Abstract
The circadian clock in the mammalian hypothalamic suprachiasmatic nucleus (SCN) is entrained by the ambient light/dark cycle, which differentially acts to cause the clock to advance or delay. Light-induced changes in the rhythmic expression of SCN clock genes are believed to be a critical step in this process, but how the two entrainment modalities—advances vs. delays—engage the molecular clockwork remains incompletely understood. We investigated molecular substrates of photic entrainment of the clock in the SCN by stably entraining hamsters to T cycles (non–24-h light/dark cycles) consisting of a single 1-h light pulse repeated as either a short (23.33-h) or a long (24.67-h) cycle; under these conditions, the light pulse of the short cycle acts as “dawn,” whereas that of the long cycle acts as “dusk.” Analyses of the expression of the photoinducible and rhythmic clock genes Period 1 and 2 (Per1 and Per2) in the SCN revealed fundamental differences under these two entrainment modes. Light at dawn advanced the clock, advancing the onset of the Per1 mRNA rhythm and acutely increasing mRNA transcription, whereas light at dusk delayed the clock, delaying the offset of the Per2 mRNA rhythm and tonically increasing mRNA stability. The results suggest that the underlying molecular mechanisms of circadian entrainment differ with morning (advancing) or evening (delaying) light exposure, and such differences may reflect how entrainment takes place in nocturnal animals under natural conditions.
Keywords: circadian synchronization, circadian clock resetting, light entrainment
A fundamental feature of the circadian clock that regulates daily behavioral and physiological rhythmicity is its entrainment to the environmental cycle of light and darkness. By matching the clock's endogenous (“free-running”) period to that of the ambient day/night cycle, entrainment ensures that the phase relationship between clock and external day remains stable over time, thereby synchronizing body rhythms to local time. Light is the clock's preeminent entraining cue (Zeitgeber), and the clock responds to light by changing its angular velocity and shifting its phase. To match its oscillation to that of the Zeitgeber, the clock can increase its velocity and advance its phase if the clock's free-running period is too long, or it can decrease its velocity and delay its phase if its free-running period is too short (1, 2).
In mammals, the master circadian clock is located in the suprachiasmatic nucleus (SCN) in the anterior hypothalamus (3). The SCN is composed of multiple, coupled single-cell circadian oscillators (4), and analyses of induced and spontaneous mutations, gene sequence homologies, and protein-protein interactions have identified candidate regulatory molecules and biochemical processes that are likely to constitute the basic intracellular oscillatory mechanism (5). Genes at the core of the clock function within autoregulatory feedback loops, with proteins rhythmically suppressing the transcription of their own mRNAs. When the basic helix–loop–helix transcription factors CLOCK (or NPAS2) and BMAL1 dimerize and bind to E-box enhancer elements, the transcription of the Period (Per1, Per2, and Per3) genes is activated. PER proteins accumulate in the cytoplasm, are regulated through their phosphorylation and their association with proteins encoded by the Cryptochrome (Cry1 and Cry2) genes, and translocate to the nucleus to negatively regulate their own transcription by interfering with the activity of the CLOCK:BMAL1 dimers. PER2 and Rev-erb-α proteins are part of a second feedback loop that regulates rhythmic Bmal1 gene transcription. Photic entrainment of the clock must impact the activities or levels of the molecular components of these loops, and the induction of Per1 and Per2 gene expression is believed to be a critical step in this process (for references and discussion, see ref. 6), but the precise mechanisms underlying shifts of the phase of the clock or changes in its velocity are not certain.
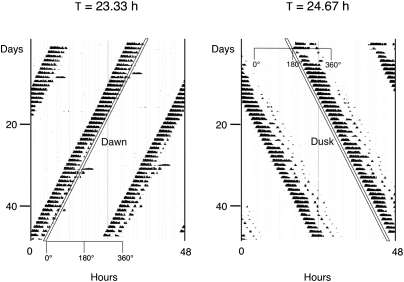
Here we report our analysis of molecular substrates of entrainment in the SCN of animals photically entrained to T cycles, i.e., non–24-h Zeitgeber cycles. We have used a previously described paradigm (7, 8) in which circadian locomotor (wheel running) activity of Syrian hamsters can be stably entrained by a single 1-h light pulse repeated as a T cycle that is either shorter or longer than 24 h by 40 min per cycle. As shown in Fig. 1, the light pulse in the short (23.33-h) or long (24.67-h) cycle falls at a different phase of the clock's oscillation to attain steady-state entrainment, specifically as a phase-advancing/period-accelerating pulse acting as the short cycle's “dawn” (following the locomotor bout for the nocturnally active hamster) or as a phase-delaying/period-decelerating pulse acting as the long cycle's “dusk” (preceding the locomotor bout). To determine how differential entrainment by a morning or an evening light pulse affects SCN clock genes, we analyzed the expression of the rhythmic, photoinducible genes Per1 and Per2 in groups of hamsters killed at different phases of these cycles. Our results indicate that Per gene expression patterns differ under entrainment to short or long T cycles, and suggest that distinct molecular mechanisms underlie these contrasting entrainment modes.
Fig. 1.
