Abstract
During protein synthesis, deacylated transfer RNAs leave the ribosome via an exit (E) site after mRNA translocation. How the ribosome regulates tRNA dissociation and whether functional linkages between the aminoacyl (A) and E sites modulate the dynamics of protein synthesis have long been debated. Using single molecule fluorescence resonance energy transfer experiments, we find that, during early cycles of protein elongation, tRNAs are often held in the E site until being allosterically released when the next aminoacyl tRNA binds to the A site. This process is regulated by the length and sequence of the nascent peptide and by the conformational state, detected by tRNA proximity, prior to translocation. In later cycles, E-site tRNA dissociates spontaneously. Our results suggest that the distribution of pretranslocation tRNA states and posttranslocation pathways are correlated within each elongation cycle via communication between distant subdomains in the ribosome, but that this correlation between elongation cycle intermediates does not persist into succeeding cycles.
Keywords: FRET, alternating wavelength laser excitation (ALEX), multi-translation cycles, classical state, hybrid state
Understanding of the mechanism of protein synthesis has improved dramatically through application of a multidisciplinary approach involving X-ray crystallography (1, 2), cryoelectron microscopy (3, 4), chemical footprinting (5), and ensemble (6–13) and single molecule kinetic studies (14–17). However, understanding the interplay of ribosome conformation and function during translation of mRNA sequences into proteins requires detailed real-time detection of the structural dynamics of polypeptide elongation.
Three tRNA binding sites, designated aminoacyl (A), peptidyl (P), and exit (E) sites, are present in all prokaryotic and eukaryotic ribosomes. In the elongation cycle of a bacterial ribosome, the A site binds an aminoacyl-tRNA (aa-tRNA) as a ternary complex (TC), elongation factor Tu (EF-Tu)·GTP·aa-tRNA. This is followed by peptide bond formation that transfers the nascent peptide from peptidyl-tRNA bound in the P site to the aa-tRNA in the A site, resulting in formation of the pretranslocation (PRE) complex. Elongation factor G (EF-G)·GTP binding to the PRE complex induces translocation of mRNA and peptidyl-tRNA and deacylated tRNA into the P and E sites, respectively, leading to formation of the posttranslocation (POST) complex. The function of the E site has been much debated because the E-site tRNA has been observed, in different ensemble studies, to dissociate either before or after binding of the next TC (18–20). This point is important for the translation mechanism because communication of E-site occupancy to other parts of ribosome has been postulated to increase fidelity of cognate tRNA selection at the ribosomal decoding site (the A site), to preserve reading frame (21, 22), to regulate programmed frameshifting (23, 24) and to determine the preference of the ribosome for binding either EF-Tu or EF-G (25).
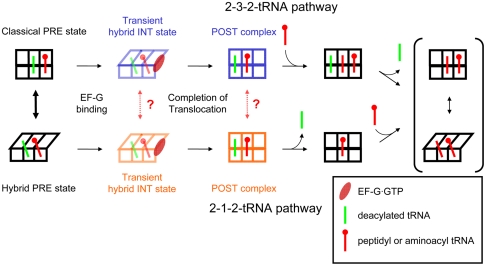
Following translocation, aminoacyl-tRNA binding to the A site of the POST complex to form the next PRE complex can occur either prior to or following E-site tRNA dissociation from the ribosome. We denote the two options as the 2-3-2 tRNA and 2-1-2 tRNA pathways, respectively, where the integers indicate the number of tRNAs occupying the ribosome during the conversion of the POST complex to the subsequent PRE complex (Fig. 1). Prior studies seeking to determine the partitioning between these two pathways have been limited by the transient nature of the putative 3-tRNA state. Averaging among individual ribosomes in ensemble experiments blurs the distribution among ribosomes containing zero to three tRNAs (18, 19). Single molecule fluorescence techniques have become powerful tools to study the translation mechanism in individual ribosomes, including tracking the bound ligands, such as tRNAs (14–17, 26). Here we use single molecule fluorescence resonance energy transfer (smFRET) experiments with alternating wavelength laser excitation (ALEX) (17, 27) to determine conditions in which the ribosome follows the 2-1-2 and 2-3-2 pathways and to identify the mechanisms that control A-site/E-site interaction.
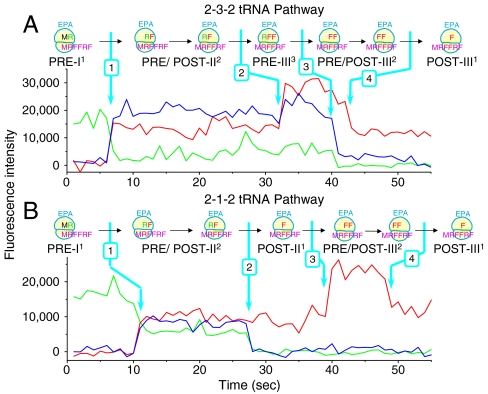
Fig. 1.
