Abstract
In species-rich assemblages of acoustically communicating animals, heterospecific sounds may constrain not only the evolution of signal traits but also the much less-studied signal-processing mechanisms that define the recognition space of a signal. To test the hypothesis that the recognition space is optimally designed, i.e., that it is narrower toward the species that represent the higher potential for acoustic interference, we studied an acoustic assemblage of 10 diurnally active frog species. We characterized their calls, estimated pairwise correlations in calling activity, and, to model the recognition spaces of five species, conducted playback experiments with 577 synthetic signals on 531 males. Acoustic co-occurrence was not related to multivariate distance in call parameters, suggesting a minor role for spectral or temporal segregation among species uttering similar calls. In most cases, the recognition space overlapped but was greater than the signal space, indicating that signal-processing traits do not act as strictly matched filters against sounds other than homospecific calls. Indeed, the range of the recognition space was strongly predicted by the acoustic distance to neighboring species in the signal space. Thus, our data provide compelling evidence of a role of heterospecific calls in evolutionarily shaping the frogs' recognition space within a complex acoustic assemblage without obvious concomitant effects on the signal.
Keywords: Amazonia, animal communication, bioacoustics, perception
Mate-recognition signals mediate successful breeding and, therefore, are often affected by strong selective forces that may lead to adaptation and reproductive isolation (1, 2). Although the causes (3, 4) and consequences (5, 6) of signal evolutionary divergence are relatively well studied, much less is known about the evolution of physiological mechanisms underlying the sensory processing of signals (7, 8). At the most basic level, signal detection and signal recognition are prerequisites for the appropriate reaction of receivers, rendering signal-processing traits at least as important as signal traits in potentially affecting the evolutionary fate of a lineage (9–11).
Signals must encode a minimum amount of information allowing for species recognition because of the high costs of reacting toward heterospecific signals as if they were homospecific (“false alarms”) (12, 13). Signal recognition occurs when receivers compare detected signals to preexisting templates, often in the central nervous system. It is usually tested with playback experiments and is assumed when tested receivers exhibit overt behavioral responses (14, 15), i.e., when they “treat as valid” the stimulus signal (16). The recognition space of a given signal is thus estimated as the range of signal values that elicit effective (meeting some minimum criterion) behavioral responses in the receivers (13).
The recognition space may be larger than necessary for the recognition of conspecific signals (17, 18), presumably because sensory systems trade off their communicative function with other ecological functions such as predator or prey detection (19). The role of heterospecific signals in shaping the recognition space has received less attention (20). One example would be the effect of masking interference on acoustic communication in birds (21), insects (22), and frogs (23, 24). Because heterospecific sounds may represent noise that interferes with signal detection (25, 26), the communication systems should reveal adaptations that reduce its effects. To date, most research to validate this idea has been conducted on the characteristics of the signals or on the pattern of signaling activity (27, 28), but compelling evidence suggests that recognition systems can evolve without concomitant variation in the signals (20, 29). We present here a manipulative study on a species-rich assemblage of diurnal frogs to test the general hypothesis that the recognition space is shaped in a way that minimizes the overlapping of signal space with those of co-occurring species.
The importance of acoustic communication for anuran reproduction is well established (30, 31). Typically, male breeding success depends on producing advertisement calls that attract females. In a fairly less-studied scenario, male breeding success depends on the successful and prolonged defense (against other males) of a territory containing resources essential for reproduction (32). Males of the latter species announce territory ownership by producing redundant series of advertisement calls throughout the breeding season; they also react toward conspecific calling intruders by increasing signaling rate or intensity and by attacking them (33). Because both levels of reaction increase energy expenditure and conspicuousness to potential predators, natural selection on signal-detection and signal-recognition mechanisms should be evident in the male–male communication systems of territorial species (20).
