Abstract
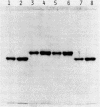
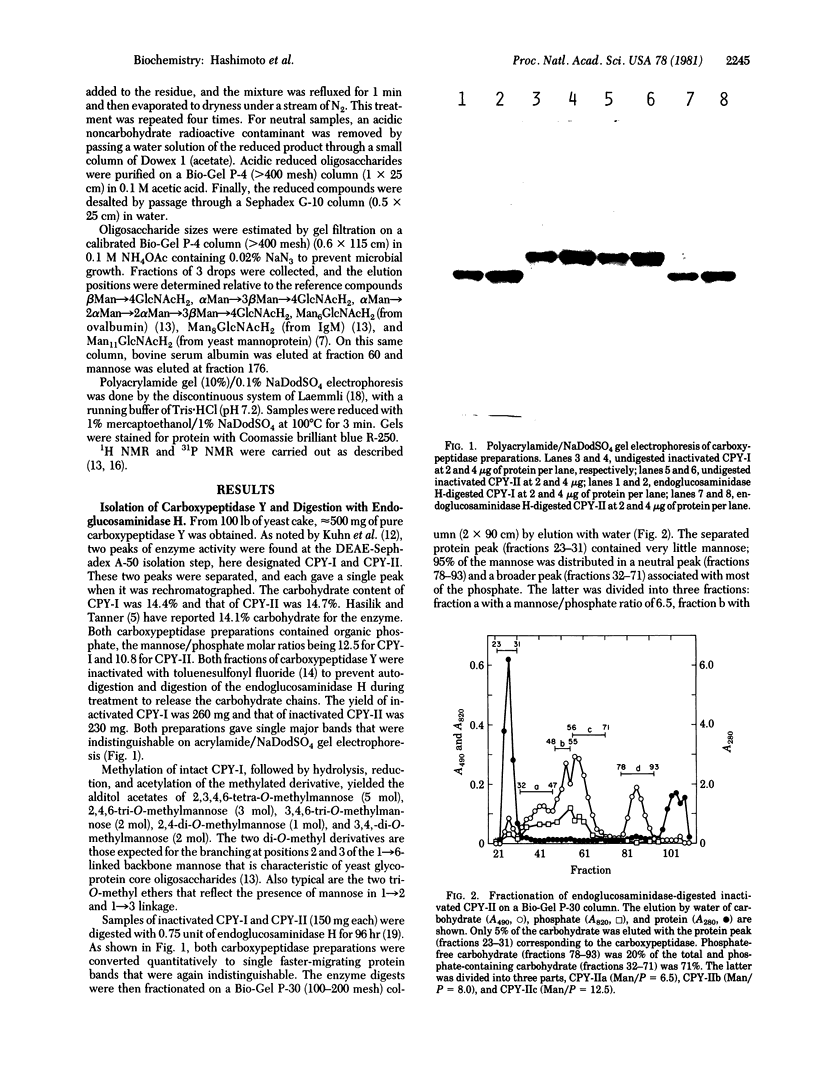
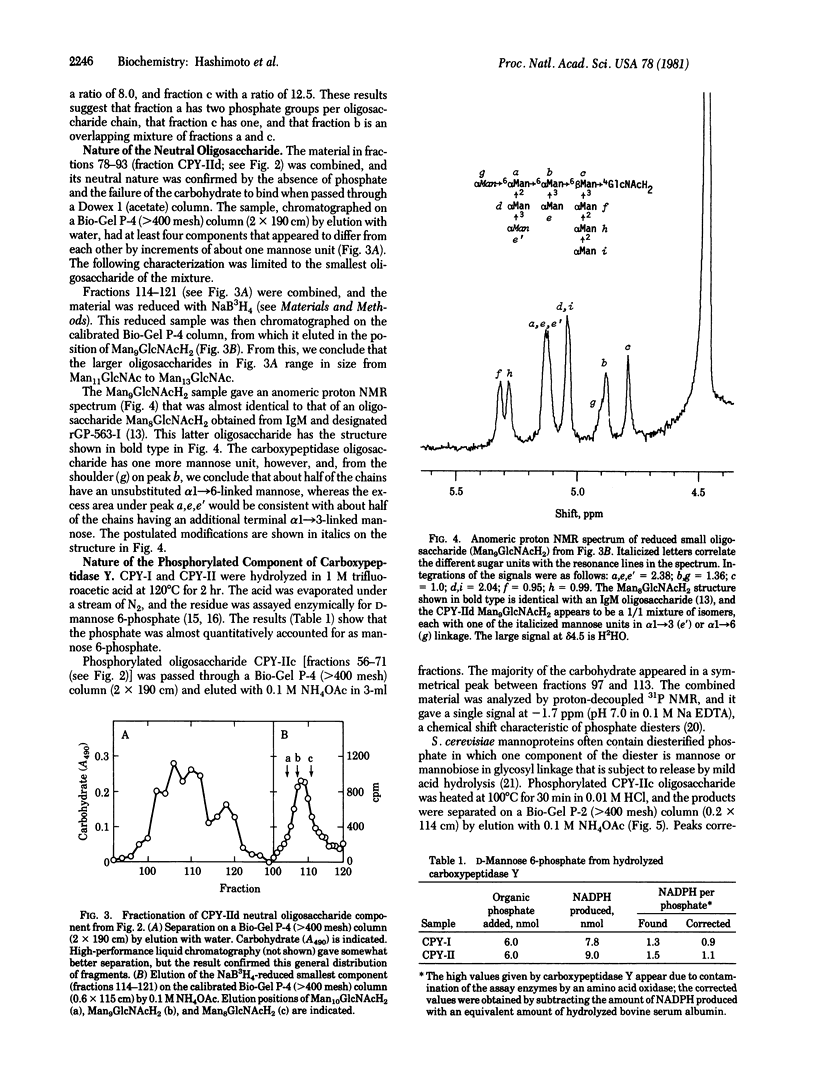
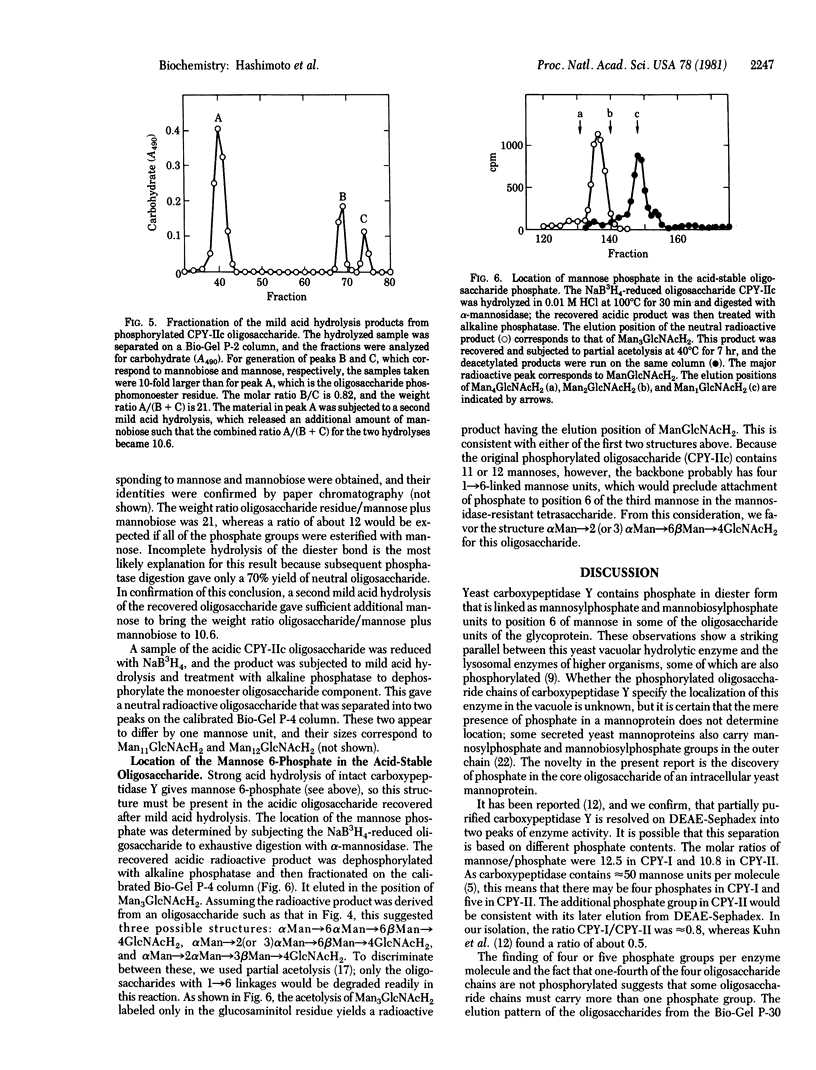
Carboxypeptidase Y, a vacuolar enzyme from Saccharomyces cerevisiae, was digested with endo-beta-N-acetyl-D-glucosaminidase H to release the four oligosaccharide chains that are linked to asparagine in the glycoprotein. The oligosaccharides were fractionated into a neutral and acidic component, and the latter proved to phosphorylated. From its gel filtration pattern, the neutral fraction was shown to be a mixture of at least four homologs, the smallest of which had a proton NMR spectrum almost identical to that given by an IgM oligosaccharide with eight mannoses and one N-acetylglucosamine [Cohen, R. E. & Ballou, C. E. (1980) Biochemistry 19, 4345--4358]. The yeast oligosaccharide has one additional mannose unit in an alpha 1 leads to 3 or alpha 1 leads to 6 linkage, whereas the larger homologs appear to have two, three, and four more mannose units. One phosphorylated oligosaccharides with a mannose/phosphate ratio of 12.5 was reduced with NaB3H4 and then subjected to mild acid hydrolysis. This released mannose and mannobiose that were glycosidically linked to the phosphate group, whereas complete acid hydrolysis yielded D-mannose 6-phosphate. The recovered oligosaccharide phosphomonoester, which contained 11 or 12 mannose units, was digested exhaustively with alpha-mannosidase, and the product of this reaction was treated