Two representative single molecule fluorescence traces. (A) The 2-3-2-tRNA pathway and (B) 2-1-2-tRNA pathway. Initiation complexes (ICs) programmed by mRNA MRFFRFYRF (Table S1; single letter amino acid code) were premixed with Arg-tRNAArg(Cy3) TC. The resulting PRE-I1 complex (in which PRE, I, and 1 indicate pretranslocation complex, first translation cycle after formation of the IC, and presence of one labeled tRNA, respectively) was immobilized on a glass cover slip. ALEX fluorescence traces were collected after injecting 10 nM Phe-tRNAPhe(Cy5) TC, 2 μM EF-G and 2 mM GTP to complete the first three elongation cycles (Table S2). Traces were collected at 24 °C. Cy3 fluorescence (green) and sensitized emission of Cy5 (FRET; blue) under 532 nm excitation, alternating with Cy5 fluorescence (red) under 640 nm excitation, were collected and displayed. The TC binding rate constants (8–10 × 106 M-1 s-1) measured here are consistent with in vitro ensemble experiment measurements [5–15 s-1 at 1–10 μM TC (28, 29)]. Formation of different complexes due to tRNA association or dissociation, indicated by changes of Cy3 and Cy5 fluorescence intensity, are marked by arrows. As the contributions of photobleaching to Cy3 and Cy5 disappearance are 0.56% and 1.6%, respectively (Fig. S1), the disappearance of Cy3 and Cy5 fluorescence is due almost entirely to dissociation of labeled tRNAs from the ribosome.
Results
In the first few elongation cycles, the ribosome can follow either the 2-3-2 pathway of tRNA dissociation (ALEX smFRET recording; Fig. 1A) or the 2-1-2 pathway (Fig. 1B). The total fluorescence intensities of Cy3- and Cy5-labeled tRNAs and FRET from adjacent tRNAs in the A and P or P and E sites unambiguously indicate the number and species of tRNAs bound. At the beginning of the traces in Fig. 1, the ribosomes have fluorescent tRNAArg(Cy3) (green intensity trace) base-paired to the second codon of the mRNA. Binding of the third codon Phe-tRNAPhe(Cy5) is indicated by the appearance of Cy5 fluorescence intensity (red trace) concomitant with FRET (sensitized Cy5 emission, blue trace). Binding of the fourth codon Phe-tRNAPhe(Cy5) and dissociation of the second codon tRNAArg(Cy3) are indicated by the doubling of the Cy5 fluorescence intensity and the disappearance of the Cy3 and FRET intensities, respectively. Dissociation of the third codon tRNAPhe(Cy5) is signaled by reduction of the Cy5 fluorescence intensity by half. All four events are marked in both traces in Fig. 1. In Fig. 1A the fourth codon Phe-tRNAPhe binds before dissociation of the second codon Arg-tRNAArg (the 2-3-2 pathway), whereas in B, those two events are reversed (the 2-1-2 pathway). Fig. 2 shows a representative ALEX-smFRET fluorescence trace exhibiting a 2-3-2 elongation event in cycle II followed by two 2-1-2 events in cycles III and IV.
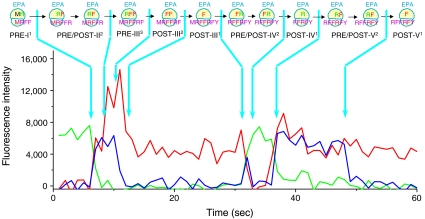
Fig. 2.
A multiple turnover trace, in which the ribosome follows the 2-3-2 pathway in Cycle II and the 2-1-2 pathway in Cycles III and IV. ICs were premixed with Arg-tRNAArg(Cy3) TC, and the resulting PRE-I1 complex was translated up to the fifth elongation cycle by adding 10 nM Arg-tRNAArg(Cy3) TC, 10 nM Phe-tRNAPhe(Cy5) TC, 2 μM EF-G and 2 mM GTP. For traces displaying all five expected labeled tRNA binding events, the 2-3-2-tRNA and 2-1-2-tRNA pathways are seen 42% and 58% of the time, respectively, in Cycle II. In contrast, only the 2-1-2-tRNA pathway is observed for Cycles III and IV. Conditions as in Fig. 1.
How do POST (posttranslocation) complexes choose between the 2-3-2 and 2-1-2 pathways? By comparing the occupancy of two known PRE (pretranslocation) tRNA states with the likelihood of adopting the 2-1-2 vs. the 2-3-2 pathway, we found that the nature of tRNA binding within the PRE state determines the posttranslocational pathway. The ribosomal PRE complex exists in two major conformational states (5, 15, 17, 30). In one PRE state, tRNAs are bound to the classical A site and P sites in both the 30S and 50S subunits (termed A/A- and P/P-site binding). In the other PRE state, the tRNAs are shifted into the P and E sites on the 50S ribosomal subunit, while retaining A- and P-site binding on the 30S subunit (31, 32), placing the tRNAs in hybrid positions designated as A/P and P/E. The overall structure of the ribosome also varies between the classical and hybrid PRE states, but as our FRET measurements in this paper are focused on the ribosome-bound tRNAs, below we refer to the two PRE complexes as classical and hybrid tRNA conformations.
FRET efficiency between adjacent tRNAs within the ribosome (17, 30) decreases in the classical to hybrid transition, allowing us to identify individual PRE complexes as having classical or hybrid conformations. Ribosomes can dynamically fluctuate between these conformations (17, 30) or populate them as noninterconvertible stable conformations (17). We have shown elsewhere that the EF-G·GTP binds directly to both the classical and hybrid PRE states and promotes translocation from each (17).
