vivo Inhibition of β-Catenin in APC-Mutant Colorectal Cancer Cells Results in Cell Cycle Arrest.
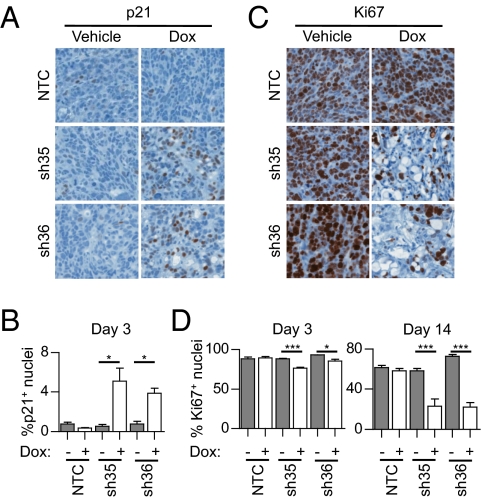
We next studied the mechanisms underlying the requirement of the active Wnt pathway for tumor maintenance and analyzed the effect of β-catenin shRNAs on tumor cell cycle in vivo. Because c-MYC represses expression of the cyclin-dependent kinase inhibitor p21 (22), we evaluated p21 expression 3 d after doxycycline treatment. Doxycycline induction of β-catenin shRNA caused a significant induction of p21 expression in vivo. Although p21 induction occurred in both LS411N and SW403 β-catenin shRNA xenografts, p21 induction was stronger in SW403 tumors where β-catenin shRNAs had the most profound effect on c-MYC expression (Fig. 2 A and B and Fig. S2 A and B). To determine whether p21 induction resulted in proliferation effects, we next analyzed Ki67 expression. β-Catenin silencing in tumors led to a decreased percentage of nuclei positive for Ki67, as early as day 3, that was further decreased at day 14 (Fig. 2 C and D). The effect of β-catenin silencing on Ki67 expression was more robust in SW403 xenografts (Fig. S2 C and D). These results are consistent with previous studies reporting that disruption of endogenous β-catenin/TCF4 activity in colorectal cancer cells in vitro induces rapid cell cycle arrest (7, 14, 15). In our study, no change in cleaved caspase 3 staining intensity was detected at day 3 (Fig. S3 A–D), suggesting that the cell cycle arrest was not coupled to apoptotic cell death. Although the mechanisms are poorly understood, cell cycle regulation may also crosstalk with autophagy (23). Wnt pathway inhibition may activate autophagy as total LC3 protein level rose at day 3 of β-catenin silencing. However, no change in the LC3-II/LC3-I ratio or in p62 expression was observed (Fig. S3 E–G). Our results demonstrate that a major consequence of inhibition of β-catenin in vivo is cell cycle arrest of APC-mutant colorectal cancer cells.
Fig. 2.
β-Catenin shRNA induces cell cycle arrest of colorectal cancer cells in vivo. (A–D) LS411N cancer cells containing NTC, sh35, or sh36 β-catenin shRNA were inoculated into mice. Tumor-bearing mice were treated for 3 or 14 d with either vehicle or doxycycline. (A) Representative images of p21 staining by IHC after 3 d of treatment. (B) Percentage of nuclei positive for p21 after 3 d of treatment. Graphs represent mean ± SEM values. (C) Representative images of Ki67 staining by IHC after 14 d of treatment. (D) Percentage of nuclei positive for Ki67 after 3 d (Left) or 14 d (Right) of treatment. Graphs represent mean ± SEM values. Two independent experiments are represented (n = 3–8 per treatment group).
In Vivo Inhibition of β-Catenin Induces APC-Mutant Colorectal Cancer Cell Differentiation.
