Abstract
Tumor microenvironment is characterized by chronic inflammation represented by infiltrating leukocytes and soluble mediators, which lead to a local and systemic immunosuppression associated with cancer progression. Here, we used the ret transgenic spontaneous murine melanoma model that mimics human melanoma. Skin tumors and metastatic lymph nodes showed increased levels of inflammatory factors such as IL-1β, GM-CSF, and IFN-γ, which correlated with tumor progression. Moreover, Gr1+CD11b+ myeloid-derived suppressor cells (MDSCs), known to inhibit tumor reactive T cells, were enriched in melanoma lesions and lymphatic organs during tumor progression. MDSC infiltration was associated with a strong TCR ζ-chain down-regulation in all T cells. Coculturing normal splenocytes with tumor-derived MDSC induced a decreased T-cell proliferation and ζ-chain expression, verifying the MDSC immunosuppressive function and suggesting that the tumor inflammatory microenvironment supports MDSC recruitment and immunosuppressive activity. Indeed, upon manipulation of the melanoma microenvironment with the phosphodiesterase-5 inhibitor sildenafil, we observed reduced levels of numerous inflammatory mediators (e.g., IL-1β, IL-6, VEGF, S100A9) in association with decreased MDSC amounts and immunosuppressive function, indicating an antiinflammatory effect of sildenafil. This led to a partial restoration of ζ-chain expression in T cells and to a significantly increased survival of tumor-bearing mice. CD8 T-cell depletion resulted in an abrogation of sildenafil beneficial outcome, suggesting the involvement of MDSC and CD8 T cells in the observed therapeutic effects. Our data imply that inhibition of chronic inflammation in the tumor microenvironment should be applied in conjunction with melanoma immunotherapies to increase their efficacy.
Keywords: therapy, cytokines
Malignant melanoma is notorious for aggressive clinical behavior, proclivity for distant metastasis, and poor response to currently available therapeutic agents (1). Immunotherapy appears to be especially promising as a result of the well known intrinsic immunogenicity of melanoma (2–4). However, despite the initial promising observations, the overall results of clinical immunotherapeutic trials are not satisfactory (3, 5). Insufficient antitumor reactivity is thought to result from the formation of a complex immunosuppressive network induced by the chronic inflammation developing in the tumor microenvironment (6–8). Indeed, chronic inflammation has been demonstrated to correlate with tumor onset and progression (6, 7). An inflammatory microenvironment ensues during tumor growth as a result of the secretion of inflammatory mediators (cytokines, chemokines, growth factors, reactive oxygen and nitrogen species, prostaglandins) by the tumor and/or stroma cells (8–10). These mediators were found to support tumor development by stimulating protumor mutations, resistance to apoptosis, and angiogenesis (7, 8). Moreover, some of the aforementioned factors were reported to induce the accumulation and activation of myeloid-derived suppressor cells (MDSCs) in tumor lesions, lymphatic organs, and the peripheral blood (9, 11–15).
MDSCs have been characterized as a heterogeneous population of immature myeloid cells that are precursors of dendritic cells, macrophages, and granulocytes and coexpress (in mice) Gr1 and CD11b (9, 11, 12). MDSC immunosuppressive activity in tumor-bearing hosts inhibiting antitumor T-cell responses was reported to be a result of the activation of inducible NOS and arginase (ARG)-1, leading to L-arginine depletion and increased production of NO and reactive oxygen species (11, 16–18). One of the major features of MDSC-induced impairment of T-cell functions is associated with a pronounced down-regulation of the T-cell receptor (TCR) ζ-chain expression (19, 20), which plays a key role in coupling the TCR-mediated antigen recognition to diverse signal transduction pathways (21).