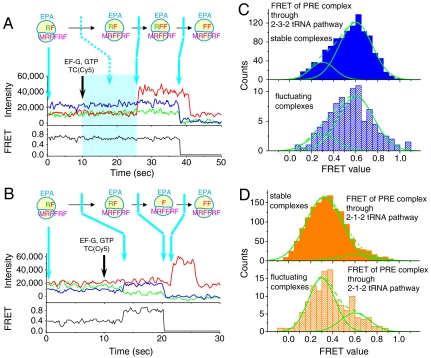
To test for correlation between the PRE state conformation and POST state pathway, cycle II PRE complexes with deacylated tRNAArg(Cy3) in the P site and peptidyl tRNAPhe(Cy5) in the A site were immobilized on the microscope coverslip and their fluorescence and FRET values were used to assign tRNAs in the PRE complex to either the classical or hybrid state. Injection of Phe-tRNAPhe(Cy5) TC and EF-G·GTP then restarted elongation (Fig. 3 A and B). Fluorescence intensities and tRNA-tRNA FRET were again used to determine subsequent E-site occupancy. The major fraction of POST complexes that follow the 2-3-2 pathway are formed by translocation from the classical PRE state (fluctuating ribosomes, 80%; stable ribosomes, 81%; Fig. 3C). Conversely, the major fraction of POST complexes that follow the 2-1-2 pathway are formed by translocation from the hybrid PRE state (fluctuating, 72%; stable, 91%; Fig. 3D). Thus, the tRNA pathway of the POST complex is largely determined by the structural conformation of the PRE complex from which it is formed via translocation.
Fig. 3.
Two representative single molecule translation traces initiated from preformed PRE-II2 complexes with mRNA fMRFFRFYRF (A and B) recorded at 100 ms integration time per frame with ALEX between 532 nm and 640 nm lasers. POST-II2 complexes translocated from the classical PRE state (A; high FRET before EF-G·GTP injection) and from the hybrid PRE state (B, low FRET before EF-G·GTP injection) go through 2-3-2 (A) and 2-1-2 pathways (B), respectively. Translocation, tRNA association and dissociation, indicated by changes of Cy3 and Cy5 fluorescence intensities, are marked by arrows. In A, the exact time following EF-G·GTP and TC addition at which the classical PRE-II2 complex translocates to POST-II2 complex (period marked by a cyan block) is not clear because these states have similar FRET values (classical PRE, 0.6; hybrid PRE, 0.3; POST, 0.55), so that transition is indicated by a dashed arrow. The traces were smoothed by applying a three-frame moving average. Experimental conditions as in Fig. 1. (C and D) FRET distributions of PRE-II2 states of the ribosomes that ultimately went through either the 2-3-2-tRNA pathway or the 2-1-2-tRNA pathway, respectively. Assignments of stable and fluctuating complexes were based on the transition between classical and hybrid PRE states before EF-G·GTP injection. For the fluctuating complexes, FRET values from the last second before EF-G·GTP injection were used. The solid fitted lines are Gaussian distributions and the dashed lines are the sums of two Gaussian components. The component with high FRET efficiency (0.6) is assigned as the classical state and the component with lower FRET (0.3) is assigned to the hybrid state (17, 30).
The strong correlation between the tRNA conformation of the PRE complex (classical or hybrid) and the pathway (2-3-2 or 2-1-2, respectively) followed by a POST complex formed by translocation from that PRE state implies that POST complexes arising by translocation of classical and hybrid PRE complexes are conformationally distinct and do not freely interconvert. Ribosomes thus appear to retain PRE state conformational information during translocation that largely determines partitioning of POST complexes between the 2-3-2 and 2-1-2 pathways. Structural variations that persist beyond one enzymatic reaction step have been described in other systems (33, 34). Here we use the term “conformational memory” to designate this PRE to POST state correlation. The correlation persists only within one elongation cycle, because ribosomes can convert from the 2-3-2 to the 2-1-2 pathway in the next cycle (Fig. 2). Such conversion also demonstrates that the correlation between PRE state and POST pathway does not arise from ribosomal heterogeneity that might cause ribosomes to proceed solely by one or the other pathway. Conversion from a 2-1-2 pathway to a 2-3-2 pathway is difficult to demonstrate due to a strong bias in favor of the 2-1-2 pathway in later cycles (see below).
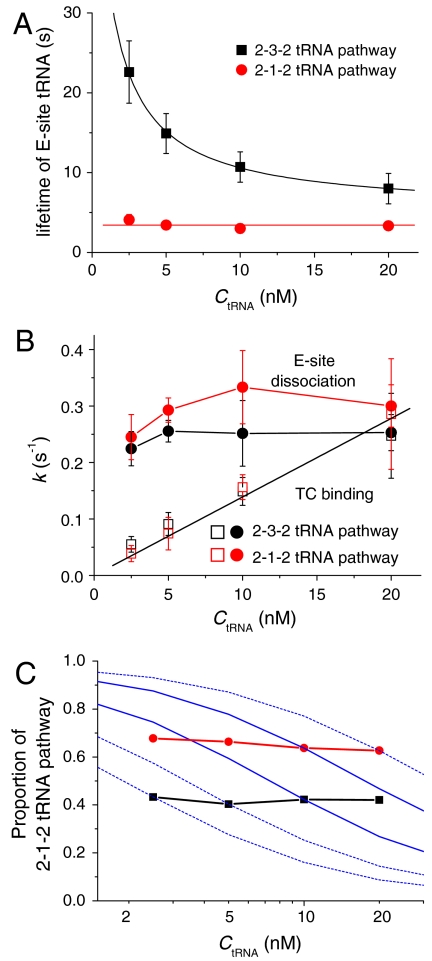
Some earlier reports (19) suggested that allosteric cooperation between the A and E sites traps the E-site tRNA on the ribosome until the A site becomes occupied by the next TC. We tested for such cooperativity by measuring the duration of E-site occupancy at various TC concentrations. For ribosomes that follow the 2-3-2 pathway, the dwell time of tRNA in the E site prior to dissociation depends strongly on TC concentration (Fig. 4A, black squares), indicating an allosteric release of E-site tRNA on occupation of the A site. In contrast, in the 2-1-2 pathway, the lifetime of E-site tRNA is almost independent of the TC concentration (Fig. 4A, red circles). The association rate of A-site tRNA increases linearly with TC concentration for both pathways, as expected (Fig. 4B, open symbols). If the dissociation of E-site tRNA and the association of A-site tRNA were spontaneous reactions independent of each other, the proportion of the 2-1-2 pathway would be expected to decrease with increased TC concentration, as the duration of the POST complexes with empty A sites decreases (blue simulated curves in Fig. 4C). In fact, the partitioning of ribosomes between the 2-1-2 and 2-3-2 pathways is independent of TC concentration (Fig. 4C). This result demonstrates that E-site tRNA dissociation before or after A-site TC binding is not governed simply by a stochastic competition between the tRNA dissociation and TC association reactions, and is consistent with the observation, presented above, that the translation pathway is mainly determined by the PRE conformational states,
Fig. 4.
