Abstract
Sustainably managing ecosystems is challenging, especially for complex systems such as coral reefs. This study develops critical reference points for sustainable management by using a large empirical dataset on the coral reefs of the western Indian Ocean to investigate associations between levels of target fish biomass (as an indicator of fishing intensity) and eight metrics of ecosystem state. These eight ecological metrics each exhibited specific thresholds along a continuum of fishable biomass ranging from heavily fished sites to old fisheries closures. Three thresholds lay above and five below a hypothesized window of fishable biomass expected to produce a maximum multispecies sustainable yield (BMMSY). Evaluating three management systems in nine countries, we found that unregulated fisheries often operate below the BMMSY, whereas fisheries closures and, less frequently, gear-restricted fisheries were within or above this window. These findings provide tangible management targets for multispecies coral reef fisheries and highlight key tradeoffs required to achieve different fisheries and conservation goals.
Keywords: human–environment interactions, sustainable fisheries, marine protected areas, resilience, social–ecological systems
Coral reefs have proven difficult to manage sustainably, in part because the multispecies nature of reef fisheries, the complexity of trophic interactions, and the times scales on which processes manifest may allow coral reefs to appear healthy long after serious degradation has occurred (1, 2). This also means that signs of degradation may not be readily apparent in the information that many managers use to evaluate the condition of reef systems [metrics such as catch data or coral cover (2)]. In addition, most reefs are located in developing countries (3) where food security concerns often take priority over conservation. In the absence of reference points that signal when further exploitation may have serious consequences for reef ecosystems, managers are often unlikely to make the socially and politically difficult decisions to restrict fishing activities. A question of critical relevance to scientists, managers, and resource users alike is whether meaningful limits or reference points can provide effective warning of conditions beyond which coral reef social–ecological systems incur a risk of serious degradation and lost value (4, 5).
Here we examine where major changes in coral reef systems occur along a gradient of fishable biomass, a readily measured and managed variable, and explore how this can be used to define key reference points that can help inform management decisions (4, 6). We compiled information from more than 300 surveys of shallow coral reefs from nine countries across the Indian Ocean (Table S1 ). Survey sites spanned some 35° of latitude and 52° of longitude and were evenly distributed among unregulated, restricted, and fisheries closure management areas (details in SI Methods). We used a suite of four statistical models (null, linear, switch-point, and piecewise; SI Methods) to identify whether broad trend and variance thresholds exist for eight ecological metrics of benthic cover, herbivory, predation, and diversity along a gradient of fishable biomass. We define a threshold as a marked change in the variance or relationship between an ecosystem driver and associated state variable. The ecosystem metrics assessed here represent key ecological states and processes (Table 1). Although some of these metrics are interrelated, each captures distinct information in terms of reef accretion, productivity, and resilience.
Table 1.
Ecological indicator metrics and processes represented by each metric
| Metric | Ecological change |
| Macroalgae, % cover | Greater macrolagal cover indicative of declining palatable algal production and calcification, declining herbivory, and possibly increased nutrient inputs |
| Ratio of macroalgae vs. hard coral. | Increased macroalgae relative to coral indicative of rates of algal production and declining calcification |
| Urchin predation index | The metric is indicative of top-down control of processes influenced by sea urchin predators (e.g., grazing). Lower index indicates lower rates of predation on invertebrates |
| Fish species richness | Reflects changes and loses in functional groups important for ecological redundancy and maintaining key processes |
| Sea urchin biomass | Increasing biomass suggests increased biological erosion rates of reef substratum, loss of coralline algae, and reef decay |
| Herbivorous fish as % of total fishable biomass | Reduced % suggests declining secondary production available to fisheries and reduced herbivory processes |
| Calcifying substrates % (hard coral and calcifying algae) | Lower % indicative of declining reef accretion and loss of reef complexity and habitat structure |
| Hard coral substrate, % cover | Lower % indicative of declining reef accretion, reef topographic complexity, benthic diversity, and abundance of coral-dependent species and associated processes, including larval recruitment |
Based on coral reef ecological studies (20–25) (SI Methods).