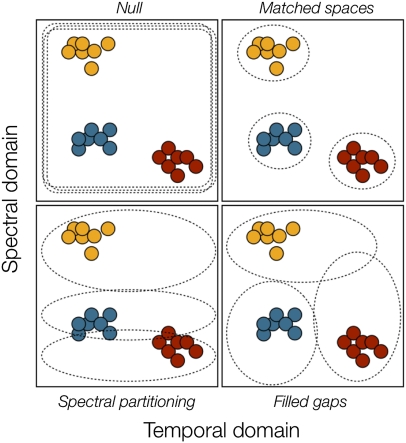
Our null hypothesis states that the recognition space of each species occupies the available signal space of the whole frog assemblage; males of each species should then indiscriminately react to every species’ signal. We anticipated at least three scenarios that falsify the null hypothesis (Fig. 1). In our matched-spaces hypothesis, the recognition space is perfectly matched to the signal space, indicating pleiotropic relationships between signal-producing and signal-recognition traits or strong natural selection against detecting sounds other than the signals. Our matched-spaces hypothesis differs from the original matched-filter hypothesis (34) in including the whole signal space rather than only the spectral parameters and in emphasizing the signal parameters’ range instead of the average values. In the spectral-partitioning hypothesis, the recognition space is partitioned in the spectral but not in the temporal domain of call parameters, suggesting an effect of masking interference in shaping the recognition space. The filled-gaps hypothesis adds to the spectral-partitioning hypothesis by including the temporal domain of the signal. The recognition space should then extend beyond the limits of the species-specific signals, depending on the multivariate distance to the neighboring species in the signal space; this result would indicate that recognition spaces are constrained by the probability of heterospecific interference. Because the last two hypotheses imply a role for heterospecific signals, we quantified the actual magnitude of this influence by estimating species acoustic co-occurrence from recordings of the acoustic environment.
Fig. 1.
Four hypotheses on the shape of the recognition space (dotted lines) and its relationship with the signal space defined by individual signals (filled circles) of three hypothetically co-occurring species (colored circles).
Our aims were thus (i) to estimate the degree of signaling co-occurrence between every pair of species in an assemblage of diurnal frogs, (ii) to characterize the parameters of their advertisement calls, (iii) to estimate the potential of call parameters to allow discrimination between homospecific and heterospecific signals, (iv) to estimate the recognition space of five species by conducting playback experiments, and, finally, (vi) to test the four hypotheses (Fig. 1) by mapping the recognition spaces onto the signal spaces estimated from the call traits.
Results
We registered 10 acoustically active frog species at the study site, nine of them calling during daytime hours (all belonging to the family Dendrobatidae) and one during the late afternoon (Leptodactylus andreae, Leptodactylidae). Regarding spatiotemporal segregation, only the calling activity of Allobates femoralis was inversely related to the calling activity of Ameerega petersi (r = −0.62, P < 0.0001, n = 53 acoustic environments). The whole pattern of species co-occurrence was successfully represented in two dimensions by multidimensional scaling (MDS; stress = 0.02). The transformed proximities between species pairs, our estimate of species co-occurrence, were not related to the multivariate difference in advertisement calls (Mantel r = 0.17, P = 0.8011, n = 45 species pairs, 9,999 permutations).
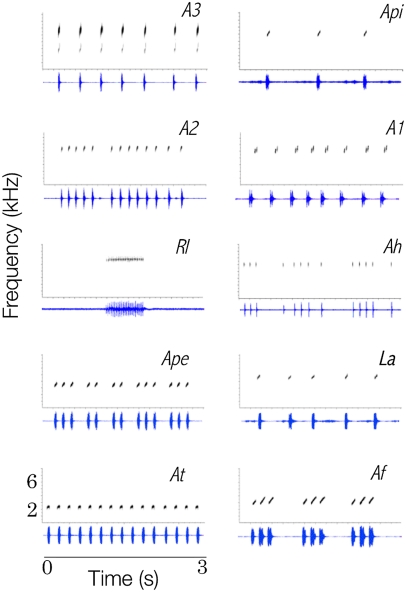
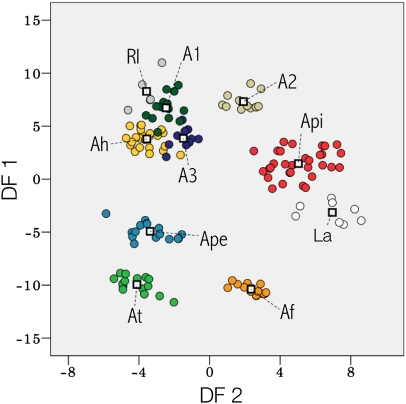
Each species’ call (Fig. 2) consisted basically of frequency-modulated notes uttered in seven species as long series of uninote calls and arranged in three species as multinote calls. Call parameters were successfully combined in two discriminant axes that explained 92.0% of variance and classified very well (98.7% of correct classifications) each species’ call (Fig. 3). The variables that most contributed to discriminate calls were low frequency (discrimination coefficient = 0.63) in the first discriminant function (explaining 66.2% of variance) and note duration (discrimination coefficient = 0.62) on the second discriminant function (25.8%). Therefore, we estimated a 2D recognition space by manipulating note frequency and note duration.