Various approaches were used during the last years to decrease MDSC amounts and immunosuppressive function under different pathological conditions (22). Recently, phosphodiesterase (PDE)-5 inhibitors [sildenafil (Viagra) and tadalafil (Cialis)], which are used for the treatment of erectile dysfunction, pulmonary hypertension, and cardiac hypertrophy (23), have been shown to exert antitumor effects in various transplantation mouse models by abrogating MDSC immunosuppressive functions that resulted in the accumulation and activation of tumor infiltrating lymphocytes (TILs) (24–26). However, the disease development and the tumor–stroma interactions in transplantation tumor models are not comparable with clinical conditions. In contrast, the ret transgenic mouse melanoma model closely resembles human disease as to tumor genetics, histopathology, and clinical development (27, 28). Mice expressing the human ret transgene in melanocytes develop spontaneously malignant skin melanoma with metastases in lymph nodes (LNs), lungs, liver, and brain (27, 28), similar to the metastatic pattern observed in melanoma in humans (29).
In this study, we investigated the role of the inflammatory tumor microenvironment in MDSC enrichment, immunosuppressive activity, and melanoma progression in ret transgenic mice. We found that an accumulation of functionally active MDSC in melanoma lesions and lymphoid organs was strongly associated with a dramatic reduction of TCR ζ-chain expression in T cells. Oral administration of sildenafil decreased inflammatory mediators in the tumor microenvironment, abrogated immunosuppressive activities of MDSC, and restored ζ-chain expression levels in TILs, which resulted in significantly increased mouse survival. This provides a new therapeutic strategy for the neutralization of inflammatory immunosuppressive environment that can overcome T-cell anergy and enhance the efficacy of T cell-mediated melanoma immunotherapy.
Results
Elevation of Inflammatory Cytokines and Growth Factors in Melanoma Lesions from Ret Transgenic Mice.
In the present study, we analyzed ret transgenic mice that spontaneously develop skin malignant melanomas and distant metastases in LN, liver, and lungs. To address the role of inflammatory mediators in the tumor microenvironment during melanoma progression, we investigated the production of relevant cytokines and chemokines in lysates prepared from skin melanomas and metastatic LN by using a Bio-Plex assay. Tumor progression was assessed as the duration between first visible tumor manifestations and mouse death. As shown in Fig. 1 A–C, concentrations of IFN-γ, IL-1β, and GM-CSF were higher in quickly growing tumors than in slowly growing ones. Analyzing cytokine amounts in metastatic LNs from the same animals revealed a similar correlation with the duration of tumor progression (Fig. 1 D–F), which was statistically significant for IL-1β (Fig. 1E). Together with the accumulation of VEGF, IL-6, and TGF-β in melanoma lesions described by us earlier (30), these observations support conditions for MDSC recruitment at the tumor site.
Fig. 1.
Inflammatory mediators in tumor lesions of transgenic mice. IFN-γ, IL-1β, and GM-CSF were measured in primary tumors (A–C) and metastatic LN (D–F) by Bio-Plex and plotted against the duration of tumor growth between first visible tumor signs and mouse death. Each group included seven to 12 mice. The correlation between two variables was calculated by using a linear regression analysis.
MDSC Are Accumulated in Skin Tumors, Metastatic LNs, and Lymphatic Organs.
Gr1+CD11b+ MDSCs were quantified during tumor development in skin tumors, metastatic LN, spleen, and bone marrow (BM; Fig. 2 A–D and Fig. S1 A and B). Accumulation of MDSCs among tumor infiltrating leukocytes significantly correlated with the increasing weight of these tumors (Fig. 2B). Furthermore, quickly progressing tumors showed elevated MDSC proportions within infiltrating leukocytes (Fig. 2C). These findings suggest that the observed enrichment of inflammatory factors in melanoma microenvironment may attract MDSCs during tumor progression.
Fig. 2.
Analysis of MDSCs and TCR ζ-chain in tumors and lymphatic organs of transgenic mice. Cells from mice with (ret tu) or without (ret) macroscopic tumors and nontransgenic littermates (wt) were assessed by flow cytometry. (A) A representative dot plot of primary tumor is shown. (B) The weight of each tumor sample was plotted against the percentage of tumor-infiltrating Gr1+CD11b+ MDSCs within CD45.2+ leukocytes. (C) The duration of tumor progression was plotted against the percentage of MDSCs among live cells. The correlation was calculated by a linear regression analysis. (D) Cumulative data for MDSCs in metastatic LNs are expressed as the percentage of leukocytes (mean and SE from 10–31 mice). (E) ζ-Chain level in CD3+CD4+ TILs expressed as mean florescence intensity (MFI) and plotted against the tumor weight (n = 12). The correlation was analyzed by using a linear regression analysis. (F) Cumulative data for ζ-chain expression in metastatic LNs are shown as MFI (mean and SE; n = 5–12 mice per group; *P < 0.05).