T-cycle entrainment by advances or delays in Syrian hamsters. (Left) Representative double-plotted actogram of wheel-running for an animal entrained to a 1-h light pulse (white diagonal bar) every 23.33 h; the light pulse falls in the late subjective night (as dawn, at the end of the locomotor activity bout). (Right) Representative actogram of wheel-running for an animal entrained to a 1-h light pulse every 24.67 h; the light pulse falls in the early subjective night (as dusk, at the beginning of the locomotor activity bout). Note that under both T cycles, time 0° corresponds to the end of subjective night.
Results
Differential Phase of Entrainment to Short and Long T Cycles.
The different patterns of entrainment to short and long T cycles shown in Fig. 1 are predicted by entrainment theory and previously confirmed in several species, including Syrian hamsters (7, 9, 10). To collect and compare data from groups of animals entrained to these two schedules, we converted time (in hours) to degrees of arc, where one circadian cycle equals 360° (thus, 1° is ∼0.0648 h for animals in the short 23.33-h T cycle and 0.0685 h for animals in the long 24.67-h T cycle). By convention for nocturnally active animals, 0° represents dawn (following the subjective night, coinciding with the end of the light pulse in the short T cycle) and 180° represents dusk (preceding the subjective night, coinciding with the end of the light pulse in the long T cycle; Fig. 1). Adult male hamsters entrained to these cycles were killed in groups at 45° intervals across the circadian cycle (n = 3 animals at each of eight time points for each T-cycle group, with time points from 0° to 135° designated as subjective day and those from 180° to 315° as subjective night), and their brains were cut and SCN tissue sections processed for in situ hybridization using 35S-labeled antisense cRNA probes and film autoradiography.
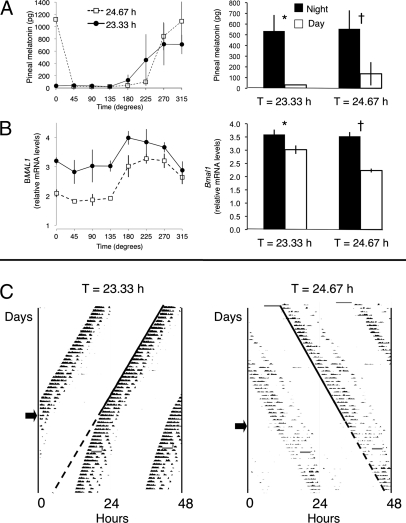
To first verify that the opposite phases of locomotor rhythmicity in the short and long T cycles actually reflected opposite phases of the circadian system as a whole, and to validate our subjective day and subjective night phase assignments, we checked two additional rhythms in the killed animals. We assayed levels of the nocturnally produced hormone melatonin in pineal glands (Fig. 2A) and mRNA levels of SCN Bmal1 (known to be photo-insensitive and rhythmically higher during the night than the day) by in situ hybridization of SCN tissue sections (Fig. 2B). These temporal profiles confirmed that the light pulse in the 23.33-h T cycle acts as dawn, marking the end of the subjective night and beginning of subjective day; and that the light pulse in the 24.67-h T cycle acts as dusk, marking the end of the subjective day and beginning of subjective night. Thus, it is the locomotor activity bout that is phased differently in the two T cycles, with offset at dawn in the 23.33-h T cycle and onset at dusk in the 24.67-h T cycle. Comparison of melatonin levels from glands pooled from subjective day with those from subjective night confirmed a significant rhythm in both T-cycle groups, with higher levels during the night (P < 0.002 and < 0.03 for 23.33-h and 24.67-h groups, respectively; Student t test; Fig. 2A). Similarly, there was a significant subjective day/night rhythm in SCN Bmal1 levels, with higher levels during the night (P < 0.02 and < 0.0001 for 23.33-h and 24.67-h groups, respectively; Student t test; Fig. 2B).
Fig. 2.
Characterization of T-cycle entrainment by advances or delays. (A) Pineal melatonin content of glands pooled from animals entrained to either the 23.33-h or the 24.67-h T cycle and killed during subjective day (at 0°, 45°, 90°, and 135°) or subjective night (at 180°, 225°, 270°, and 315°; n = 2–3 animals per time point for each T-cycle group, as a few glands were lost at some time points; mean ± SEM). Temporal profiles (Left) and subjective day and subjective night pools (Right) show higher levels during the night for both T-cycle groups; as expected, levels were high at 0° for the 24.67-h but not for the 23.33-h T-cycle group, due to the acute suppressive effect of light at dawn on melatonin production. *P < 0.002; †P < 0.03; Student t test. (B) SCN Bmal1 relative mRNA levels of tissue sections pooled from animals entrained to either the 23.33-h or the 24.67-h T cycle and killed during subjective day or subjective night (n = 3 animals per time point for each T cycle). Temporal profiles (Left) and subjective day and subjective night pools (Right) show higher levels during the night for both T-cycle groups. *P < 0.02; †P < 0.0001; Student t test. (C Left) Representative actogram of an animal entrained to the 23.33-h T cycle that upon release into constant darkness (arrow) shows a free-running period that is shorter than 24 h but longer than the previous entraining cycle. (Right) Representative actogram of an animal entrained to a 24.67-h T cycle that upon release into constant darkness (arrow) shows a free-running period that is longer than 24 h and similar to the previous entraining cycle. In both cases the solid line marks the onset of activity during entrainment, and the dotted line the extrapolated onset after release into constant darkness.