Dependence of various parameters on the concentration of Phe-tRNAPhe(Cy5) TC for ribosomes programmed with mRNA fMRFFRFYRF and initiated with either uncharged tRNAfMet or with charged fMet-tRNAfMet as indicated. (A) Overall lifetime for E-site tRNA occupancy within POST-II2 complexes going through either the 2-3-2 pathway [black squares, measured between arrows (1) and (3), as in Fig. 1A] or 2-1-2 pathway [red circles, measured between arrows (1) and (2), as in Fig. 1B]. (B) Association rate constant of Phe-tRNAPhe(Cy5) TC paired with the 4th codon [Phe; red (2-1-2 pathway) and black (2-3-2 pathway) open squares] and dissociation rate constant of the E-site tRNAArg(Cy3) paired with the 2nd codon [Arg; red (2-1-2 pathway) and black (2-3-2 pathway) filled circles]. For the 2-3-2 pathway, rate constants for TC binding and E-site dissociation after the A site becomes occupied are measured as the reciprocals of the times between arrows (1) and (2), and (2) and (3), respectively (traces as in Fig. 1A). For the 2-1-2 pathway, rate constants for E-site dissociation and TC binding after the E site becomes empty are measured as the reciprocals of the times between arrows (1) and (2), and (2) and (3), respectively (traces as in Fig. 1B). The intervals between arrows (1) and (2) in Fig 1 A and B are dominated by the rates of TC binding or E-site dissociation, respectively, because accommodation and translocation are considerably faster (SI Text, Translocation Rate Constants at Elongation Cycles II, III, and IV). Errors bars in A and C indicate SD. The red circles in A and C are the same data plotted as reciprocals for comparison; in A the SD values are within the size of the symbols. (C) Fraction of the 2-1-2 tRNA pathway of POST-II2 complexes for ribosomes initiated with either 74% (red circles) or 0% (black squares) fMet-charged initiator tRNAfMet. The blue lines are theoretical predictions based on the assumption that the 2-1-2 vs. 2-3-2 tRNA pathway of the POST complex is determined by a competition between stochastic reactions TC association and E-site tRNA dissociation.
Interestingly, the E-site dissociation rate from the 3-tRNA complex (i.e., after TC binding) is similar to the spontaneous E-site dissociation rate in the 2-1-2 pathway (Fig. 4B, closed symbols) and, as expected, relatively independent of TC concentration. The similarity of E-site tRNA dissociation rates implies that the occupancy of E site by tRNA prevents EF-G promoted translocation. Otherwise, after the A site became occupied to form the 3-tRNA complex, a relatively rapid translocation (1.3 ± 0.1 s-1) (SI Results, Translocation Rate Constants at Elongation Cycles II, III, and IV and Figs. S2 and S3) catalyzed by EF-G would have resulted in an accelerated E-site dissociation.
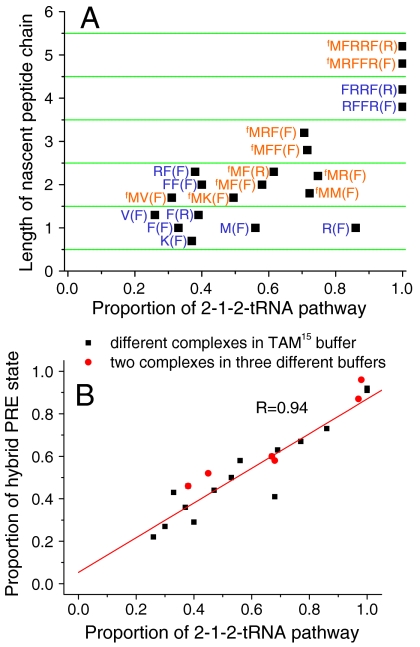
The proportion of 2-1-2 vs. 2-3-2 events was measured as a function of several variables that modulate the partition between classical and hybrid PRE conformational states, including the length of the nascent peptide chain, the specific mRNA codon sequences, the presence or absence of an fMet at the peptide N terminus, and the composition of the bathing buffer (Fig. 5A, Fig. S4, and Table S3). Although the classical/hybrid partitioning in PRE states varies markedly in these conditions, the correlation of classical and hybrid PRE states with the 2-3-2 and 2-1-2 pathways, respectively, is maintained (Fig. 5B and Table S3).
Fig. 5.
(A) Distribution of translation pathways is affected by the length and sequence of the nascent peptide chain. Letter(s) before parentheses indicate the amino acid sequence of the nascent chain bound to the ribosome in the POST complex and the letter within parentheses is the next cognate aa-tRNA. M and fM represent elongator Met and initiator fMet, respectively. The values for complexes without fMet (blue sequences) are measured from the ribosomes initiated with uncharged tRNAfMet. The 2-1-2 pathway values for complexes with N-terminal fMet (red sequences) are linearly extrapolated from the results of ribosomes initiated from uncharged and highly charged (89% or 80%) tRNAfMet, as explained in Figs. S5 and S6. B. Correlation between the proportion of ribosomes in the hybrid PRE state and subsequent 2-1-2-tRNA pathway selection. Black squares: various complexes measured in TAM15 buffer and shown in Fig. 5A and Fig. S4, Red diamonds: two of the complexes were measured in TAM15 buffer and also in buffers B and C (see also Table S3). The proportions of hybrid PRE state were measured from FRET between tRNAs in the P and A sites within preformed immobilized PRE complexes. Both fluctuating and stable PRE complexes were counted into the FRET distributions (17).