Next we studied Gr1+CD11b+ MDSCs in lymphatic organs. Tumor-bearing mice showed significantly increased MDSC frequencies in metastatic LN (Fig. 2D) compared with nontransgenic littermates and/or transgenic mice without macroscopically visible tumors (i.e., control groups). Similar changes were found in the spleen and BM (Fig. S1 A and B). When testing other potential MDSC markers such as IL-4Rα (14) or F4/80 (31), we found no significant changes in their expression compared with control groups. Recent reports have shown that MDSCs consist of granulocytic CD11b+Ly6G+Ly6Clow and monocytic CD11b+Ly6G+/−Ly6Chigh subsets (32, 33). We detected a reduction in monocytic MDSC numbers among total Gr1+CD11b+ cells in the BM of tumor-bearing mice compared with nontransgenic littermates (18% and 11%, respectively; P < 0.05), with a slight increase in the frequency of the granulocytic subpopulation. No changes in MDSC subsets were observed in spleen and LN from these mice.
We next investigated key factors involved in the induction of immunosuppression such as NO and ARG-1 (9, 11) and found a profound elevation of NO production by MDSCs in the BM, metastatic LN, and spleen from melanoma-bearing mice compared with both control groups (Fig. S2 A–C). Furthermore, amounts of ARG-1–expressing cells within MDSCs in metastatic LNs from tumor-bearing mice were significantly higher than in WT animals (Fig. S2D).
TCR ζ-Chain Is Down-Regulated in TILs and Lymphatic Organs.
To study the effect of MDSC on T cells in tumor-bearing mice, we tested TCR ζ-chain expression in primary tumors, LN, spleen, and BM by intracellular staining. A decrease in the ζ-chain expression in CD3+CD4+ TILs significantly correlated with tumor progression as indicated by an augmented tumor weight (Fig. 2E). For CD3+CD8+ TILs, this effect was observed as a strong tendency. When comparing the lymphocyte infiltration of larger (>700 mg) and smaller (<700 mg) tumors, we detected much lower ζ-chain expression in both CD4+ and CD8+ TILs from larger tumors (Fig. S3A). Moreover, both CD4+ and CD8+ T cells from metastatic LNs (Fig. 2F) and spleens (Fig. S3B) from mice with tumors of different weight showed significantly lower ζ-chain expression compared with tumor-free transgenic and WT mice, indicating the suppression of TCR ζ-chain expression in tissues with enriched MDSC amounts.
MDSC Suppress T-Cell Proliferation and TCR ζ-Chain Expression ex Vivo.
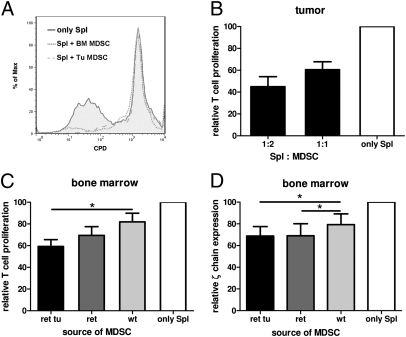
To test the direct impact of MDSCs from melanoma-bearing mice on T-cell activity and ζ-chain expression, MDSCs were isolated from skin tumors by using CD11b MicroBeads (80% of Gr1+CD11b+ cell purity) and from the BM by FACS sorting (99% of Gr1+CD11b+ cell purity). The selected cells were then cocultured with C57BL/6 splenocytes stimulated by anti-CD3 and anti-CD28 mAbs or with OT-1 splenocytes activated by ovalbumin (OVA). Tumor-derived MDSCs profoundly inhibited T-cell proliferation tested by the reduced expression of the cell proliferation dye (CPD) eFluor 670 (Fig. 3 A and B). Interestingly, MDSCs isolated from the BM of the same tumor-bearing animals also showed a considerably higher immunosuppressive activity than those from WT mice detected by the CPD eFluor 670 (Fig. 3 A and C) and Ki67 expression on stimulated T cells. As ζ-chain is an essential molecule transmitting downstream signals after TCR ligation, we investigated its expression in nonstimulated C57BL/6 spleen T lymphocytes upon overnight coculture with BM Gr1+CD11b+ cells sorted by FACS. Similar to the proliferation assay, MDSCs from transgenic tumor-bearing mice inhibited ζ-chain significantly stronger than WT MDSCs (Fig. 4D). Interestingly, even MDSCs from transgenic mice without macroscopic tumors induced a significant down-regulation of ζ-chain expression (Fig. 3D).