Last, to demonstrate that animals were actually entrained to the short and long T cycles, a separate set of entrained animals was released into constant darkness (DD); upon release into DD, the phase of the free-running locomotor rhythm was predicted by the phase under entrained conditions for both the 23.33-h and 24.67-h T cycles (Fig. 2C), confirming true entrainment. Of note, the free-running period in DD (τ) showed prominent aftereffects of the previous entraining cycle, i.e., it was relatively short in the 23.33-h T-cycle group (23.58 ± 0.03 h, n = 3) and relatively long in the 24.67-h T-cycle group (24.58 ± 0.04 h, n = 4) compared with the species-typical τ close to 24 h (as documented in the literature over the past few decades) (11, 12). Interestingly, τ was statistically indistinguishable from the period of the previous T cycle for the 24.67-h animals (95% confidence interval for τ of 24.45–24.71 h) but not for the 23.33-h animals (23.45–23.70 h).
SCN Per1 and Per2 mRNA Rhythms During Photoentrainment to T Cycles.
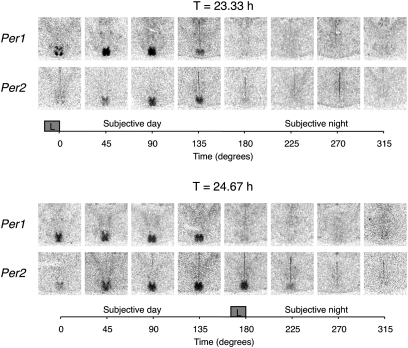
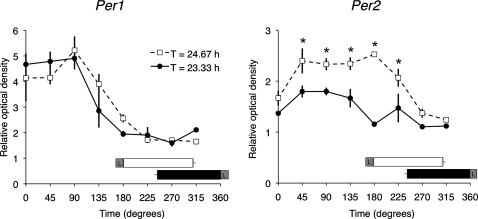
Fig. 3 shows representative SCN coronal tissue sections from animals killed at 45° intervals and processed for Per1 and Per2 by in situ hybridization. As expected, SCN Per1 and Per2 mRNA levels were rhythmic in both short and long T-cycle groups, with relatively higher levels during the subjective day than subjective night, but with notable differences in localization and timing. During the 23.33-h T cycle, Per1 expression was high at 0°, with elevated signal in discrete dorsomedial and ventrolateral SCN subregions; by 45° the signal encompassed the entire dorsoventral extent of the SCN; and by 135° it was clearly present but diminished. Expression of Per2 lagged Per1, first clearly appearing at 45°, but last visible (like Per1) at 135°. During the 24.67-h T cycle, Per1 was also relatively high at 0°, but elevated signal was not seen in the ventrolateral subregion; over the course of the day the signal encompassed the full extent of the SCN and was last visible at 135°. Again, increased expression of Per2 lagged Per1, first clearly appearing at 45°, but its expression was extended (unlike Per1) to the end of the dusk light pulse at 180° and remained present but diminished at 225°. A semiquantitative analysis of these temporal patterns for the whole SCN is shown in Fig. 4. There was no difference in either the duration or the amplitude of rhythmic Per1 expression during entrainment to the 23.33-h or 24.67-h T cycle; two-way ANOVA yielded a significant effect of time (P < 0.0001) but no effect of T-cycle group (P = 0.87) or of the interaction (P = 0.36). In contrast, both the duration and the amplitude of rhythmic Per2 expression were greater under the 24.67-h than under the 23.33-h T cycle; two-way ANOVA yielded a significant effect of time (P < 0.0001), of T-cycle group (P = 0.0001), and of the interaction (P = 0.0028).
Fig. 3.
Expression of the clock genes Per1 and Per2 in the SCN under stable entrainment to 23.33-h (Upper) or 24.67-h (Lower) T cycles. Representative autoradiograms of SCN-level coronal hypothalamic sections from animals killed at the indicated time point relative to the dawn (0° for T = 23.33 h) or the dusk (180° for T = 24.67 h) light pulse (L within gray square) and hybridized with radiolabeled riboprobes for each transcript using exonic probes.
Fig. 4.
Expression levels of Per1 (Left) and Per2 (Right) in the SCN under stable entrainment to 23.33-h or 24.67-h T cycles. Values represent the mean ± SEM (n = 3 for each time point and each T cycle) of the OD within the whole SCN, normalized to the OD within the surrounding hypothalamus. Both the duration and the amplitude of rhythmic Per2 expression were greater under the 24.67-h than under the 23.33-h T cycle; two-way ANOVA yielded a significant effect of time, of T-cycle group, and of the interaction (see text). Also shown are the mean locomotor activity bout durations and phases for animals in the 23.33-h (black bar) and 24.67-h (white bar) T-cycle groups. L within gray square indicates the phase of the 1-h light pulse. *P = 0.05, post hoc Student t test contrasts.