Fig. 2.
Oscillogram (lower, blue) and sonogram (upper, gray) of the advertisement calls of 10 acoustically co-occurring frog species at Panguana, Peru. A1, Allobates sp. 1; A2, Allobates sp. 2; A3, Allobates sp. 3; Af, Allobates femoralis; Ah, Ameerega hahneli; Ape, Ameerega petersi; Api, Ameerega picta; At, Ameerega trivittata; La, Leptodactylus andreae; RI, Ranitomeya lamasi.
Fig. 3.
Discriminant plot summarizing between individuals’ (dots) and species’ (colors) differences in five parameters of the advertisement calls. The first discriminant function (DF1; 66.2%) is correlated with high values of low (r = 0.86), peak (r = 0.76), and high (r = 0.73) frequency, whereas the second (DF2; 25.8%) is mainly correlated with note duration (r = 0.69). The discriminant axes were switched to improve data visualization and comparison with Figs. 1 and 5. Species abbreviations are as described in Fig. 2.
The effect of manipulating call parameters on the probability of male reaction differed among species (Table 1 and Fig. 4). Whereas A. femoralis males reacted to synthetic stimuli with deviations up to 10–15 SD, Ameerega picta, males restricted their reactions to stimuli within 2 SD of the average values. In addition, three of four species tolerated lower deviations in peak frequency than in note duration (Fig. 4).
Table 1.
Summary of the GAM analyses on binary data to test the effect of note duration and note frequency on male reaction to the playback of synthetic calls in five frog species
| Species | n | DE, % | Predictor | df | Deviance | P |
| Am. hahneli | 57 | 16.2 | Peak frequency | −2.9 | −6.23 | 0.0925 |
| Note duration | −3.0 | −10.9 | 0.0118 | |||
| Am. picta | 31 | 20.9 | Peak frequency | −3.1 | −6.40 | 0.0974 |
| Note duration | −2.8 | −5.45 | 0.1243 | |||
| Am. trivittata | 51 | 33.2 | Peak frequency | −0.6 | −4.6 | 0.0148 |
| Note duration | −4.3 | −19.0 | 0.0011 | |||
| Allobates sp. 1 | 51 | 36.8 | Peak frequency | −3.5 | −17.4 | 0.0010 |
| Note duration | −3.0 | −11.9 | 0.0075 | |||
| A. femoralis | 63 | 43.8 | Peak frequency | −3.0 | −18.6 | 0.0003 |
| Note duration | −3.5 | −19.1 | 0.0004 |
The ability of the model to predict the outcome of the playback experiments is expressed as the percentage of deviation explained (DE).
Fig. 4.
Recognition space of five co-occurring frog species as estimated by a GAM on the phonotactic reaction of males to the playback of synthetic advertisement calls. The variables were manipulated beyond the natural range of variation (expressed as SD) until most individuals failed to react to the corresponding stimulus call.