Fig. 3.
Immunosuppressive activity of MDSCs from transgenic mice. (A–C) MDSCs were isolated from skin tumors and BM, cocultured with C57BL/6 splenocytes labeled with CPD eFluor 670, and stimulated with anti-CD3 and anti-CD28 mAbs. (A) A histogram from one representative experiment of four is shown. Cumulative data (mean and SE; n = 4–6 mice per group) for the activity of MDSCs isolated from tumors (B) or BM (C) are shown as a ratio between T-cell proliferation levels with and without MDSCs. The proliferation of splenocytes alone (only Spl) was considered as 100%. Spl:MDSC ratios were as indicated (B) or were 1:1 (C). (D) FACS-sorted MDSCs were coincubated with nonstimulated C57BL/6 splenocytes at the Spl:MDSC ratio of 0.6:1 followed by the ζ-chain detection. Results are depicted as a ratio between ζ-chain levels (measured as MFI) in samples with and without MDSC. ζ-Chain levels in T cells cultured alone was considered as 100%. Mean and SE from three independent experiments (n = 4 mice per group) are shown (*P < 0.05).
Fig. 4.
Effect of sildenafil on melanoma progression. Tumor-bearing mice received sildenafil with drinking water (20 mg/kg/24 h) for 6 wk. (A) Survival of mice (n = 12 mice per group) is shown as a Kaplan–Meier curve. (B) Levels of IL-1β, VEGF, GM-CSF, Ccl2, and Ccl3 were detected in metastatic LNs by using Bio-Plex and expressed as pg/mg protein (mean and SE; n = 6–8 mice per group). (C) IL-6 in tumor lysates was measured by Bio-Plex assay and depicted as pg/mg protein (mean and SE; n = 5–8 mice per group). (D) MDSCs were measured in metastatic LNs from treated and untreated mice by flow cytometry and presented as the percentage of leukocytes (mean and SE; n = 6–11 mice per group). (E) S100A9 expression in MDSCs of metastatic LNs was detected by flow cytometry and shown as the percentage of S100A9+ cells among total MDSCs (mean and SE; n = 6–8 mice per group; *P < 0.05 and **P < 0.01).
Sildenafil Delays Tumor Progression and Impairs MDSC Function in Vivo.
To neutralize MDSC-related immunosuppressive effects in melanoma-bearing mice, we used the PDE-5 inhibitor sildenafil. By using Western blot analysis, we demonstrated that the PDE-5 protein is expressed in MDSCs isolated from skin tumors but not in cells established from primary melanoma (Ret cells; Fig. S4). Chronic administration of sildenafil (with drinking water for 6 wk) resulted in the profound prolongation of mouse survival compared with the untreated (i.e., control) group (P = 0.002; Fig. 4A). Interestingly, PDE-5 expression levels in tumor MDSCs from treated mice seemed to be similar to those of control animals (Fig. S4). As sildenafil could also partially inhibit the activity of PDE-6 expressed in photoreceptor cells of mouse retina (34), we tested PDE-6 by using immunohistology and found no significant changes in the retina from treated mice compared with controls. Analysis of the PDE-5 inhibition revealed a strong elevation of cGMP levels in skin tumors and metastatic LNs from treated mice compared with untreated tumor-bearing mice (Fig. S5A). Moreover, as expected, this cGMP accumulation was accompanied by a significant decrease in the NO content in primary tumors (Fig. S5B). A similar tendency was observed in metastatic LNs.