As measured in TAM15 buffer, ribosomes follow both pathways when the nascent peptide is up to three amino acids long. With increasing peptide length, the proportion of ribosomes following the 2-1-2 pathway increases, reaching essentially 100% when the nascent peptide length is > 3 amino acids (Figs. 2 and 5A). Both acylated (charged) and deacylated (uncharged) tRNAfMet in the P site can initiate peptide synthesis, allowing examination of the influence of an N-terminal fMet on the proportion of ribosomes following the two pathways (Fig. 5A and Table S3). For a given elongation cycle, the 2-1-2 pathway was generally favored for POST complexes containing a N-terminal fMet, in accordance with the observation that longer nascent peptide chains favor this pathway. For example, in the POST-II complex with a Phe codon in the P site (fMRF), a linear relationship was found between the charging level of tRNAfMet and the 2-3-2:2-1-2 distribution, decreasing from 62:38 in the absence of fMet to 33:67 when tRNAfMet was 89% charged with fMet, (Fig. S5A and Table S3). The proportions of 2-1-2 and 2-3-2 events for POST complexes initiated with fully charged fMet-tRNAfMet (Fig. 5A, Fig. S4 and Table S3) were estimated by short extrapolations of the linear relationships (Fig. S5) between charging and pathway distributions in experiments using uncharged tRNAfMet and up to 80–89% fMet-tRNAfMet. For all of the test mRNAs examined (Fig. 5A) save one [fMR(F) vs. R(F)] (SI Results, The Linear Relationship Between tRNAfMet Charging and Pathway Selection, Fig. S6, SI Results, The fMet Charging and Sequence Effects on Net Rate Constants, and Table S4), higher fMet charging led to a lower proportion of 2-3-2 events (see Table S3 and Fig. S5).
Besides TAM15 buffer, two other buffers commonly used in previous studies of ribosome function were tested. Lowering the Mg2+ concentration from 15 to 7 mM (Methods, Buffer B) drastically decreased the proportion of classical cycle II PRE (PRE-II) complexes, and the proportion of POST-II complexes following the 2-3-2 pathway, in both the presence and absence of fMet at the N-terminus of the nascent peptide. At still lower Mg2+ concentration (4.5 mM), addition of polyamines (Buffer C), restored both the occupancy of classical PRE states and the proportion of the 2-3-2 pathway (Table S3). These results are consistent with previous observations (18, 19), that both high Mg2+ concentration and the presence of polyamines increase the affinity of deacylated tRNA for the E site.
Discussion
Here we show that early elongation cycles proceed by at least two routes (classical PRE state → translocation → 2-3-2 tRNA pathway and hybrid PRE state → translocation → 2-1-2 tRNA pathway). Partitioning between these routes depends on both the sequence and length of the nascent peptide, which, along with results of other single molecule and structural studies (31, 32, 35–37), suggests that the route may be determined, at least in part, by interactions between the nascent peptide and the ribosome. The relationships between the dynamics of PRE and POST complexes are incorporated in a reaction scheme that applies to the first few elongation cycles (Fig. 6). In this scheme, PRE complexes reversibly interconvert between the classical and hybrid states, but this conformational flexibility is largely blocked in the transient intermediate and POST complexes that follow EF-G·GTP binding, conferring a short-term conformational memory on the ribosome. The observation that ribosomes that go through 2-3-2 pathway in cycle II mostly follow the 2-1-2 pathway in subsequent cycles (Fig. 2), as expected from the peptide length dependence, demonstrates that such conformational memory is retained only within an elongation cycle. It remains to be determined to what extent such memory is related to progress or restriction of motions such as ratcheting between the 30S and 50S subunits (38–40), swiveling of the 30S head region (41, 42), and conformational changes of other ribosomal components (43–45).
Fig. 6.
The initial mode of elongation. In the first few elongation cycles, elongation proceeds via both 2-3-2-tRNA and 2-1-2-tRNA pathways. Within many PRE complexes, the classical and hybrid tRNA states can reversibly interconvert. The transient INT states and POST complexes arising from EF-G·GTP facilitated translocation of classical and hybrid PRE complexes, however, are conformationally distinct and do not freely interconvert (dashed arrows), thereby retaining conformational memory throughout an elongation cycle.
The tight correlation between the PRE and POST events as peptide length and sequence and other factors vary further suggests that PRE conformational states determining the subsequent posttranslocation pathways is a general feature of the elongation cycle. The virtual disappearance of the classical PRE → 2-3-2 POST route when the nascent peptide is longer than three residues supports the hypothesis (46) that the bacterial ribosome has an initiation mode in the first few cycles and a more stable elongation mode thereafter. The switch between these modes occurs at approximately cycle 3, although reappearance of the 2-3-2 tRNA pathway at peptide chain lengths > 3 might be possible as a result of specific mRNA sequences that elicit special ribosomal responses, such as programmed frameshifting (23, 24) and stalling (47).