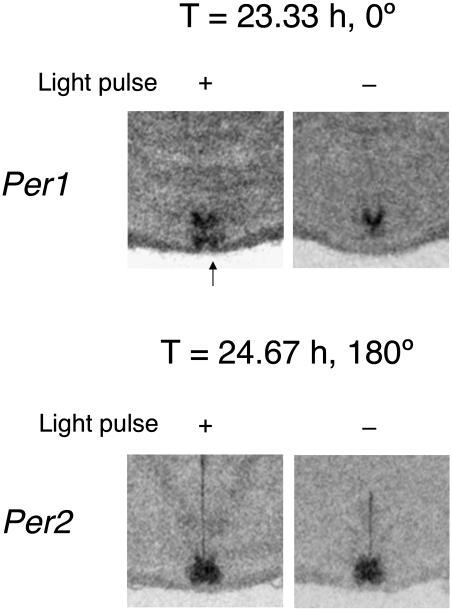
We determined the photic dependence of Per1 expression at the end of the light pulse at 0° during 23.33-h T-cycle entrainment (dawn) and of Per2 expression at the end of the light pulse at 180° during 24.67-h T-cycle entrainment (dusk). A separate set of entrained animals was killed at 0° or 180°, either at the end of the entraining light pulses or at the same circadian phases but with the lights not turned on (n = 3 for each group). As illustrated in Fig. 5, dawn expression of Per1 at 0° during the 23.33-h T cycle did depend on the presence of light, especially and predictably in the ventral subregion, but, surprisingly, dusk expression of Per2 at 180° during the 24.67-h T cycle did not. Relative OD measurements of dawn Per1 in the ventral and dorsal SCN with lights on were significantly greater than with lights off (P = 0.002 and 0.009, respectively, two-tailed Student t test), whereas measurements of dusk Per2 in the two SCN subdivisions were no different in the presence or absence of light (P = 0.12 and 0.28, respectively, two-tailed Student t test).
Fig. 5.
Differential dependence of SCN Per1 and Per2 expression on the presence of light. (Upper) Representative autoradiograms of SCN-level coronal hypothalamic sections from an animal killed immediately after the light pulse under a 23.33-h T cycle (+) or at the same circadian phase after the light pulse was not presented (−). Relative OD measurements for Per1 in the ventral and dorsal SCN with lights on were significantly greater than with lights off (see text), and ventral expression of Per1 (arrow) was absent when the light pulse was not presented (n = 3 for lights-on and lights-off groups; ventral, 1.50 ± 0.05 lights on vs. 1.15 ± 0.02 lights off; dorsal, 1.78 ± 0.03 lights on vs. 1.52 ± 0.03 lights off; mean ± SEM). (Lower) Representative autoradiograms of SCN-level coronal hypothalamic sections from an animal killed immediately after the light pulse under a 24.67-h T cycle (+) or at the same circadian phase after the light pulse was not presented (−). Relative OD measurements for Per2 were not significantly different in the presence or absence of light (see text; n = 3 for lights-on and lights-off groups; ventral, 2.36 ± 0.09 lights on vs. 1.99 ± 0.12 lights off; dorsal, 2.84 ± 0.29 lights on vs. 2.47 ± 0.06 lights off).
Acute Nighttime Photic Induction of SCN Per1 and Per2 mRNA During T-Cycle Entrainment.
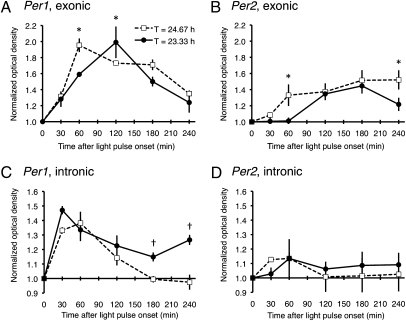
Our data indicate that entrainment to short or long T cycles differentially affects rhythmic SCN Per1 and Per2 gene expression. The dawn (0°) light pulse of the 23.33-h T cycle acutely induced Per1 mRNA. Though the dusk (180°) light pulse of the 24.67-h T cycle did not acutely induce Per2 mRNA, entrainment of the clock to the long T cycle somehow altered Per2 steady-state regulation, resulting in a Per2 rhythm with increased mRNA levels during subjective day and with an extended duration of elevation. To probe the state of the circadian system under entrainment to each of the T cycles, we analyzed the dynamics of Per gene expression after a light pulse administered in the middle of the subjective night, when gene expression was low (Fig. 4); subjective-night light pulses are known to acutely induce Per1 and Per2 gene expression with different temporal profiles (13–16). An additional set of animals was entrained to short or long T cycles and then exposed to a single 30-min light pulse at 270°; animals were killed in groups (n = 3 animals at each of six time points for each T-cycle group), and their brains were cut and SCN tissue sections processed for in situ hybridization using film autoradiography and 35S-labeled antisense cRNA probes against Per1 and Per2 intronic and exonic sequences. Because splicing of introns from heteronuclear mRNA is relatively rapid, a rise in hybridization signal with the intronic probe likely reflects de novo synthesis of immature transcript; however, elevated exonic expression without concomitantly elevated intronic expression suggests increased stability of mature transcript. The subjective-night light pulse induced both Per1 and Per2 gene expression in the SCN as measured by both probes, and a semiquantitative analysis of the temporal pattern of increased expression is plotted in Fig. 6, normalized for each probe as the relative change from baseline at time 0 in darkness. For the Per1 exonic probe, two-way ANOVA yielded no effect of T-cycle group (P = 0.13), but there was an effect of time (P < 0.0001) and of the interaction (P < 0.03); the peak increase in hybridization signal occurred earlier at 60 min under the 24.67-h T cycle and later at 120 min under the 23.33-h T cycle (Fig. 6A). For the Per1 intronic probe, there was a significant effect of T-cycle group (P = 0.001), of time (P < 0.0001), and of the interaction (P < 0.03), with elevated expression at later time points under the 23.33-h T cycle (Fig. 6C). For the Per2 exonic probe, there was a significant effect of T-cycle group (P = 0.01) and of time (P < 0.0001), but no effect of the interaction (P = 0.24); hybridization signal continued to increase after the light pulse even to include the last time point (Fig. 6B). However, for the Per2 intronic probe, there was a significant effect of time only (P < 0.03), with no effect of T-cycle group (P = 0.47) or of the interaction (P = 0.20; Fig. 6D). Thus, for Per2, persistently elevated exonic expression in the 24.67-h T-cycle group was unaccompanied by elevated intronic expression, suggesting a more stable Per2 transcript under the 24.67-h T cycle.