with alkaline phosphatase, which yielded radioactive Man3GlcNAcH2. These results suggest that the mannosidase-resistant phosphorylated oligosaccharide has the structure Man leads to P leads to 6 alpha Man leads to alpha Man leads to 6 beta Man leads to 4GlcNAcH2, in which some of the phosphate groups are substituted with mannobiose instead of mannose. A second phosphorylated oligosaccharide with a mannose/phosphate ratio of 6.5 probably contains two phosphodiester groups, but its structure has not been investigated in detail.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Ballou L., Cohen R. E., Ballou C. E. Saccharomyces cerevisiae mutants that make mannoproteins with a truncated carbohydrate outer chain. J Biol Chem. 1980 Jun 25;255(12):5986–5991. [PubMed] [Google Scholar]
- Cohen R. E., Ballou C. E. Linkage and sequence analysis of mannose-rich glycoprotein core oligosaccharides by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Sep 2;19(18):4345–4358. doi: 10.1021/bi00559a031. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Hashimoto C., Cohen R. E., Ballou C. E. Characterization of phosphorylated oligomannosides from Hansenula wingei mannoprotein. Biochemistry. 1980 Dec 9;19(25):5932–5938. doi: 10.1021/bi00566a042. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur J Biochem. 1978 Nov 15;91(2):567–575. doi: 10.1111/j.1432-1033.1978.tb12710.x. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Kuhn R. W., Walsh K. A., Neurath H. Isolation and partial characterization of an acid carboxypeptidase from yeast. Biochemistry. 1974 Sep 10;13(19):3871–3877. doi: 10.1021/bi00716a008. [DOI] [PubMed] [Google Scholar]