Dominance of the 2-1-2 pathway after two or three elongation cycles is consistent with recently reported real-time single molecule translation measurements of Uemura et al. (16). However, those workers reported no evidence for a 2-3-2 pathway during the first three cycles of elongation, in contrast to the results obtained here. Our results demonstrate that mRNA sequence and buffer (Fig. 5 and Table S3), which differ between the two studies, can strongly affect the observed partitioning between pathways. Differences both in the tRNAs employed (five elongator tRNAs in the current study vs. two in ref. 16) and in the attachment sites of fluorescent labels on the tRNAs (the D loop in the current study vs. position 47 in ref. 16) might also affect partitioning. Moreover, photobleaching of fluorescent probes on the tRNAs could bias the observations toward the 2-1-2 pathway. Uemura et al. (16) considered this issue and performed experiments at lower laser power than their standard conditions. This reduced photobleaching, albeit not entirely. In the present study, the lasers were adjusted to 0.06–0.09 μW/μm2 (laser power at the sample) and contribution of photobleaching to disappearance of fluorescent signals from the ribosome was determined to be less than 1.6% (Fig. S1), the remainder largely resulting from dissociation of E-site tRNAs. A full understanding of why Uemura et al. (16) detected many fewer 2-3-2 pathway events in the first two or three elongation cycles will require a detailed consideration of these and perhaps additional factors. However, it is important to emphasize that our results are likely to underestimate the proportion of 2-3-2 events occurring in vivo. This is because the quite low TC concentrations (≤ 20 nM) we used would be expected to favor the 2-1-2 pathway, whereas in vivo TC concentrations generally exceed several μM. Indeed, Uemura et al. (16) stressed that the rare 2-3-2 events that they did observe were only seen in the presence of high (μM) concentrations of labeled tRNA.
Our results provide direct evidence for a long-range positive allosteric effect of A-site binding on E-site dissociation, a point that has been highly controversial (18–20). E-site dissociation rates and partitioning between the POST pathways over a range of TC concentrations show that ribosomes on the 2-3-2 pathway effectively trap the deacylated tRNA in the E site until the A site becomes occupied. The tRNA occupancy of the E site inhibits EF-G assisted translocation (shown above) and has been implicated in preventing amino acid misincorporation and random frame-shifting (21, 48, 49), as well as in assisting programmed frame-shifting (23, 24). Future single molecule studies will provide direct tests of the functional effects of E-site binding, and how A-site binding modulates these effects, on these and other processes [e.g., binding of accessory protein factors (50–54), entry of the nascent peptide chain into the exit tunnel (46) and formation of the Shine–Dalgarno helix (46, 55)].
Methods
The 70S initiation complexes or 70S PRE complexes formed from a 5′-biotinylated mRNA (Dharmacon RNAi Tech.; Table S1) were immobilized on a biotin/PEG-streptavidin coated glass surface (56). After washing away unbound complexes, collection of real-time translation traces began 10 s prior to injecting 10 nM fluorescent-labeled ternary complexes, 2 μM EF-G and 2 mM GTP into the flow cell. Fluorescent-labeled ternary complexes were preformed from EF-Tu, GTP, and charged tRNAs labeled with either Cy3 or Cy5 at dihydrouridine positions in the D loop (57, 58). Recording continued for 10 min without further washing. The details of immobilized 70S complexes and ternary complexes added during the recordings for different experiments are listed in Table S2. Cy3 and Cy5 fluorescence intensities as well as the FRET signal between Cy3 and Cy5 were excited and collected by ALEX (27) between a 532 nm laser and a 640 nm laser on a custom-built objective-type total internal reflection fluorescence microscope (17). Except as otherwise indicated (Fig. 3), fluorescence intensities were recorded with alternating 532 nm and 640 nm laser excitation (ALEX) at 500 ms integration time per frame with resulting in a net time resolution of 1 s. The power densities of the 532 nm and 640 nm lasers were 0.09 and 0.06 μW/μm2, respectively, to keep the photobleaching rates of both Cy3 and Cy5 below 1.0 × 10-3 s-1 (Fig. S1). Unless specifically mentioned, translation experiments were carried out with buffer TAM15 (15 mM MgAc2, 50 mM Tris-HCl pH 7.5, 30 mM NH4Cl, 70 mM KCl, and 1 mM DTT) at 23 °C. Buffer B (7 mM MgCl2, 50 mM Tris-HCl pH 7.5, 70 mM NH4Cl, 30 mM KCl, and 1 mM DTT) and buffer C (4.5 mM MgAc2, 2 mM spermidine, 0.05 mM spermine, 20 mM Hepes-KOH pH 7.3, 150 mM NH4Ac, 4 mM β-mercaptoethanol) were also used. Other details of materials preparation, the experimental setup, tRNA-tRNA FRET measurement, and data analysis were described in a previous publication (17).
Supplementary Material
Acknowledgments.
We thank Dr. Haibo Zhang, Dr. Hanqing Liu, and Xiaonan Cui for preparing ribosomes and proteins. This work was supported by National Institutes of Health (NIH) R01 Grants GM080376 (B.S.C. and Y.E.G.) and GM071014 (B.S.C.), and National Institute of Standards and Technology Advanced Technology Program (ATP) Grant 70NANB7H7011 through Anima Cell Metrology, Inc.
Footnotes
Conflict of interest statement: B.S.C. and Y.E.G. are paid consultants of Anima Cell Metrology, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106999108/-/DCSupplemental.