Fig. 6.
Acute photic induction of SCN Per1 and Per2 expression in the middle of the subjective night under 23.33-h or 24.67-h T cycles. Per1 gene expression measured by exonic (A) or intronic (C) probes, normalized for each probe as the relative change from baseline at time 0 in darkness. For the exonic probe, two-way ANOVA showed no effect of T-cycle group, but there was an effect of time and of the interaction; for the intronic probe, there was a significant effect of T-cycle group, of time, and of the interaction (see text). *, †P = 0.05, post hoc Student t test contrasts. Per2 gene expression measured by exonic (B) or intronic (D) probes, normalized for each probe as the relative change from baseline at time 0 in darkness. For the exonic probe, there was a significant effect of T-cycle group and of time, but no effect of the interaction; for the intronic probe, there was a significant effect of time only, with no effect of T-cycle group or of the interaction (see text). *P = 0.05, post hoc Student t test contrasts.
Discussion
Entraining hamsters to a single 1-h light pulse repeated as a short or long T cycle has enabled us to analyze steady-state SCN Per1 and Per2 rhythms during differential entrainment to either simulated dawn or dusk; true entrainment under these conditions was confirmed by measuring the phases of rhythms of pineal melatonin content, SCN Bmal1 gene expression, and locomotor activity (and for the latter, its onset after animals were released into DD). This T-cycle paradigm, taken from the work of Elliott (7, 8), is distinct from previous studies of SCN Per1 and Per2 gene expression, which have relied on patterns of synchronization to complete photoperiods, responses to an acute light pulse during DD, or examination of animals genetically deficient in one or more clock genes. Our study is unique in that it analyzes the expression of such genes by cyclic light pulses rather than by complete light/dark cycles (17) that induce stable entrainment; light exposure at dawn and dusk likely represents a more accurate reflection of how entrainment takes place in nocturnal animals under natural conditions (18, 19).
As expected for both entrainment groups, SCN Per1 and Per2 mRNA levels were rhythmic, with relatively higher levels during the subjective day than night. The expression of Per2 lagged that of Per1, so that Per1 (but not Per2) was expressed immediately at the end of the dawn (0°) light pulse of the 23.33-h T cycle, whereas Per2 (but not Per1) was expressed at the end of the dusk (180°) light pulse of the 24.67-h T cycle. Importantly, our results revealed fundamental differences in the effects of light on Per expression when entrainment was accomplished by advances or delays. The dawn (advancing) light pulse of the short T cycle acted to acutely elevate Per1 mRNA from its low baseline level characteristic of the preceding subjective night; this was especially evident in the ventrolateral subregion of the SCN, as would be predicted in hamsters (20–22). In contrast, and surprisingly, the dusk (delaying) light pulse of the long T cycle had no acute effect on either Per1 or Per2 mRNA, but entrainment of the clock to the long T cycle somehow altered Per2 steady-state regulation, resulting in a Per2 mRNA rhythm with subjective day levels of increased amplitude and extended duration. In this way, morning light advanced the onset of the Per1 mRNA rhythm, whereas evening light delayed the offset of the Per2 mRNA rhythm. We do not believe that these contrasting effects represented an artifact of the specific T cycles we selected, e.g., that one of the cycles might have been further from the species-natural τ than the other. Both cycles led to entrainment of 100% of our hamsters, and lie just within the limits of stable entrainment of hamsters to a single 1-h light pulse (with estimated 50% of animals entrained under 24.7- to 24.8-h and 23.1- to 23.2-h cycles) (8, 23).