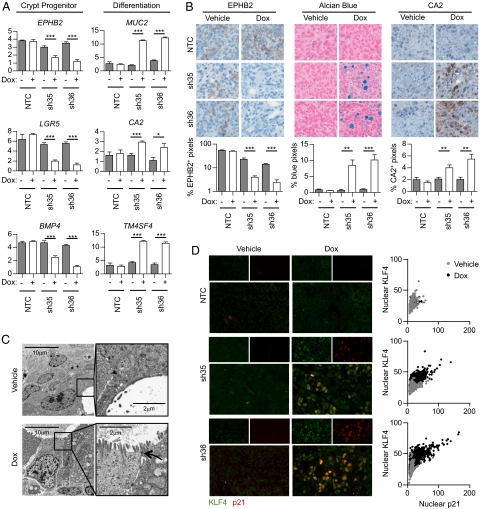
Cell cycle arrest is often sufficient to induce differentiation. Similarly, induction of a differentiation program can trigger cell cycle withdrawal (24). We, therefore, assessed whether inhibition of β-catenin induces differentiation of APC-mutant colorectal cancer cells in vivo. To do so, we analyzed the expression of various markers of the colon crypt progenitor/stem cells and of differentiation of intestinal epithelial cells by RT-qPCR. Three genes that have been previously described as crypt progenitor/stem cell markers, ephrin type-B receptor 2 (EPHB2) (Fig. S4A), bone morphogenetic protein 4 (BMP4) (14), and leucine-rich repeat-containing G-protein–coupled receptor 5 (LGR5) (25), were significantly down-regulated in LS411N β-catenin shRNA tumors after 3 d doxycycline treatment, suggesting that β-catenin imposes a crypt progenitor/stem cell phenotype on LS411N cells. Concomitantly, differentiation markers such as carbonic anhydrase 2 (CA2) (Fig. S4B), mucin 2 (MUC2) (26), and transmembrane 4 L six family member 4 (TM4SF4) (27) were significantly up-regulated (Fig. 3A). The changes in EPHB2, mucins, and CA2 protein levels were confirmed by immunohistochemistry. Doxycycline treatment led to decreased EPHB2 and increased CA2 protein expression as well as mucin production (Fig. 3B), confirming that β-catenin inhibition induced LS411N cell differentiation. Similarly, β-catenin silencing induced differentiation of SW403 cells in vivo (Fig. S2 E and F). We also noted the appearance of gland-like structures upon β-catenin shRNA induction, suggesting that as the colorectal cancer cells acquire phenotypic characteristics of mucosecretory intestinal cells, they may undergo major morphological changes. Electron microscopy analysis of cellular morphology revealed that cancer cells in the vehicle-treated tumors were unpolarized and rarely formed gland-like structures. The rare gland-like structures present had a paucity of microvilli and lacked apical–lateral junctions (Fig. 3C). In contrast, after 3 d of doxycycline-induced β-catenin knockdown, the tumor cells became polarized and displayed numerous microvilli and apical–lateral junctions, acquiring morphology similar to that of normal intestinal epithelial cells (Fig. 3C). Consistent with differentiation toward an intestinal epithelial phenotype, we found no evidence of an epithelial–mesenchymal transition (Fig. S5). Costaining for the differentiation marker KLF4 (28) (Fig. S4C) and p21 revealed that differentiation and cell cycle arrest were highly correlated on the cellular level (Fig. 3D). Immunohistochemical staining of serial sections with CA2 and p21 showed a similar trend (Fig. S6). Thus, whereas β-catenin imposes a proliferative crypt progenitor phenotype on APC-mutant colorectal cancer cells, its depletion induces a rapid and dramatic differentiation process accompanied by cell cycle arrest.
Fig. 3.
Colorectal cancer cell differentiation is induced by β-catenin shRNA in vivo. (A–D) LS411N cancer cells containing NTC, sh35, or sh36 β-catenin shRNA were inoculated into mice. Tumor-bearing mice were treated for 3 d with either vehicle or doxycycline. (A) Quantitative RT-qPCR of tumor expression of crypt progenitor markers including EPHB2, LGR5, and BMP4 and differentiation markers including MUC2, CA2, and TM4SF4 after 3 d of treatment. Expression was normalized to 18S mRNA. Graphs represent mean ± SEM values. Arbitrary units are shown. (B) Representative images of EPHB2 staining (Left) by IHC after 3 d of treatment. Percentages of positive pixels are indicated. Graphs represent mean ± SEM values. (Center) Representative images of Alcian blue staining after 3 d of treatment. Percentages of blue pixels are indicated. Graphs represent mean ± SEM values. (Right) Representative images of CA2 staining by IHC after 3 d of treatment. Percentages of positive pixels are indicated. Graphs represent mean ± SEM values. Two independent experiments are represented (n = 3 per treatment group). (C) Representative electron microscopy images of sh36 β-catenin shRNA tumors treated for 3 d with either vehicle (Upper) or doxycyline (Lower). Arrow indicates apical–lateral junction. (D, Left) Representative images of KLF4 (green) and p21 (red) costaining by immunofluorescence after 3 d of treatment. (Right) Mean intensity of nuclear KLF4 as a function of mean intensity of nuclear p21 in the indicated conditions. Each dot represents one nucleus. At least 1,000 nuclei were analyzed per condition. Correlation between mean intensity of nuclear KLF4 and mean intensity of nuclear p21: P < 0.0001 (Spearman's correlation test).