We next investigated sildenafil-mediated effects on inflammatory factors in melanoma lesions. The concentration of IL-β, VEGF, and GM-CSF, as well as chemokines Ccl2 (MCP-1) and Ccl3 (MIP-1α), was significantly diminished in metastatic LNs from treated compared with untreated mice (Fig. 4B). Moreover, decreased levels of IL-6 in primary melanomas upon sildenafil therapy were detected (Fig. 4C). Importantly, these alterations in inflammatory mediators were associated with a reduction in numbers of MDSCs infiltrating metastatic LNs (Fig. 4D). Furthermore, the amount of MDSCs expressing the myeloid-related protein S100A9 was significantly decreased in skin tumors from treated mice (Fig. 4E). Metastatic LNs also displayed, under sildenafil therapy, a reduction in the proportion of MDSCs producing NO and in levels of NO production (Fig. S6 A and B). In addition, the intracellular expression level of ARG-1 in MDSCs from treated mice was considerably lower than in the control group (Fig. S6C). Similar sildenafil-induced changes were also detected in primary skin tumors.
Restoration of T-Cell Activity Upon in Vivo Sildenafil Therapy.
Next, we asked whether PDE-5 inhibition in MDSCs could alter their ability to suppress T-cell functions. To this end, MDSCs isolated from primary tumors of transgenic animals treated with sildenafil for 5 wk were ex vivo coincubated with stimulated C57BL/6 splenocytes. Such MDSCs exerted a weaker ability to suppress T-cell proliferation than those isolated from untreated tumor-bearing mice (Fig. 5A). Sildenafil also induced a partial recovery of TCR ζ-chain expression both in CD4+ and CD8+ T cells infiltrating primary tumors (Fig. 5B) and metastatic LNs (Fig. 5C). Moreover, we observed an elevated frequency of CD3+CD4+ and CD3+CD8+ T cells in metastatic LNs (Fig. 5D) and primary tumors compared with untreated mice. In addition, an accumulation of the key T-cell growth factor IL-2 was found in metastatic LNs upon the therapy (Fig. 5E). To investigate the proportion of tumor-specific CD8+ TILs upon the sildenafil therapy, we performed stainings with tetramers containing Kb and the peptide SVYDFFVWL derived from tyrosinase-related protein (TRP)-2 as a model melanoma antigen. We detected no significant increase in frequencies of TRP-2–specific CD8+ T cells within total CD8+ population in primary tumors and metastatic LNs upon the treatment; these melanoma-specific cells displayed also no differences in ζ-chain levels compared with total CD8+ TILs. Finally, to study if sildenafil's therapeutic outcome is a result of an effects on T cells, we depleted CD8+ T cells in sildenafil-treated mice by using corresponding mAbs (Fig. 5F). The survival of these mice was similar to that of the untreated group and significantly shorter compared with animals receiving sildenafil without depleting mAbs (P = 0.025).
Fig. 5.
Sildenafil decreases MDSC-induced immunosuppression and enhances TILs. (A) MDSCs were isolated from tumors of treated and untreated mice and coincubated with activated normal splenocytes at the Spl:MDSC ratio of 1:1. Means and SEs from three independent experiments (n = 4 mice per group) are presented as a proportion between the proliferation of T cells cultured alone or with MDSCs and expressed as percentage. (B and C) ζ-Chain expression in TILs from skin tumors (B) and metastatic LNs (C) are expressed as MFI (mean and SE; n = 10–13 mice per group). (D) T cells in metastatic LNs were detected by flow cytometry. Results (mean and SE; n = 11–14 mice per group) are shown as the percentage of CD3+CD4+ and CD3+CD8+ T cells among mononuclear cells. (E) IL-2 was measured in metastatic LNs by Bio-Plex assay and expressed as pg/mg protein (mean and SE; n = 6–8 mice per group). (F) Some sildenafil-treated mice were injected with rat anti-mouse mAbs depleting CD8+ T cells. IgG from rat serum were inoculated in untreated mice. Survival (n = 8 mice per group) is shown as a Kaplan–Meier curve (*P < 0.05 and **P < 0.01).