To begin to understand the molecular mechanisms that might underlie the change in the Per2 mRNA expression pattern, we probed the state of the circadian clock in the SCN by analyzing the photoresponsiveness of Per1 and Per2 to a brief light pulse administered in the middle of the subjective night, when levels of both transcripts are at their minimum. For this experiment, we assumed that the durations of subjective day and night were compressed and decompressed equally under short and long T; we also ignored the possible complication of the animals' locomotor activity per se, which in the long T cycle began and ended earlier than in the short T cycle (Fig. 4). Our results using intronic and exonic probes hint that Per1 transcription was sustained longer after administration of the photic stimulus during the short T cycle, although this was not associated with a T-cycle group difference in the rise and fall of the mature mRNA. Notably, entrainment to the long T cycle was associated with an apparent increase in Per2 mRNA stability, raising the possibility that the increased amplitude and extended duration of subjective-day Per2 under this T cycle was due to decreased mRNA degradation rather than elevated transcription. We note that regulation of Per2 mRNA degradation has been described previously in cell culture (24), and also that the Per2 gene (25) and the level and stabilization of PER2 protein (26–28) have been related to the length of τ. Further studies will be required to determine the expression patterns of SCN PER2 protein under T-cycle entrainment, their possible relationships to entrainment aftereffects on τ, and the potential role of behavioral activity per se on gene regulation.
There currently exists a large literature on the circadian phase-shifting effects of an acute light pulse given to animals in DD, with advances or delays of rhythm phase depending on the phase of light administration (in the late or early subjective night, respectively). A recurring theme of this work is that advance and delay phase shifts are not simply mirror images of each other but instead appear to exhibit some fundamentally different features, e.g., in the duration of transients, pharmacological sensitivity, and underlying signal transduction pathways (29). Here we show that T-cycle entrainment by advances or delays relies on dawn or dusk light pulses that also exhibit essentially different features: a pulse at dawn advances the clock, advancing the onset of the Per1 rhythm and acutely increasing mRNA transcription, whereas a pulse at dusk delays the clock, delaying the offset of the Per2 rhythm and tonically increasing mRNA stability. We anticipate that the signaling pathways mediating these contrasting effects should be distinct; indeed, a recent report is consistent with this view, demonstrating that entrainment to short or long T cycles is differentially inhibited by pharmacological blockade of SCN nitric oxide levels (23).
Our finding of differential aftereffects in DD after entrainment to the short or long T cycles is interesting. Animals entrained to the short T cycle and placed in DD quickly lengthened their rhythm toward the species-natural τ, whereas those entrained to the long T cycle maintained their long cycle length even in DD. This dichotomy—that entrainment to the short T cycle must require a daily advance of the clock's phase, whereas entrainment to the long T cycle entails a matching change of the clock's period—recalls the two major models for circadian photoentrainment (1, 2, 30). The discrete (nonparametric, phasic) model proposes that entrainment is the result of daily light-induced phase shifts, as predicted by a phase–response curve. The continuous (parametric, tonic) model, in contrast, proposes that entrainment occurs by a change in the oscillator's velocity that approximates its period to that of the entraining photocycle. Natural entrainment to the light/dark cycle may involve both phasic and tonic effects of light, and we speculate that the former might act in the morning on Per1 transcription and the latter might act in the evening on Per2 degradation.
The clock in the SCN has been modeled as a complex pacemaker consisting of two mutually coupled circadian oscillators, a morning oscillator (M) accelerated by light and synchronized to dawn and an evening oscillator (E) decelerated by light and synchronized to dusk (31, 32). It has been proposed that M and E oscillators might represent transcription-translation loops of clock genes, with Per1 a part of M and Per2 a part of E (33). Evidence both for and against this proposition has been gathered using animals genetically deficient in Per and Cry genes (6, 34–37), although Per1- and Per2-deficient mice can show intact phase-shifting responses. Though our results do link Per1 with dawn and Per2 with dusk, they intimate that M and E oscillator components are not only affected by different genes but also may be entrained by distinct molecular mechanisms.
Experimental Procedures
Animals.
Male Syrian hamsters (Mesocricetus auratus; Charles River), 21 d old at time of delivery to the laboratory, were housed individually in clear polycarbonate cages contained within well-ventilated, light-proof environmental cabinets isolated in an animal facility. Food and water were freely available and replenished at irregular hours. Light (300–400 l× at the midcage level) was provided by 15-watt cool-white fluorescent tubes mounted above the shelves and automatically controlled by a programmable timer. Animals were exposed to a 1-h light pulse presented every 23.33 h (23.33-h T cycle) or every 24.67 h (24.67-h T cycle) and maintained (entrained) for 12–16 wk unless otherwise indicated. Dim red light was used for animal care and husbandry when necessary. All behavioral experiments were conducted at the University of Massachusetts Medical School and performed in compliance with the Animal Care and Use Committees of the University of Massachusetts Medical School and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
For measurement of locomotor (wheel-running) activity, a microswitch on the outside of each cage was activated by the rotation of a 125-mm (diameter) metal running wheel; the number of switch closures per 15-min interval was automatically recorded, stored on hard disk by an IBM computer-based data acquisition system (Dataquest; Mini-Mitter), and plotted for each animal as an actogram.
Melatonin Assays.