Reversibility of Cell Cycle Arrest and Differentiation Induced by β-Catenin Silencing.
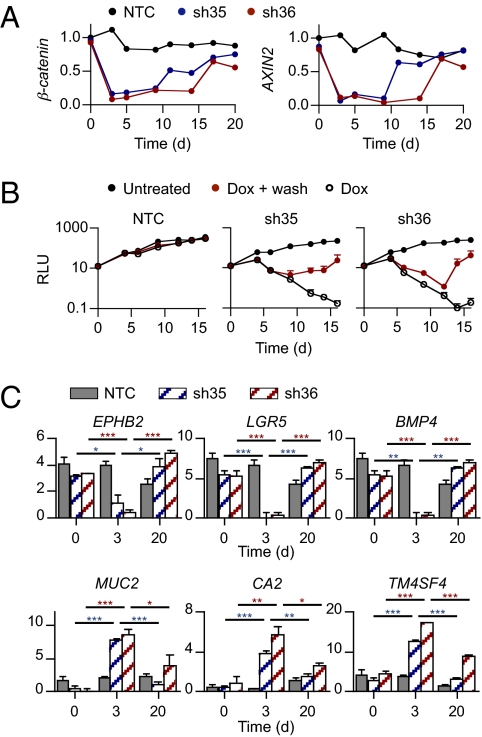
To determine whether the cell cycle arrest and differentiation induced by Wnt/β-catenin pathway inhibition are reversible, we took advantage of our inducible β-catenin shRNAs that allow us to inhibit (upon doxycycline treatment) and then restore (upon doxycycline withdrawal) β-catenin expression in APC-mutant colorectal cancer cells. Similarly to what we observed in vivo, doxycycline treatment for 3 d in vitro induced marked down-regulation of β-catenin, AXIN2, and c-MYC expression in LS411N cells containing β-catenin shRNA but not NTC shRNA (Fig. 4A and Fig. S7 A–C). Consequently, LS411N cells showed reduced in vitro proliferation (Fig. 4B and Fig. S7D) and significant decrease in expression of crypt progenitor/stem cell markers (EPHB2, BMP4, and LGR5) concomitant with increased expression of differentiation markers (CA2, MUC2, and TM4SF4) (Fig. 4C and Fig. S7 E–G). To determine whether such differentiated cancer cells could regain their proliferative potential and dedifferentiate upon Wnt/β-catenin pathway reactivation, after 3 d of doxycycline treatment, LS411N cells were either kept in the presence of doxycycline or further cultured in doxycycline-free medium. Within 8–14 d after doxycycline withdrawal, β-catenin expression was restored and the Wnt/β-catenin signaling pathway was reactivated as determined by AXIN2 mRNA expression (Fig. 4A). Interestingly, upon doxycycline withdrawal, LS411N β-catenin shRNA cells resumed their proliferation (Fig. 4B) and appeared to reacquire a crypt progenitor/stem cell phenotype, losing differentiation markers (CA2, MUC2, and TM4SF4) and reexpressing crypt progenitor/stem cell markers (EPHB2, BMP4, and LGR5) (Fig. 4C). In contrast, cells maintained under continuous doxycycline treatment did not proliferate. This observation excludes the possibility that the growth observed upon doxycycline withdrawal was due to cells that were resistant to knockdown, as those cells would also be present in the population treated continuously with doxycycline. We cannot exclude the possibility that knockdown within a subset of the LS411N β-catenin shRNA cells was inefficient, giving rise to partially differentiated cells that may cycle slowly but not completely arrest, and that it is this subpopulation that appears to revert upon doxycycline withdrawal. Although our results suggest that the cell cycle arrest and differentiation induced by Wnt/β-catenin pathway inhibition may be reversible, future studies designed to track the fate of individual tumor cells are needed to definitively prove reversibility.