Results and Discussion
For the majority of thresholds, the piecewise linear model was selected (Table S2), whereby the slope of the linear fit became markedly steeper below a specific fishable biomass. Variance around the identified threshold points was greatest for macroalgae cover, followed by the predation index, but was generally small (Fig. 1 and Table S3), supporting the notion that these are definitive threshold points for the region. The first, and most variable, thresholds to be crossed were increases in the variance of macroalgal cover at fishable biomass levels less than 1,130 kg/ha, followed by a related increase in variance of the ratio of macroalgae to living hard coral at 850 kg/ha, above which macroalgae rarely dominated space over coral cover (Fig. 1). This finding suggests that the appearance of macroalgal-dominated sites may become more prevalent as fish biomass falls and that this is the earliest warning point of change toward reef degradation in the Indian Ocean. This finding fits with theory and studies of terrestrial ecosystems where changes in state patchiness can precede catastrophic shifts among ecosystem states (7, 8). It also supports findings related to increased variance of fish populations being an early indicator of instability (9) and other empirical and theoretical work suggesting that increased variability is an early indicator of a state change (10). Note, however, that sites having high coverage of macroalgae (i.e., >30%) were largely found when fishable biomass was below ≈500 kg/ha. A third threshold indicated a sharp decline in predation rates on tethered sea urchins when fishable biomass was less than 640 kg/ha. Thus, changes in macroalgal cover and a decline in predation of sea urchins are likely to be the first indicators of fishing affecting ecosystem processes, occurring with even moderate declines in fish biomass.
Fig. 1.
State of eight ecological indicator metrics among closed (red circles), fishing-restricted (orange triangles), and unregulated (green circles) locations along a gradient of fishable biomass for reefs in the Indian Ocean. (A) Percentage cover of macroalgae; (B) ratio of macroalgae to coral; (C) urchin predation index; (D) numbers of fish species; (E) proportion of herbivorous fish in total fishable biomass; (F) urchin biomass (kg/ha); (G) percentage cover of calcifying substrates; (H) percentage cover of hard coral. Blue lines represent the best-fit model, based on DIC (Methods), and solid black lines indicate the thresholds (with 95% confidence intervals as dotted black lines) predicted by the Bayesian models.
Thresholds for five additional metrics were at fishable biomass levels below 300 kg/ha, including fish species richness; the proportion of herbivorous fish in the fishable biomass; sea urchin biomass; calcifying benthic organisms; and hard coral cover. Although the biomass of herbivorous fish correlates with total fishable biomass (suggesting that fishable biomass broadly relates to herbivory), the proportion of herbivore biomass declines rapidly at low levels of total fishable biomass, suggesting that the ratio captures unique, process-related information (Fig. 1E). Typically, herbivores are one of the last remaining fishery-target groups in catches of heavily exploited locations (11), as supported by the proportion of herbivore biomass threshold at very low fishable biomass levels shown here. The remaining species composition is mainly microinvertebrate feeding fish, small planktivores, and other small, nontarget fish species. Thresholds related to calcifying organisms, herbivorous fish, sea urchins, and numbers of fish species are relatively close to the final threshold, coral cover. Coral cover is highly variable, likely owing to the impact of the widespread 1998 El Niño Southern Oscillation (ENSO)-driven coral mortality event (12); however, coral cover declined substantially at fishable biomass below 90 kg/ha. The fact that coral cover was the last threshold supports previous assertions that coral cover is a poor metric of impending ecosystem collapse, because it may be robust to reductions of underlying key processes (2). When this threshold is crossed, we hypothesize that ecosystem functioning is so altered that feedback mechanisms may limit recovery on the short time scales relevant to management (1, 2, 7, 8).
Ironically, coral cover is one of the most commonly measured metrics for coral reef condition but was the least sensitive metric to losses in fishable biomass. There is broad correspondence between this observed pattern and the documented trajectory of Caribbean reefs, where severely overfished reefs were dominated by very high cover of live corals until their sudden shift to dominance by macroalgae following a die-off of the most abundant grazing sea urchin (1). Together, these studies indicate that reefs may appear healthy long after fishable biomass has been reduced to the point that ecosystem function is jeopardized. This emphasizes the need for reference points that ensure management actions are implemented before it is too late.