In conclusion, the ability of sildenafil to prolong survival of mice with spontaneously arising melanoma was associated with inhibition of MDSC immunosuppressive functions and concomitant restoration of T-cell function in association with recovery of ζ-chain expression in cells infiltrating skin melanomas and metastatic LNs.
Discussion
The fundamental role of chronic inflammation in suppressing T cell-mediated antitumor immune responses and promoting tumor progression has been recently documented (6–10). However, the previously suggested interplay among inflammation, immunosuppression, and tumor progression has been based primarily on studies performed by using conventional transplantation tumor mouse models that do not adequately mimic human tumor development.
To address the role of chronic inflammatory mediators in tumor progression in more clinically relevant conditions, we used the ret transgenic mouse model of spontaneous skin melanoma, which resembles human melanoma with respect to clinical development ensuring natural tumor-stroma interactions (27, 28). We found an association between increased concentrations of IL-1β and GM-CSF in melanoma lesions and accelerated melanoma progression. Recently we have reported similar data on the correlation of melanoma progression with VEGF and IL-6 levels (30). It has been well documented that various cytokines, chemokines, and growth factors including IL-1β, IL-6, IL-10, Ccl2 (MCP-1), Ccl3 (MIP-1α), GM-CSF, VEGF, and TGF-β are needed to drive MDSC migration into tumor lesions and to keep their suppressive phenotype in tumor-bearing hosts (6, 9, 11–13, 35). We also detected an accumulation of IFN-γ in melanoma lesions, which is known to be released by activated T cells, leading to the MDSC recruitment into the chronic inflammatory area and to the stimulation of NO production by these cells (6, 9, 11, 14, 36).
Analyzing Gr1+CD11b+ MDSCs in melanoma lesions and lymphoid organs from transgenic mice revealed a remarkable elevation of MDSC frequencies as demonstrated in different transplantation tumor models and patients with cancer (9, 11, 12, 17, 32, 33, 37, 38). Although an expansion of MDSCs was reported also for HER-2/neu transgenic mice spontaneously developing mammary carcinomas (39), it was studied only in the spleen and not in the tumor microenvironment. Our data indicate that an increased production of various inflammatory mediators can attract MDSCs into melanoma lesions in ret transgenic mice. Similar MDSC accumulation has been described in mice developing chronic inflammation (19) that resembles the tumor microenvironment (6, 40). The enhanced levels of MDSCs may cause decreased amounts of mature dendritic cells (41). Indeed, we have previously reported a significant decrease in numbers of these cells in melanoma lesions and lymphoid organs from ret transgenic mice (30). These cumulative data suggest a linkage among developing tumors, chronic inflammation, and immunosuppression. MDSC enrichment in tumor-bearing transgenic mice was associated with increased immunosuppressive activity, NO production, and ARG-1 expression in these cells, which is in agreement with earlier studies on tumor-bearing mice (9, 11–13). We also demonstrated that MDSCs in transgenic tumor-bearing mice consisted of granulocytic Ly6Clow and monocytic Ly6Chigh subpopulations as previously shown for other models (9, 32, 33). However, the granulocytic subset frequency was not increased as observed in transplantation tumor models (32). In addition, we detected no significant expression of IL-4Rα, which is considered as an important MDSC marker in some mouse tumor systems (14, 42). These discrepancies might occur as a result of differences between transplantation models and the autochthonous model used in our study.
A remarkable MDSC accumulation obtained herein was accompanied by a strong decrease in TCR ζ-chain expression in TILs from tumors and T cells in lymphoid organs of transgenic mice. A down-regulation of ζ-chain expression has been previously described not only in cancer (43, 44), but also in chronic inflammation associated with sustained antigenic stimulation (19, 21), suggesting again a striking resemblance of these two pathological conditions. We observed a direct effect of MDSCs on TCR ζ-chain expression in coculture experiments. In the presence of MDSCs derived from tumor-bearing transgenic mice, T cells showed not only decreased proliferative capacity but also lower ζ-chain expression. This impairment of ζ-chain expression was associated with elevated MDSC immunosuppressive activity as reflected by enhanced ARG-1 expression and NO production, factors shown to induce the anergy of reactive T cells (16, 17, 20, 21). We suggest that measuring ζ-chain expression levels in T cells from tumor-bearing host, without their removal from the suppressive microenvironment, could provide an accurate indication of their activity.