Fig. 4.
Reversibility of cell cycle arrest and differentiation induced by β-catenin silencing. (A–C) LS411N cancer cells containing NTC, sh35, or sh36 β-catenin shRNA were grown in vitro in the absence or presence of doxycycline for 3 d. At day 3, doxycycline was washed out and cells were further incubated in the absence or presence of doxycycline for 15 d. (A) Quantitative RT-qPCR of remaining β-catenin and AXIN2 at indicated time points. Expression was normalized to 18S mRNA. Graphs represent mean ± SEM values. Arbitrary units are shown. (B) In vitro cell growth of LS411N cancer cells containing NTC (Left), sh35 (Center), or sh36 (Right) β-catenin shRNA. The number of viable cells was assessed at indicated time points. Graphs represent mean ± SEM Relative Luminescence Unit (RLU). Three independent experiments are represented. (C) Quantitative RT-qPCR of expression of crypt progenitor markers including EPHB2, LGR5, and BMP4 and of differentiation markers including MUC2, CA2, and TM4SF4 at indicated time points in LS411N cancer cells containing NTC (gray), sh35 (blue), or sh36 (red) β-catenin shRNA. Expression was normalized to 18S mRNA. Graphs represent mean ± SEM values. Arbitrary units are shown.
Nuclear β-Catenin Correlates with Crypt Progenitor and Differentiation Markers in Human Colonic Adenocarcinomas.
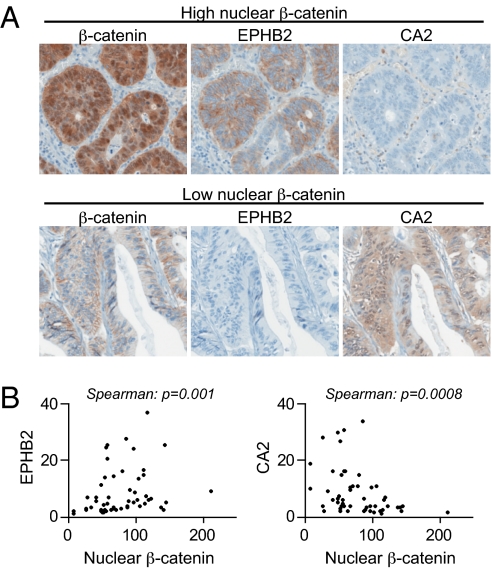
Next, we asked whether activation of the Wnt/β-catenin pathway correlates with a crypt progenitor/stem cell phenotype in colorectal tumors in patients. We analyzed the expression of nuclear β-catenin, the crypt progenitor/stem cell marker EPHB2, and the differentiation marker CA2 in 52 human colorectal adenocarcinomas. These parameters did not correlate with tumor stage or histologic grade (Fig. S8). However, we observed that human colorectal tumors with high nuclear β-catenin often had strong expression of the crypt progenitor/stem cell marker EPHB2 and weak expression of the differentiation marker CA2. In contrast, tumors with weak nuclear β-catenin staining had the opposite profile (Fig. 5). Correlations between nuclear β-catenin intensity and EPHB2 and CA2 expression were significant (respective P values: 0.0009 and 0.001). Our findings in human colorectal adenocarcinomas are similar to those of van de Wetering et al. in early neoplastic lesions (aberrant crypt foci) (14), strongly suggesting that the Wnt/β-catenin pathway continues to control colorectal tumor cell fate in established tumors in patients.
Fig. 5.
In human colonic adenocarcinomas, high levels of nuclear β-catenin correlate with crypt progenitor not differentiation markers. (A) Representative images of β-catenin, EPHB2, and CA2 staining by IHC on serial sections from tumors presenting either high (Upper) or low (Lower) levels of nuclear β-catenin. (B) Mean signal intensity of EPHB2 staining (Left) and CA2 staining (Right) as a function of the mean intensity of nuclear β-catenin staining. Each dot represents one patient. n = 52 patients.