To examine how our results fit with fisheries management concepts and to begin exploring whether this combination of ecosystem and fisheries approaches could inform potential reference points for managers, we calculated a multispecies extension of the well-known maximum sustainable yield for single stocks (13). Setting fisheries targets on the basis of historical biomass and catch data has been a common approach to defining reference points in multispecies fisheries (6, 13) and would also be appropriate for coral reefs. We estimated unfished reef fish biomass (B0) at ≈1,200 kg/ha (±110 95% confidence interval; n = 47) by using unfished reference areas and the oldest no-take marine parks in the region where asymptotes or fishable biomass equilibriums were known to have occurred (14) (SI Methods). From this, we hypothesized a window of biomass-based multispecies maximum sustainable yield (BMMSY) as ≈0.25–0.50 B0 (300 ± 28 to 600 ± 54 kg/ha) (6, 13). At the upper end, this heuristic window matches the inflection of a logistic biomass model within a given system (0.5 B0) and at the lower end matches length-based estimates of optimal yield in stock-recruitment models (0.25 B0; SI Methods). A broad window of BMMSY also acknowledges our current uncertainty, particularly because tropical coral reef fisheries are likely to differ from lower diversity temperate fisheries.
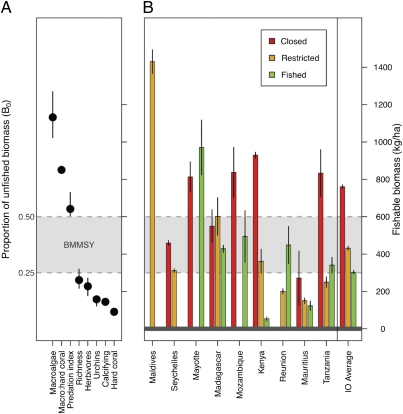
We then compared our BMMSY window with the threshold values for various ecosystem attributes (Fig. 2A). A key finding from this study is that the lower end of the BMMSY window occurs at biomass levels just above the five thresholds that occur in rapid succession. Thus, at or below the lower limits of BMMSY, small decreases in biomass may trigger a sequence of events leading to declines in key ecosystem processes. Avoiding the associated losses of ecosystem services requires monitoring of fishable biomass and implementing fisheries restrictions to reduce effort before biomass falls below ≈300 kg/ha and crosses the remaining thresholds. One possible interpretation of these results is that the three thresholds that fell above the BMMSY window could be considered “warning points” of ecosystem decline, whereas the five that fell below generate cause for alarm (Fig. 2A).
Fig. 2.
Linking ecological thresholds to fisheries management. (A) Estimated fishable biomass thresholds (±SE) among Indian Ocean (IO) reefs. Filled circles are posterior mean estimates, and vertical lines are 95% confidence intervals. Horizontal dotted lines define the boundaries of the hypothesized 0.25–0.5 BMMSY window. (B) Mean (±95% confidence intervals) biomass of reef fishes by country and fisheries management, for the studied regions based on sites. Countries on the x axis are ordered by the log of national population per kilometer of coastline, increasing from left to right.
Despite the strong inferential power of this framework, using one of the most readily measured and managed coral reef variables (fishable biomass), maintaining reef fisheries above desired fishable biomass levels may be challenging. Many of the fisheries management methods used in Western and industrialized fisheries, such as limiting fishing effort, have seldom succeeded in tropical fisheries (4, 15). In contrast, gear restrictions and temporary or small closures are more common and tend to be more acceptable in the socioeconomic context of many tropical countries (16). As expected, pooling biomass data into the dominant forms of tropical fisheries management by country—closures, gear-restricted, and unregulated fished areas—shows that fishable biomass generally increases as fishing restrictions become more stringent (Fig. 2B). The dominant pattern is best represented at the national scale by Kenyan reefs. Here biomass is above BMMSY in moderately sized permanent fisheries closures, falls within the BMMSY limits when fishing is restricted by gear, and below BMMSY in unregulated fisheries.
Overall, fisheries closures tend to have fishable biomass levels at or above the BMMSY window and most of the thresholds. Among countries, conditions in both unregulated and gear-restricted fisheries displayed high variability and in some countries have crossed many key thresholds. Restrictions on gear had variable outcomes, but in several countries these management systems operate at or below the BMMSY window. Very few gear-restricted and unregulated fisheries were above the upper BMMSY fishery target and urchin predation threshold. Deviations from these ecological patterns are due to well-known effects, such as low compliance with fisheries restrictions (Mauritius and Reunion) and low fishing pressure (Madagascar, Maldives, and Mayotte). For example, Maldivian fishable biomass was above all thresholds, likely reflecting the large reef area and small human populations, the traditional focus on pelagic tuna fisheries, and a national priority for marine ecotourism (17).