- Kuhn R. W., Walsh K. A., Neurath H. Reaction of yeast carboxypeptidase C1 with group-specific reagents. Biochemistry. 1976 Nov 2;15(22):4881–4885. doi: 10.1021/bi00667a020. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Cohen R. E., Ballou C. E. Carbohydrate structure of yeast invertase. Demonstration of a form with only core oligosaccharides and a form with completed polysaccharide chains. J Biol Chem. 1979 Dec 10;254(23):12209–12218. [PubMed] [Google Scholar]
- Li Y. T. Studies on the glycosidases in jack bean meal. I. Isolation and properties of alpha-mannosidase. J Biol Chem. 1967 Dec 10;242(23):5474–5480. [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Rosenfeld L., Ballou C. E. Genetic control of yeast mannan structure. Biochemical basis for the transformation of saccharomyces cerevisiae somatic antigen. J Biol Chem. 1974 Apr 10;249(7):2319–2321. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Biosynthetic intermediates of beta-glucuronidase contain high mannose oligosaccharides with blocked phosphate residues. J Biol Chem. 1980 Jul 25;255(14):6633–6639. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. The use of endo-beta-N-acetylglucosaminidase H in characterizing the structure and function of glycoproteins. Biochem Biophys Res Commun. 1977 Oct 10;78(3):935–944. doi: 10.1016/0006-291x(77)90512-5. [DOI] [PubMed] [Google Scholar]