Discussion
The role of aberrant Wnt pathway activation in the initiation of colon tumorigenesis is well recognized. However, the degree to which established colorectal tumors that have acquired additional mutations depend on sustained Wnt pathway activity is unclear. Here we show that Wnt/β-catenin pathway activation is required for maintenance of colorectal tumors harboring APC mutations, as β-catenin inhibition markedly reduced the growth of established colorectal tumor xenografts in mice. Consistent with previous in vitro studies, β-catenin inhibition resulted in cell cycle arrest and differentiation of the colorectal cancer cells. In some cases inactivation of oncogenes or restoration of tumor suppressor genes in established tumors has irreversible consequences (e.g., restoration of p53 expression in murine lymphomas results in apoptosis) (26). In contrast, we report that upon Wnt/β-catenin pathway reactivation, colorectal cancer cells in vitro resumed their proliferation and dedifferentiated, indicating that, in this experimental setting, the effects of transient Wnt pathway inhibition appeared reversible.
Interestingly, whereas we observed that Wnt/β-catenin pathway activation is required for the maintenance of APC-mutant colorectal tumors, Mologni et al. recently reported that β-catenin inhibition had no significant effect on the maintenance of colorectal tumors harboring β-catenin mutations (18). Similarly, an early study by Chan et al. showed that targeted deletion of the mutant allele of β-catenin in HCT116 colorectal cancer cells did not affect growth or survival in vitro (29). Although the number of cell lines analyzed so far is limited, there are several mechanisms by which mutations in APC and β-catenin may differentially affect the degree of Wnt pathway dependency. First, the magnitude of aberrant Wnt pathway activity may differ in colorectal tumors containing APC versus β-catenin mutations. A comparison of colorectal cancer cell lines harboring either APC or β-catenin mutations did not reveal obvious differences in the extent of Wnt pathway activation (Fig. S9 A–C). Second, the β-catenin–independent functions of APC may contribute to the differences in Wnt pathway dependency, as loss of APC may also affect mitotic progression and reduce apoptosis (27). Finally, APC and β-catenin–mutant colorectal tumors may select for different profiles of cooperating mutations. Consistently, colorectal cancer cell lines mutant for either APC or β-catenin do show different cooperating mutations (Fig. S9D). Specifically, whereas both LS411N and SW403 colorectal cancer cells are wild type for phosphoinositide-3-kinase, catalytic, α-polypeptide (PIK3CA), the β-catenin–mutant colorectal cancer cell lines used by Mologni et al. have activating PIK3CA mutations. Given the important role of phosphoinositide-3-kinase (PI3K) in the regulation of cell proliferation and survival (30), activating mutations of PIK3CA may represent an alternative driver of tumor growth in colorectal cancer cells harboring β-catenin mutations, thereby leading to Wnt pathway independence for tumor maintenance. Further studies will be required to test these hypotheses. Other potential differences between the colorectal tumor xenografts used by Mologni et al. and those in the present study may include growth factor expression or tumor–stromal interactions.
Mirroring findings in early neoplastic lesions (14), we report that high levels of nuclear β-catenin in human colorectal adenocarcinomas correlate with crypt progenitor but not differentiation markers, suggesting that the Wnt/β-catenin pathway continues to control colorectal tumor cell fate in established tumors in patients. Together with the requirement of sustained Wnt/β-catenin pathway activation for the maintenance of APC-mutant colorectal tumor xenografts, these observations support efforts to treat APC-mutant colorectal cancer by pharmacological inhibition of the Wnt/β-catenin pathway.
Materials and Methods
Cell Culture.
LS411N, SW403, and RKO cancer cells were obtained from the American Type Culture Collection. Cells were cultured as indicated in SI Methods.
Lentivirus and Infection.
Lentiviral supernatant production and cell infection were performed as described in SI Methods.
RNA Extraction and Quantitative Reverse Transcription–PCR.
Total RNA was isolated as described in SI Methods. An ABI taqman gene expression assay was performed as indicated in SI Methods.
Growth Assay.
Cell proliferation assays upon shRNA-mediated knockdown of β-catenin were performed by seeding 1,000 cells/well in a 96-well plate (triplicate) in the absence and presence of doxycycline. Cell Titer Glo measurements were taken at several time points according to the manufacturer's (Promega) instruction to track cell proliferation.
Immunohistochemistry, Immunofluorescence, and Image Analysis.