Tradeoffs between fish yield, employment, and biodiversity are common in fisheries (4, 6). However, in multispecies artisanal fisheries, such as those on coral reefs, defining these tradeoffs is particularly challenging. Our results provide some insight into how different management options result in important socioeconomic and ecological tradeoffs. We found that nonextractive uses, as often found in protected areas, generally maintain reef systems dominated by high calcification, with high predation and species diversity. Such management systems may maximize benefits to tourism markets and potentially provide benefits to fishers from spillover and recruitment (18), but they also have high management costs that can result in conflict and displacement (4, 19). Additionally, in the western Indian Ocean, reef fisheries closures are frequently small, surrounded by heavily fished areas, can have limited compliance, and their rapid expansion and adoption will be challenged by social and political realities. Unregulated and gear-restricted fisheries tend to favor maximum employment policies, but often with ecological impacts leading to losses in critical ecosystem services. Where fishing is intense, biomass may be reduced below critical thresholds, resulting in severe degradation. Therefore, appropriate gear-based management, and effort and catch controls tailored to the local socioeconomic context are required to complement closures and contribute to the maintenance of reef ecosystems throughout the broader seascape.
Spatial environmental variation, time lags, nonequilibrium dynamics, and food web complexity contribute to the observed variation among these study sites, and this adds to the challenge of predicting the responses of management actions. For example, feedbacks and time lags among reef ecosystem processes are thought to slow the return time from sea urchin and macroalgal states to dominance by calcifying coral and algae, even if stressors are reduced (20, 21). Fish catch derived from nearshore fisheries often come from multiple habitats, including seagrass, sand, and pelagic areas. This adds to the difficulty of determining reference points from fish catch—the most commonly collected metric in fisheries. Consequently, ecological field studies and simple diagnostic metrics are needed to provide information about the state of the ecosystem and to assist management decisions. The thresholds we found integrate much ecological complexity across processes that influence resource productivity—a critical advancement in theory and practice. For managers charged with maintaining desirable ecosystems, simple empirical information based on the hypothetical–deductive scientific process is needed to make decisions. The results in this study help inform tangible targets for managers and provide new direction for further research on the sustainability of coral reef fisheries.
It is not clear how appropriate the specific fisheries reference points and thresholds we identified for the Indian Ocean are for other coral reef regions. Given the similarity of dominant reef species, functional groups, and food-web structure between the Pacific and Indian oceans, it is likely that our findings have some general applicability in the Pacific; however, the effects of variable productivity and diversity may change relationships and shift the biomass thresholds (22). The Caribbean and Indo-Pacific reefs are different; Caribbean reefs are less productive and lack good historical and contemporary reference points (23), and it is therefore expected that BMSSY and ecological thresholds will reflect these differences. Importantly, the data and techniques we present here provide one of the first frameworks for ecosystem-based fisheries management of coral reefs that can be developed and tested in other locations. Such development may include alternate, context-specific, ecosystem state variables and processes. Finally, our framework, where harvesting yields and ecological threshold estimates are combined, should be applicable to ecosystem management beyond coral reefs.
Methods
Details are provided in SI Methods.
Data.
Coral reef benthic habitat and associated mobile fauna were surveyed at 157 individual sites across nine countries in the Indian Ocean, spanning ≈35° latitude and ≈52° longitude. Data were collected from 1988 to 2009, resulting in a database of 335 site–time combinations (Table S1). Of the 335 individual surveys, 109 were in no-take fisheries closures, 109 were in areas with restrictions on gear use, and 117 were in areas fully open to all fishing gears.
Eight types of ecological data were collected and evaluated; biomass of reef fish (kg/ha); species richness of reef fish (number per 500 m2); biomass of herbivorous reef fish (scarids, acanthurids, and siganids; kg/ha); biomass of reef fish capable of preying on sea urchins (balistids and labrids >30 cm; kg/ha); sea urchin biomass (kg/ha); a predation index based on tethered sea urchin assays (proportion of urchins preyed upon); cover of macroalgae (%); hard coral cover (%); and cover of all calcifying organisms (hard corals, coralline, and calcareous algae; %). These were used to calculate eight metrics that were indicative of key reef states and processes. Bo was calculated from surveys of marine parks and unfished reefs in the region (14).
Analysis.
To identify and quantify the presence of potential thresholds in the relationship between fishable biomass and each of the ecological metrics, we compared four candidate linear models: a null model, linear model, switch-point model, and piecewise linear model (Table S2). All models were run in a Bayesian framework, and relative model support was assessed using the deviance information criterion (DIC), whereby lower-valued DIC scores provided support for one model over another.