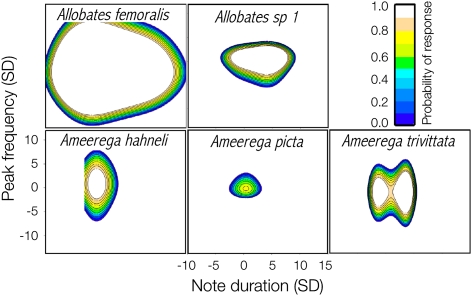
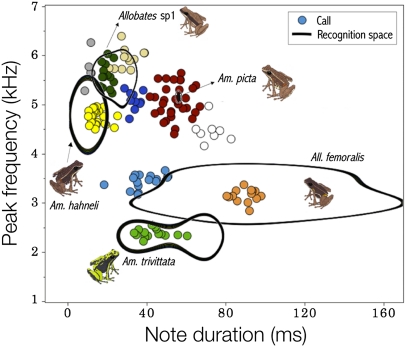
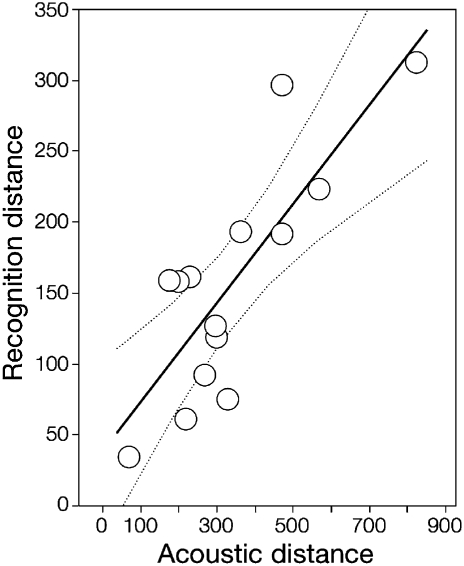
The recognition and signal spaces overlapped but did not have the same boundaries or shape (Fig. 5). Four species reacted to supernormal stimuli, but the limits of their recognition spaces did not overlap. Only in Allobates sp. 1 did the recognition space include most calls of an acoustically neighboring species, Allobates sp. 2. We did not consider Am. petersi to be a neighboring species of A. femoralis in the communication space because both species appear segregated in the analysis of co-occurrence (see above). Finally, acoustic distance was a strong predictor of recognition distance [permutational regression, likelihood ratio test (LRT) = 8.5, P = 0.0090, n = 18 species pairs, 10,000 permutations] after controlling for concomitant variation in co-occurrence (LRT = 0.2, P = 0.7012). Because Am. picta males exhibited a conspicuously atypical and statistically nonsignificant recognition space (Figs. 4 and 5), we repeated the regression analysis, excluding the species pairs involving it; the pattern became even stronger (Fig. 6; acoustic distance: LRT = 21.2, P = 0.0007; co-occurrence: LRT = 3.1, P = 0.1065; n = 14 species pairs, 10,000 permutations).
Fig. 5.
Communication space depicting the relationships between the recognition space (5 species shown) and the signal space (10 species shown) as defined by two call parameters in an assemblage of diurnal frogs. Each dot represent average values of call parameters for a single frog. The line delimitates the call parameter values at which the probability of male reaction was estimated as 0.99 by a GAM.
Fig. 6.
Relationship between the projection of the recognition space (recognition distance) and pairwise differences in call traits (acoustic distance) while controlling for the degree of species co-occurrence in an acoustic community of diurnal frogs.
Discussion
Our study provides sufficient evidence to reject two of the four initial hypotheses: the recognition spaces did not occupy the whole signal space of the frog assemblage (null hypothesis) nor did they match in range the signal space of the same species’ call (matched-spaces hypothesis). Statistically, the matched-spaces hypothesis predicts that the recognition space should reach up to 3 SD from the bivariate centroid defined by peak frequency and note duration. Instead, the models (Fig. 5 and Table 1) show that they reached between 4 and 15 SD away from the centroid, often in an asymmetrical fashion. In one species (Am. picta), the model was not statistically significant as to reject or accept any hypothesis about the recognition space.
The lack of sender–receiver match apparently conflicts with the matched-filter hypothesis (34). The latter predicts an association between the peak frequency of an auditory signal and the peak spectral sensitivity of the peripheral auditory system (13), which would improve the signal-to-noise ratio and thereby reduce masking by heterospecific or abiotic noise. A direct comparison between the matched-filter hypothesis as it was applied to the tuning of auditory neurons and our matched-spaces hypothesis is misleading, however, because (i) we have emphasized the range and not the center of the recognition space, (ii) we have included temporal and not merely spectral parameters of the signal, and (iii) we have studied behavioral rather than physiological reactions to auditory stimuli.
Acoustically communicating species may not interfere with each other if their signaling activity is segregated in space and time (24, 35, 36). Our data do not support this explanation. Should species tend to segregate, then their calling activities would be negatively correlated across our recordings of acoustic environment; this happened for just 1 (A. femoralis and Am. petersi) of 18 species pairs. In addition, the MDS proximity, our estimate of pairwise species co-occurrence, was unrelated to concomitant differences in advertisement calls. Thus, contrary to another work (21), we found no general evidence that frogs uttering more similar calls are also better segregated in space and time.