Melatonin content from individual sonicated pineal glands was measured by ELISA (IBL International), according to the protocol of von Gall et al. (38). Briefly, duplicate supernatants of each homogenized pineal were incubated overnight with a rabbit polyclonal antibody and a known amount of biotinylated melatonin. After several rinses, the bound melatonin was exposed to a colorimetric reaction and the concentration calculated according to a standard curve. The assay sensitivity was 1.6 pg/mL. All samples were assayed in a single assay with an intra-assay variability (SD relative to the mean) of 9.2%.
In Situ Hybridization.
Animals were decapitated under dim red light. Brains were rapidly removed and frozen in 2-methylbutane cooled to −30 °C. The 15-μm–thick coronal sections through the SCN were cut on a cryostat and mounted onto slides coated with Vectabond (Vector Laboratories).
Plasmids used to generate antisense cRNA probes for hamster Per1 and Per2 (exonic probes) were generously provided by H. Okamura (Kyoto University, Kyoto, Japan) and S. Shibata (Waseda University, Tokyo, Japan), respectively. A PCR amplicon with RNA polymerase promoter regions was used for Bmal1 (template kindly provided by C. Weitz, Harvard Medical School, Boston, MA).
Syrian hamster Per1 intron 17 and Per2 intron 2 probes were obtained by first amplifying hamster genomic DNA with primer pairs located in the flanking exons to obtain intron sequences. Subsequently, nested primers were used to isolate an intronic fragment. For Per1, exonic primers (corresponding to nt 2215–2234 and 2454–2477 of the hamster Per1 cDNA; GenBank accession no. AY423771.1) yielded an 841-bp product containing intron 17 (GenBank accession no. JF739178). Primers based on intronic sequence were then used to amplify a 515-bp fragment (nt 154–668; GenBank accession no. JF739178), which was subcloned into Topo Dual promoter plasmid (Invitrogen) for generation of Per1 intronic sense and antisense probes. For Per2, the exonic primers (corresponding to nt 108–130 and 231–255; GenBank accession no. AF306648.1) yielded an 844-bp fragment containing intron 2 (GenBank accession no. JF739179). Nested intronic primers were then used to amplify a 625-bp fragment (nt 167–791; GenBank accession no. JF739179), which was subcloned and used to generate the Per2 intronic sense and antisense probes. Intronic probes were validated using reference SCN sections from test animals killed either in the dark or after a light pulse. As measured by relative optical density (ROD; SCN/adjacent hypothalamus), hybridization intensity with each of the antisense probes was significantly increased after light exposure compared with the dark controls (P < 0.005; Student t test), whereas ROD values from sections probed with the sense probes were low (near 1) and did not differ significantly from the values for the dark controls.
In vitro transcription reactions were performed in the presence of 35S-UTP with the appropriate RNA polymerase. In situ hybridization with the radiolabeled cRNA probes was performed as previously described (22, 39, 40). Serial coronal sections were hybridized with the same probe at intervals of 75 μm (Figs. 3 and 4) or 120 μm (Fig. 6). ODs of film autoradiograms were measured in ImageJ (National Institutes of Health). Templates were made for subregions or the whole SCN by taking the best-fit outline from several representative sections picked at random from the experimental samples. Background values were determined by sampling the adjacent hypothalamus of each section. ROD was calculated as the OD value for the region of interest divided by the background OD value for the same section; this normalized OD was measured bilaterally for each subregion in 2–4 sections and averaged for each animal. The duration of elevated Per rhythmic expression was calculated using onset and offset at a level midway between peak and trough values, the latter defined as the average level at the three highest and the three lowest contiguous time points, respectively.
Acknowledgments
We thank Drs. Jeffrey Elliott and Roelof Hut for critical discussions; Dr. Charlotte von Gall for advice on melatonin assays; Ms. Dorothy Lam for help with in situ hybridization; and Drs. Hitoshi Okamura, Shigenobu Shibata, and Charles Weitz for gifts of recombinant plasmids. Support for this work was provided in part by National Institutes of Health Grants R01 GM094109 (to W.J.S.), R01 NS056125 (to D.R.W), and R01 MH075016 (to H.O.d.l.I).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Daan S, Aschoff J. The entrainment of circadian systems. In: Takahashi JS, Turek F, editors. Handbook of Behavioral Neurobiology: Circadian Clocks. Vol 12. New York: Kluwer Academic/Plenum; 2001. pp. 7–42. [Google Scholar]
- 2.Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- 3.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford Univ Press; 1991. [Google Scholar]
- 4.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver DR, Reppert SM. Circadian timekeeping. In: Squire LR, et al., editors. Fundamental Neuroscience. San Diego: Academic; 2008. pp. 931–958. [Google Scholar]
- 6.Pendergast JS, Friday RC, Yamazaki S. Photic entrainment of period mutant mice is predicted from their phase response curves. J Neurosci. 2010;30:12179–12184. doi: 10.1523/JNEUROSCI.2607-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott JA. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976;35:2339–2346. [PubMed] [Google Scholar]
- 8.Elliott JA. Austin, TX: University of Texas at Austin; 1974. Photoperiodic regulation of testis function in the golden hamster: Relation to the circadian system. PhD dissertation. [Google Scholar]