A key aspect of our study was the development of a therapeutic strategy toward overcoming the chronic inflammatory conditions associated with MDSC activity in the tumor microenvironment of transgenic mice. To this end, we manipulated the inflammatory conditions by using the PDE-5 inhibitor sildenafil, which has been previously found to neutralize MDSC activity in transplantable tumor models (24–26). The outcome of chronic drug administration was a significant increase in the survival of tumor-bearing mice. Importantly, we demonstrated a significant reduction of key inflammatory mediators such as IL-1β, VEGF, GM-CSF, Ccl2, and Ccl3 in metastatic LN and IL-6 in primary tumors upon the treatment, demonstrating its anti-inflammatory effect. Moreover, sildenafil induced a decrease in amounts of MDSCs in LN metastases that express the proinflammatory protein S100A9, known to be critically involved in the accumulation and retention of MDSCs (45, 46). Although moderate, these changes in numerous inflammatory molecules in the melanoma microenvironment could create conditions for observed diminution in MDSC numbers and immunosuppressive activity as reflected by decreased NO production and ARG-1 expression.
Furthermore, we showed that all sildenafil effects were strongly associated not only with an increase in TIL amounts but also with an enhancement of TCR ζ-chain expression in these cells. Moreover, a systemic depletion of CD8+ T cells in treated mice using corresponding mAbs completely abrogated antitumor effects of sildenafil, highlighting the mechanism of the restoration of antitumor T-cell reactivity. Interestingly, tetramer staining revealed no accumulation of melanoma antigen-specific CD8+ T lymphocytes or increased ζ-chain expression in these cells compared with total CD8+ TILs, indicating that the T-cell recovery happens upon the treatment regardless of their antigen specificity. As ζ-chain levels in TILs have been reported as a prognostic and survival biomarker in patients with cancer (43, 44), it could be used for the evaluation of the host immune status and of the efficiency of tumor immunotherapies. Importantly, the PDE-5 expression was detected in tumor-derived MDSCs but not in the Ret melanoma cell line. Moreover, we demonstrated no impairment of PDE-6 expression in the retina, indicating the absence of possible side effects related to PDE-6 inhibition (34). Based on our model, a possibility to improve melanoma immunotherapy is currently assessed by combining sildenafil treatment with adoptive transfer of activated melanoma-specific T cells.
Taken together, by using a transgenic mouse skin melanoma model, which is highly similar to human cutaneous melanoma, we highlight a complex association of proinflammatory cells and factors playing a key role in generating immunosuppression typical for tumor-bearing hosts. We demonstrated a remarkable increase in levels of inflammatory mediators and in amounts of strongly immunosuppressive MDSCs in melanoma lesions, which correlated with diminished TCR ζ-chain expression in TILs. Moreover, the treatment with sildenafil enabled neutralization of the harmful tumor microenvironment and uncovered the mechanisms underlying its unique anti-inflammatory effects as reflected by the reduction of inflammatory mediator levels associated with decreased MDSC numbers and suppressive activity. This resulted in a partial restoration of antitumor T-cell functions and significant retardation of spontaneous melanoma progression. We thus suggest that a key prerequisite for an effective melanoma immunotherapy should involve monitoring the initial immune status and the neutralization of immunosuppressive microenvironment typical for melanoma before applying any immunologic treatments.