Species’ communication spaces might instead be partitioned along the spectral axis (spectral-partitioning hypothesis) because spectral overlapping could cause masking interference. The spectral-partitioning hypothesis received mixed support from our data. On the positive side, spectral traits performed much better than temporal traits did in discriminating species’ calls (see Fig. 3 and corresponding discriminant coefficients). Regarding the recognition space, four of the five species tolerated lower deviations in note frequency than in note duration. On the negative side, we found substantial overlap between species in the call spectral axis (Figs. 3 and 5). Also, the recognition space of Allobates sp. 1 encompassed a significant fraction of the signal space of Allobates sp. 2 and spectrally overlapped with at least two other species (Fig. 5). One explanation for the latter case would be that Allobates sp. 1 is the only species with microtemporal structuring of the call (Fig. 2), which we did not consider in our estimates of the signal space: Males utter series of calls, each consisting of two notes separated by a very short time interval.
We found unequivocal evidence favoring our filled-gaps hypothesis as the dominant pattern regarding the shape of the recognition space. The extent by which the recognition space exceeded the homospecific signal space was strongly related to the multivariate distance to other species’ signals (Fig. 6). Thus, our results support the idea that recognition spaces subserve detection of sounds other than conspecific signals and that their shape is constrained by the probability of heterospecific interference. Moreover, because the filled-gaps hypothesis implies some degree of segregation along the spectral domain of the recognition space, we could also explain the evidence partly supporting the spectral-partitioning hypothesis by accepting the filled-gaps hypothesis as valid.
The strong pattern we detected may have arisen from the negative consequences of heterospecific signals on communication, territory defense, and successful breeding. In agreement with an adaptive scenario, related species within our study system (e.g., within the genus Ameerega or Allobates) do not share similarly shaped recognition spaces, and previous studies have strongly suggested adaptive divergence in the signal spectral processing in A. femoralis without concomitant variation in the signal (29). Also, the physiological basis of intraspecific variation in spectral tuning has been previously tracked for other frog species (37). Alternatively, learning has been invoked to explain sensory biases (38) and thereby could explain the patterns we observed; the adaptive adjustment would then occur within a single generation of individuals. Although some signal characteristics are thought to be relatively conservative in frogs (39, 40) and pleiotropically linked to body size and perhaps sensory systems (13), the possibilities that signal-processing mechanisms are adjusted via learning or phenotypic plasticity deserve deeper exploration.
The recognition spaces may still depend on at least two factors that we did not manipulate here. Repeating the whole experiment at different sound-pressure levels might reveal interesting changes in recognition spaces perhaps associated to among-species differences in territory size. We chose, however, a sound-pressure level range that mimics a natural sound produced by an eventual intruder approaching within 1–2 m of the focal male. In our positive controls (synthetic sounds with the call average parameters), playbacks were recognized as “valid” intruders in every case. On the other hand, the whole experiment could also be done testing females, rather than males, in playback experiments. Females, in general, have shown to be more selective than males in the calls to which they respond (41, 42). However, territory ownership and successful defense against other males appears to be the most important factor determining reproductive success in basal dendrobatids such as the ones we studied here (32). In turn, dendrobatid females might use much more multimodal information than males do to recognize conspecific males. Courtship in dendrobatid frogs involves a complex exchange of auditory, visual, and tactile signals, often lasting several days (40). In any case, the conclusions we draw here apply to an ecologically relevant (male–male) communication system studied under realistic conditions of signal amplitude.
In sum, the co-occurrence of acoustically communicating species has been traditionally explained by invoking spatial and temporal segregation of signaling activity as well as spectral segregation of the signals. We found almost no evidence of spatiotemporal segregation and some evidence of signal spectral stratification in the male–male communication system of territorial frogs. Instead, our data support an additional explanation about how species overcome heterospecific noise while concurring for breeding purposes. They might develop a suite of sensory and perhaps cognitive processes that selectively deal with heterospecific signals according to the probability of interference: a context-dependent recognition space.
Methods
We conducted our field recordings and experiments at the Panguana Biological Field Station (−9.614° N, −74.936° W), in the Peruvian Amazon, during November 2005, November 2006, and November 2007–January 2008. Nine species of dendrobatid and one species of leptodactylid frogs were calling during daytime hours within an area of ∼2.37 km2 of old-growth forest. All dendrobatid species at Panguana appear to breed synchronously in advance of and throughout the rainy season (43). Males of these species utter relatively simple (easy to measure and synthesize) advertisement calls based on frequency-modulated tones (notes).