- 9.Pittendrigh C, Minis DH. The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am Nat. 1964;98:261–294. [Google Scholar]
- 10.Pittendrigh CS, Daan S. Functional analysis of circadian pacemakers in nocturnal rodents. 4. Entrainment: Pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- 11.Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- 12.Paul MJ, Schwartz WJ. On the chronobiology of cohabitation. Cold Spring Harb Symp Quant Biol. 2007;72:615–621. doi: 10.1101/sqb.2007.72.042. [DOI] [PubMed] [Google Scholar]
- 13.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 14.Miyake S, et al. Phase-dependent responses of Per1 and Per2 genes to a light-stimulus in the suprachiasmatic nucleus of the rat. Neurosci Lett. 2000;294:41–44. doi: 10.1016/s0304-3940(00)01545-7. [DOI] [PubMed] [Google Scholar]
- 15.Yan L, Silver R. Resetting the brain clock: Time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci. 2004;19:1105–1109. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagano M, Adachi A, Masumoto KH, Meyer-Bernstein E, Shigeyoshi Y. rPer1 and rPer2 induction during phases of the circadian cycle critical for light resetting of the circadian clock. Brain Res. 2009;1289:37–48. doi: 10.1016/j.brainres.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 18.DeCoursey PJ. Light-sampling behavior in photoentrainment of a rodent circadian rhythm. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1986;159:161–169. doi: 10.1007/BF00612299. [DOI] [PubMed] [Google Scholar]
- 19.Gattermann R, et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett. 2008;4:253–255. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: Rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21:7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto S, et al. Expression of the Per1 gene in the hamster: Brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol. 2001;430:518–532. [PubMed] [Google Scholar]
- 22.de la Iglesia HO, Meyer J, Schwartz WJ. Using Per gene expression to search for photoperiodic oscillators in the hamster suprachiasmatic nucleus. Brain Res Mol Brain Res. 2004;127:121–127. doi: 10.1016/j.molbrainres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Plano SA, Golombek DA, Chiesa JJ. Circadian entrainment to light-dark cycles involves extracellular nitric oxide communication within the suprachiasmatic nuclei. Eur J Neurosci. 2010;31:876–882. doi: 10.1111/j.1460-9568.2010.07120.x. [DOI] [PubMed] [Google Scholar]
- 24.Woo KC, et al. Mouse period 2 mRNA circadian oscillation is modulated by PTB-mediated rhythmic mRNA degradation. Nucleic Acids Res. 2009;37:26–37. doi: 10.1093/nar/gkn893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendergast JS, Friday RC, Yamazaki S. Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS ONE. 2010;5:e8552. doi: 10.1371/journal.pone.0008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz M, Peirson SN, Hankins MW, Foster RG. Long-term constant light induces constitutive elevated expression of mPER2 protein in the murine SCN: A molecular basis for Aschoff's rule? J Biol Rhythms. 2005;20:3–14. doi: 10.1177/0748730404272858. [DOI] [PubMed] [Google Scholar]
- 27.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 28.Meng QJ, et al. Setting clock speed in mammals: The CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 30.Daan S. The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jürgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms. 2000;15:195–207. doi: 10.1177/074873040001500301. [DOI] [PubMed] [Google Scholar]
- 31.Pittendrigh CS, Daan S. Functional analysis of circadian pacemakers in nocturnal rodents. 5. Pacemaker structure: Clock for all seasons. J Comp Physiol. 1976;106:333–355. [Google Scholar]
- 32.Daan S, Berde C. Two coupled oscillators: Simulations of the circadian pacemaker in mammalian activity rhythms. J Theor Biol. 1978;70:297–313. doi: 10.1016/0022-5193(78)90378-8. [DOI] [PubMed] [Google Scholar]
- 33.Daan S, et al. Assembling a clock for all seasons: Are there M and E oscillators in the genes? J Biol Rhythms. 2001;16:105–116. doi: 10.1177/074873001129001809. [DOI] [PubMed] [Google Scholar]
- 34.Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- 35.Steinlechner S, et al. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J Biol Rhythms. 2002;17:202–209. doi: 10.1177/074873040201700303. [DOI] [PubMed] [Google Scholar]
- 36.Spoelstra K, Albrecht U, van der Horst GT, Brauer V, Daan S. Phase responses to light pulses in mice lacking functional per or cry genes. J Biol Rhythms. 2004;19:518–529. doi: 10.1177/0748730404268122. [DOI] [PubMed] [Google Scholar]
- 37.Spoelstra K, Daan S. Effects of constant light on circadian rhythmicity in mice lacking functional cry genes: Dissimilar from per mutants. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:235–242. doi: 10.1007/s00359-007-0301-3. [DOI] [PubMed] [Google Scholar]
- 38.von Gall C, et al. Transcription factor dynamics and neuroendocrine signalling in the mouse pineal gland: A comparative analysis of melatonin-deficient C57BL mice and melatonin-proficient C3H mice. Eur J Neurosci. 2000;12:964–972. doi: 10.1046/j.1460-9568.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- 39.de la Iglesia HO. In situ hybridization of suprachiasmatic nucleus slices. In: Rosato E, editor. Methods Mol Biol. Vol 362. Clifton, NJ: Humana; 2007. pp. 513–531. [DOI] [PubMed] [Google Scholar]
- 40.Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus) J Biol Rhythms. 2005;20:206–218. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]