Materials and Methods
SI Materials and Methods include descriptions of used transgenic mice and reagents, flow cytometry analysis, bio-plex, in vitro proliferation and biochemical assays. They contain also therapy with sildenafil, depletion of CD8+ T cells in treated mice, immunohistology, and Western blotting. Details for each method are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank I. Nakashima for providing ret transgenic mice, B. Arnold for OT-I mice, T. Schumacher for Kb/TRP-2 peptide tetramers, S. Schmitt and M. Ficht for FACS sorting, and K. Frank and L. Umansky for technical assistance. This work was supported by the German Cancer Research Center and Ministry of Science and Technology of Israel Cooperation in Cancer Research Grant CA128 (to V.U. and M.B.), Dr. Mildred Scheel Foundation for Cancer Research Grant 108992 (to V.U. and D.S.), and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer (V.U. and D.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108121108/-/DCSupplemental.
References
- 1.Spatz A, Batist G, Eggermont AM. The biology behind prognostic factors of cutaneous melanoma. Curr Opin Oncol. 2010;22:163–168. doi: 10.1097/CCO.0b013e328337fe8f. [DOI] [PubMed] [Google Scholar]
- 2.Guevara-Patiño JA, Turk MJ, Wolchok JD, Houghton AN. Immunity to cancer through immune recognition of altered self: Studies with melanoma. Adv Cancer Res. 2003;90:157–177. doi: 10.1016/s0065-230x(03)90005-4. [DOI] [PubMed] [Google Scholar]
- 3.Parmiani G, Castelli C, Santinami M, Rivoltini L. Melanoma immunology: Past, present and future. Curr Opin Oncol. 2007;19:121–127. doi: 10.1097/CCO.0b013e32801497d7. [DOI] [PubMed] [Google Scholar]
- 4.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 5.Jandus C, Speiser D, Romero P. Recent advances and hurdles in melanoma immunotherapy. Pigment Cell Melanoma Res. 2009;22:711–723. doi: 10.1111/j.1755-148X.2009.00634.x. [DOI] [PubMed] [Google Scholar]
- 6.Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Semin Cancer Biol. 2006;16:80–88. doi: 10.1016/j.semcancer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porta C, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallina G, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez PC, Ochoa AC. T cell dysfunction in cancer: role of myeloid cells and tumor cells regulating amino acid availability and oxidative stress. Semin Cancer Biol. 2006;16:66–72. doi: 10.1016/j.semcancer.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Rivoltini L, et al. Immunity to cancer: Attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev. 2002;188:97–113. doi: 10.1034/j.1600-065x.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 19.Ezernitchi AV, et al. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez PC, et al. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 21.Baniyash M. TCR zeta-chain downregulation: Curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4:675–687. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- 22.Ugel S, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serafini P, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capuano G, Rigamonti N, Grioni M, Freschi M, Bellone M. Modulators of arginine metabolism support cancer immunosurveillance. BMC Immunol. 2009;10:1. doi: 10.1186/1471-2172-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato M, et al. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 28.Umansky V, et al. Melanoma-specific memory T cells are functionally active in Ret transgenic mice without macroscopic tumors. Cancer Res. 2008;68:9451–9458. doi: 10.1158/0008-5472.CAN-08-1464. [DOI] [PubMed] [Google Scholar]
- 29.Houghton AN, Polsky D. Focus on melanoma. Cancer Cell. 2002;2:275–278. doi: 10.1016/s1535-6108(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhao F, et al. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15:4382–4390. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 31.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peranzoni E, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Laties A, Zrenner E. Viagra (sildenafil citrate) and ophthalmology. Prog Retin Eye Res. 2002;21:485–506. doi: 10.1016/s1350-9462(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 35.Marigo I, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Bronstein-Sitton N, et al. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat Immunol. 2003;4:957–964. doi: 10.1038/ni975. [DOI] [PubMed] [Google Scholar]
- 37.Filipazzi P, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 38.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 39.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 40.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 41.Gabrilovich DI. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 42.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 43.Ishigami S, et al. CD3-zetachain expression of intratumoral lymphocytes is closely related to survival in gastric carcinoma patients. Cancer. 2002;94:1437–1442. doi: 10.1002/cncr.10346. [DOI] [PubMed] [Google Scholar]
- 44.Whiteside TL. Down-regulation of zeta-chain expression in T cells: A biomarker of prognosis in cancer? Cancer Immunol Immunother. 2004;53:865–878. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng P, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha P, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.