References
- 1.Schmeing TM, Ramakrishnan V. What recent ribosome structures have revealed about the mechanism of translation. Nature. 2009;461:1234–1242. doi: 10.1038/nature08403. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan V. Ribosome structure and the mechanism of translation. Cell. 2002;108:557–572. doi: 10.1016/s0092-8674(02)00619-0. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti A, et al. A structural view of translation initiation in bacteria. Cell Mol Life Sci. 2009;66:423–436. doi: 10.1007/s00018-008-8416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra K, Frank J. Ribosome dynamics: Insights from atomic structure modeling into cryo-electron microscopy maps. Annu Rev Biophys Biomol Struct. 2006;35:299–317. doi: 10.1146/annurev.biophys.35.040405.101950. [DOI] [PubMed] [Google Scholar]
- 5.Moazed D, Noller HF. Intermediate states in the movement of transfer-RNA in the ribosome. Nature. 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- 6.Antoun A, Pavlov MY, Lovmar M, Ehrenberg M. How initiation factors maximize the accuracy of tRNA selection in initiation of bacterial protein synthesis. Mol Cell. 2006;23:183–193. doi: 10.1016/j.molcel.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Daviter T, Gromadski KB, Rodnina MV. The ribosome’s response to codon-anticodon mismatches. Biochimie. 2006;88:1001–1011. doi: 10.1016/j.biochi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Dorner S, Brunelle JL, Sharma D, Green R. The hybrid state of tRNA binding is an authentic translation elongation intermediate. Nat Struct Mol Biol. 2006;13:234–241. doi: 10.1038/nsmb1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigoriadou C, Marzi S, Kirillov S, Gualerzi CO, Cooperman BS. A quantitative kinetic scheme for 70S translation initiation complex formation. J Mol Biol. 2007;373:562–572. doi: 10.1016/j.jmb.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan DL, Kirillov SV, Cooperman BS. Kinetically competent intermediates in the translocation step of protein synthesis. Mol Cell. 2007;25:519–529. doi: 10.1016/j.molcel.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savelsbergh A, et al. An elongation factor G-induced ribosome rearrangement precedes tRNA-mRNA translocation. Mol Cell. 2003;11:1517–1523. doi: 10.1016/s1097-2765(03)00230-2. [DOI] [PubMed] [Google Scholar]
- 12.Wintermeyer W, et al. Mechanisms of elongation on the ribosome: Dynamics of a macromolecular machine. Biochem Soc Trans. 2004;32:733–737. doi: 10.1042/BST0320733. [DOI] [PubMed] [Google Scholar]
- 13.Zavialov AV, Ehrenberg M. Peptidyl-tRNA regulates the GTPase activity of translation factors. Cell. 2003;114:113–122. doi: 10.1016/s0092-8674(03)00478-1. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard SC. Single-molecule observations of ribosome function. Curr Opin Struct Biol. 2009;19:103–109. doi: 10.1016/j.sbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank J, Gonzalez RL. Structure and dynamics of a processive Brownian motor: The translating ribosome. Annu Rev Biochem. 2010;79:381–412. doi: 10.1146/annurev-biochem-060408-173330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uemura S, et al. Real-time tRNA transit on single translating ribosomes at codon resolution. Nature. 2010;464:1012–1017. doi: 10.1038/nature08925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, et al. Single molecule fluorescence measurements of ribosomal translocation dynamics. Mol Cell. 2011;42:367–377. doi: 10.1016/j.molcel.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semenkov YP, Rodnina MV, Wintermeyer W. The “allosteric three-site model” of elongation cannot be confirmed in a well-defined ribosome system from Escherichia coli. Proc Natl Acad Sci USA. 1996;93:12183–12188. doi: 10.1073/pnas.93.22.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nierhaus KH. The allosteric 3-Site model for the ribosomal elongation cycle—features and future. Biochemistry. 1990;29:4997–5008. doi: 10.1021/bi00473a001. [DOI] [PubMed] [Google Scholar]
- 20.Nierhaus KH, et al. The elongating ribosome: Structural and functional aspects. Biochem Cell Biol. 1995;73:1011–1021. doi: 10.1139/o95-108. [DOI] [PubMed] [Google Scholar]
- 21.Nierhaus KH. Decoding errors and the involvement of the E-site. Biochimie. 2006;88:1013–1019. doi: 10.1016/j.biochi.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Zaher HS, Green R. Fidelity at the molecular level: Lessons from protein synthesis. Cell. 2009;136:746–762. doi: 10.1016/j.cell.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leger M, Dulude D, Steinberg SV, Brakier-Gingras L. The three transfer RNAs occupying the A, P, and E sites on the ribosome are involved in viral programmed-1 ribosomal frameshift. Nucleic Acids Res. 2007;35:5581–5592. doi: 10.1093/nar/gkm578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao PY, Gupta P, Petrov AN, Dinman JD, Lee KH. A new kinetic model reveals the synergistic effect of E-, P- and A-sites on +1 ribosomal frameshifting. Nucleic Acids Res. 2008;36:2619–2629. doi: 10.1093/nar/gkn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson DN, Nierhaus KH. The E-site story: The importance of maintaining two tRNAs on the ribosome during protein synthesis. Cell Mol Life Sci. 2006;63:2725–2737. doi: 10.1007/s00018-006-6125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aitken CE, Petrov A, Puglisi JD. Single ribosome dynamics and the mechanism of translation. Annu Rev Biophys. 2010;39:491–513. doi: 10.1146/annurev.biophys.093008.131427. [DOI] [PubMed] [Google Scholar]