Antibodies used are described in SI Methods. Xenograft tumor samples were fixed in 10% neutral-buffered formalin for 6–24 h, processed, and paraffin embedded. Archival formalin-fixed, paraffin-embedded human colorectal adenocarcinoma specimens were procured by Maine Medical Center Tissue Bank, Maine Medical Center Pathology Department, Portland, ME. Immunohistochemical and immunofluorescent staining was performed on the Ventana Discovery System. Images were captured using an Aperio Scanscope or an Aperio Scanscope FL (Aperio Technologies) and analyzed as described in SI Methods. The necrotic area and stromal tissue portions were manually excluded, using the tools provided by the imaging systems.
In Vivo Efficacy Study of Doxycycline-Inducible β-Catenin shRNA.
All animals were handled in accordance with Novartis Institutes for Biomedical Research Animal Care and Use Committee protocols and regulations. Mice were housed in a temperature- and humidity-controlled animal facility with ad libitum access to food and water and acclimated for at least 3 d before experimental manipulation. Female Nu/Nu mice (6–8 wk old; Taconic) were inoculated s.c. with 5 × 106 cells in the right dorsal axillary region. Tumor dimensions were measured with calipers, and tumor volume was calculated as (length × width2)/2. Mice bearing 100–300 mm3 established tumors were randomized and enrolled into treatment groups (n = 6–8 per group). Mice received either vehicle (5% dextrose in water) or doxycycline hyclate (25 mg/kg, once a day) via oral gavage for the duration of the study.
Statistical Analysis.
Unpaired t tests and Spearman's correlation tests were used to determine statistical significance. Symbols used: *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Supplementary Material
Acknowledgments
We thank Humphrey Gardner and the Maine Medical Center Tissue Bank for tissue collaborations, and Yeonju Shim for constructing the Tissue MicroArray.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104182108/-/DCSupplemental.
References
- 1.Jin LH, et al. Detection of point mutations of the Axin1 gene in colorectal cancers. Int J Cancer. 2003;107:696–699. doi: 10.1002/ijc.11435. [DOI] [PubMed] [Google Scholar]
- 2.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 3.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 4.Rubinfeld B, et al. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 6.Polakis P. Casein kinase 1: A Wnt'er of disconnect. Curr Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- 7.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 8.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 9.Powell SM, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 10.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 11.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 12.Shibata H, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 13.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 15.van de Wetering M, et al. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 2003;4:609–615. doi: 10.1038/sj.embor.embor865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 17.Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–1300. [PubMed] [Google Scholar]
- 18.Mologni L, et al. Colorectal tumors are effectively eradicated by combined inhibition of beta-catenin, KRAS, and the oncogenic transcription factor ITF2. Cancer Res. 2010;70:7253–7263. doi: 10.1158/0008-5472.CAN-10-1108. [DOI] [PubMed] [Google Scholar]
- 19.Samowitz WS, et al. Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 1999;59:1442–1444. [PubMed] [Google Scholar]
- 20.Dikovskaya D, et al. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiederschain D, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, et al. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene. 2003;22:351–360. doi: 10.1038/sj.onc.1206145. [DOI] [PubMed] [Google Scholar]
- 23.Cecconi F, Levine B. The role of autophagy in mammalian development: Cell makeover rather than cell death. Dev Cell. 2008;15:344–357. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Skoultchi AI. Coordinating cell proliferation and differentiation. Curr Opin Genet Dev. 2001;11:91–97. doi: 10.1016/s0959-437x(00)00162-3. [DOI] [PubMed] [Google Scholar]
- 25.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 26.Carrato C, et al. Differential apomucin expression in normal and neoplastic human gastrointestinal tissues. Gastroenterology. 1994;107:160–172. doi: 10.1016/0016-5085(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 27.Wice BM, Gordon JI. A tetraspan membrane glycoprotein produced in the human intestinal epithelium and liver that can regulate cell density-dependent proliferation. J Biol Chem. 1995;270:21907–21918. doi: 10.1074/jbc.270.37.21907. [DOI] [PubMed] [Google Scholar]
- 28.Shie JL, et al. Role of gut-enriched Krüppel-like factor in colonic cell growth and differentiation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G806–G814. doi: 10.1152/ajpgi.2000.279.4.G806. [DOI] [PubMed] [Google Scholar]
- 29.Chan TA, Wang Z, Dang LH, Vogelstein B, Kinzler KW. Targeted inactivation of CTNNB1 reveals unexpected effects of beta-catenin mutation. Proc Natl Acad Sci USA. 2002;99:8265–8270. doi: 10.1073/pnas.082240999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.