Supplementary Material
Acknowledgments
We thank national institutions for logistical support and the many field assistants who helped collect data. Funding was provided by the Western Indian Ocean Marine Science Association, Leverhulme Trust, John T. and Catherine D. MacArthur Foundation, World Bank/Global Environmental Facility Coral Reef Targeted Research for Management Program, and Wildlife Conservation Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.R.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106861108/-/DCSupplemental.
References
- 1.Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 2.Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25:633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Donner S, Potere D. The inequity of the global threat to coral reefs. Bioscience. 2007;57:214–215. [Google Scholar]
- 4.Beddington JR, Agnew DJ, Clark CW. Current problems in the management of marine fisheries. Science. 2007;316:1713–1716. doi: 10.1126/science.1137362. [DOI] [PubMed] [Google Scholar]
- 5.Kates RW, et al. Environment and development. Sustainability science. Science. 2001;292:641–642. doi: 10.1126/science.1059386. [DOI] [PubMed] [Google Scholar]
- 6.Worm B, et al. Rebuilding global fisheries. Science. 2009;325:578–585. doi: 10.1126/science.1173146. [DOI] [PubMed] [Google Scholar]
- 7.Rietkerk M, Dekker SC, de Ruiter PC, van de Koppel J. Self-organized patchiness and catastrophic shifts in ecosystems. Science. 2004;305:1926–1929. doi: 10.1126/science.1101867. [DOI] [PubMed] [Google Scholar]
- 8.Kéfi S, et al. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature. 2007;449:213–217. doi: 10.1038/nature06111. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh CH, et al. Fishing elevates variability in the abundance of exploited species. Nature. 2006;443:859–862. doi: 10.1038/nature05232. [DOI] [PubMed] [Google Scholar]
- 10.Scheffer M, et al. Early-warning signals for critical transitions. Nature. 2009;461:53–59. doi: 10.1038/nature08227. [DOI] [PubMed] [Google Scholar]
- 11.McClanahan TR, Hicks CC, Darling ES. Malthusian overfishing and efforts to overcome it on Kenyan coral reefs. Ecol Appl. 2008;18:1516–1529. doi: 10.1890/07-0876.1. [DOI] [PubMed] [Google Scholar]
- 12.Ateweberhan M, McClanahan TR, Graham NAJ, Sheppard CRC. Episodic heterogeneous decline and recovery of coral cover in the Indian Ocean. Coral Reefs. 2011;30:739–752. [Google Scholar]
- 13.Hilborn R. Pretty good yield and exploited fishes. Mar Policy. 2002;34:193–196. [Google Scholar]
- 14.McClanahan TR, Graham NAJ, Wilson SK, Letourneur Y, Fisher R. Effects of fisheries closure size, age, and history of compliance on coral reef fish communities in the western Indian Ocean. Mar Ecol Prog Ser. 2009;396:99–109. [Google Scholar]
- 15.Johannes RE. The renaissance of community-based marine resource management in Oceania. Annu Rev Ecol Syst. 2002;33:317–340. [Google Scholar]
- 16.Aswani S. Customary sea tenure in Oceania as a case of rights-based fishery management: Does it work? Rev Fish Biol Fish. 2005;15:285–307. [Google Scholar]
- 17.Risk MJ, Sluka B. In: Coral Reefs of the Indian Ocean: Their Ecology and Conservation. McClanahan TR, Sheppard CRC, Obura DO, editors. New York: Oxford Univ Press; 2000. pp. 325–351. [Google Scholar]
- 18.Halpern BS, Lester SE, Kellner JB. Spillover from marine reserves and the replenishment of fished stocks. Environ Conserv. 2009;36:268–276. [Google Scholar]
- 19.Mascia MB, Claus CA. A property rights approach to understanding human displacement from protected areas: The case of marine protected areas. Conserv Biol. 2009;23:16–23. doi: 10.1111/j.1523-1739.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 20.Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 21.Bellwood DR, Hughes TP, Hoey AS. Sleeping functional group drives coral-reef recovery. Curr Biol. 2006;16:2434–2439. doi: 10.1016/j.cub.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Mora C, et al. Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol. 2011;9:e1000606. doi: 10.1371/journal.pbio.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 24.Nyström M, Graham NAJ, Lokrantz J, Norström AV. Capturing the cornerstones of coral reef resilience: Linking theory to practice. Coral Reefs. 2008;27:795–809. [Google Scholar]
- 25.Norström AV, Nyström M, Lokrantz J, Folke C. Alternative states on coral reefs: Beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser. 2009;376:295–306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.