- 27.Kapanidis AN, et al. Fluorescence-aided molecule sorting: Analysis of structure and interactions by alternating-laser excitation of single molecules. Proc Natl Acad Sci USA. 2004;101:8936–8941. doi: 10.1073/pnas.0401690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pape T, Wintermeyer W, Rodnina MV. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E-coli ribosome. EMBO J. 1998;17:7490–7497. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson M, Bouakaz E, Lovmar M, Ehrenberg M. The kinetics of ribosomal peptidyl transfer revisited. Mol Cell. 2008;30:589–598. doi: 10.1016/j.molcel.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Blanchard SC, Kim HD, Gonzalez RL, Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci USA. 2004;101:12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agirrezabala X, et al. Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol Cell. 2008;32:190–197. doi: 10.1016/j.molcel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julian P, et al. Structure of ratcheted ribosomes with tRNAs in hybrid states. Proc Natl Acad Sci USA. 2008;105:16924–16927. doi: 10.1073/pnas.0809587105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penner MH, Frieden C. Substrate-induced hysteresis in the activity of Escherichia coli dihydrofolate reductase. J Biol Chem. 1985;260:5366–5369. [PubMed] [Google Scholar]
- 34.Yang H, et al. Protein conformational dynamics probed by single-molecule electron transfer. Science. 2003;302:262–266. doi: 10.1126/science.1086911. [DOI] [PubMed] [Google Scholar]
- 35.Valle M, et al. Locking and unlocking of ribosomal motions. Cell. 2003;114:123–134. doi: 10.1016/s0092-8674(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 36.Cornish PV, Ermolenko DN, Noller HF, Ha T. Spontaneous intersubunit rotation in single ribosomes. Mol Cell. 2008;30:578–588. doi: 10.1016/j.molcel.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornish PV, et al. Following movement of the L1 stalk between three functional states in single ribosomes. Proc Natl Acad Sci USA. 2009;106:2571–2576. doi: 10.1073/pnas.0813180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank J, Agrawal RK. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature. 2000;406:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 39.Horan LH, Noller HF. Intersubunit movement is required for ribosomal translocation. Proc Natl Acad Sci USA. 2007;104:4881–4885. doi: 10.1073/pnas.0700762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ermolenko DN, Noller HF. mRNA translocation occurs during the second step of ribosomal intersubunit rotation. Nat Struct Mol Biol. 2011;18:457–462. doi: 10.1038/nsmb.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratje AH, et al. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature. 2010;468:713–U143. doi: 10.1038/nature09547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Dunkle JA, Cate JHD. Structures of the ribosome in intermediate states of ratcheting. Science. 2009;325:1014–1017. doi: 10.1126/science.1175275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–333. doi: 10.1038/nature09206. [DOI] [PubMed] [Google Scholar]
- 44.Mesters JR, Potapov AP, de Graaf JM, Kraal B. Synergism between the GTPase activities of EF-Tu.GTP and EF-G.GTP on empty ribosomes: Elongation factors as stimulators of the ribosomal oscillation between two conformations. J Mol Biol. 1994;242:644–654. doi: 10.1006/jmbi.1994.1614. [DOI] [PubMed] [Google Scholar]
- 45.Szaflarski W, Nierhaus KH. Optimized energy consumption for protein synthesis. Origins Life Evol B. 2007;37:423–428. doi: 10.1007/s11084-007-9091-4. [DOI] [PubMed] [Google Scholar]
- 46.Tenson T, Hauryliuk V. Does the ribosome have initiation and elongation modes of translation? Mol Microbiol. 2009;72:1310–1315. doi: 10.1111/j.1365-2958.2009.06741.x. [DOI] [PubMed] [Google Scholar]
- 47.Ramu H, Mankin A, Vazquez-Laslop N. Programmed drug-dependent ribosome stalling. Mol Microbiol. 2009;71:811–824. doi: 10.1111/j.1365-2958.2008.06576.x. [DOI] [PubMed] [Google Scholar]
- 48.Devaraj A, Shoji S, Holbrook ED, Fredrick K. A role for the 30S subunit E site in maintenance of the translational reading frame. RNA. 2009;15:255–265. doi: 10.1261/rna.1320109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sergiev PV, et al. Function of the ribosomal E-site: A mutagenesis study. Nucleic Acids Res. 2005;33:6048–6056. doi: 10.1093/nar/gki910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaha G, Stanley RE, Steitz TA. Formation of the first peptide bond: The structure of EF-P bound to the 70S ribosome. Science. 2009;325:966–970. doi: 10.1126/science.1175800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, et al. Molecular localization of a ribosome-dependent ATPase on Escherichia coli ribosomes. Nucleic Acids Res. 2006;34:1158–1165. doi: 10.1093/nar/gkj508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin Y, et al. The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosorne. Cell. 2006;127:721–733. doi: 10.1016/j.cell.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 53.Connell SR, et al. A new tRNA intermediate revealed on the ribosome during EF4-mediated back-translocation. Nat Struct Mol Biol. 2008;15:910–915. doi: 10.1038/nsmb.1469. [DOI] [PubMed] [Google Scholar]
- 54.Liu HQ, Pan DL, Pech M, Cooperman BS. Interrupted catalysis: The EF4 (LepA) effect on back-translocation. J Mol Biol. 2010;396:1043–1052. doi: 10.1016/j.jmb.2009.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Giacco V, et al. Shine–Dalgarno interaction prevents incorporation of noncognate amino acids at the codon following the AUG. Proc Natl Acad Sci USA. 2008;105:10715–10720. doi: 10.1073/pnas.0801974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nat Methods. 2008;5:507–516. doi: 10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan DL, Qin HO, Cooperman BS. Synthesis and functional activity of tRNAs labeled with fluorescent hydrazides in the D-loop. RNA. 2009;15:346–354. doi: 10.1261/rna.1257509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaur J, Raj M, Cooperman BS. Fluorescent labeling of tRNA dihydrouridine residues: Mechanism and distribution. RNA. 2011;17:1393–400. doi: 10.1261/rna.2670811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.