Acoustic Co-Occurrence.
Throughout November 2006 and November 2007–January 2008, we recorded 53 samples of the acoustic environment to estimate the extent of overlap in calling activity between each species pair, following protocols published elsewhere (44) (SI Methods). To estimate the degree of acoustic co-occurrence, we ran an MDS analysis from the rectangular (acoustic environment sample × species) matrix of calling activity. MDS reproduces the pattern of similarity/distance among several objects (species) in two (or any number of user-defined) dimensions. We used the matrix of pairwise-transformed proximities between species as the covariate co-occurrence in further analyses.
Signal Space.
To describe each species’ advertisement call, we recorded between five and 29 spontaneously calling males by using standard methods published elsewhere (44, 45, 46) (SI Methods). To estimate the potential of measured call parameters for species recognition (25), we ran a canonical discriminant function analysis. The magnitude of between-species divergence in each call parameter was directly estimated from the standardized coefficients in the first two discriminant functions. We also used the result of discriminant function analysis to select the two call parameters that best discriminated between species and, therefore, were used as the main experimental treatments in our estimation of the recognition space.
Recognition Space.
We conducted 577 valid playback experiments with 577 synthetic signals on 531 males of five frog species to estimate their recognition spaces. In brief, we (i) used the measured properties of each species’ advertisement call to synthesize normal and supernormal (i.e., beyond the natural range of variation) stimulus calls, (ii) conducted playback experiments on territorial males, (iii) used male binary responses (to approach or not the loudspeaker) to build a statistical model of the recognition space, and, finally, (iv) mapped each species’ recognition space onto the 2D signal space created by the manipulated call features.
Call synthesis and playback experiments were conducted according to the protocols published elsewhere (29, 33, 47) (SI Methods). To estimate each species’ recognition space, we built a generalized additive model (GAM) of male binary reactions with one spectral and one temporal call parameter (see Signal Space above) as predictor variables. In contrast to general linear models, GAMs fit isotropic nonlinear smooths of a number of predictors, and the response variable can be explicitly nonnormal (48), e.g., binomial in our study. To assess the significance of each predictor, we deleted it from the model and compared the incomplete and the complete models with ANOVA (49). To avoid overparameterization in the models, we ran separate GAMs for the subset of data corresponding to each species. Graphically, each species-specific recognition space was represented as a line encompassing the range of signal variation that would elicit positive phonotactic reactions with a probability of at least 0.99.
We further created a communication space by mapping the recognition spaces onto the 2D signal space. The resulting picture was visually contrasted against our four a priori hypotheses (Fig. 1). If our matched-spaces hypothesis holds, then the recognition space should extend up to about 3 SD of each relevant call parameter, no matter the multivariate distance to the signal of other co-occurring species. If the spectral-partitioning hypothesis holds, then recognition space should be much wider in the temporal than in the spectral domain, allowing it to overlap with the other species’ signal space in the former but not in the latter case. Finally, if the filled-gaps hypothesis holds, then the projections of the recognition space should be longer toward the species that appear farther in the signal space.
To explicitly test the latter hypothesis, we tested whether the projection of the recognition space (recognition distance) could be predicted from the pairwise differences in call traits (acoustic distance) while controlling for the degree of species co-occurrence. The three variables were estimated for species pairs that were neighbors in the communication space (i.e., no other species’ signal was between them) and that included at least one species used to estimate recognition spaces. Acoustic distance was interpolated from the communication space as the straight-line unitless distance between the centroids of signal parameters of each species pair. The recognition distance was concomitantly estimated from the intersection between the line used to estimate call distance and the line used to delimit a species recognition space. Co-occurrence was estimated from the MDS analysis on the acoustic environments (see Acoustic Co-Occurrence above). Because the statistical unit of analysis is a species pair, we ran the corresponding regression model (recognition distance = A*acoustic distance + B*co-occurrence + C + ε) by using permutation techniques on the R package “glmperm” (50) after the modification by W. Werft and A. Benner (51). Briefly, the significance of the regression coefficient (e.g., acoustic distance) was tested by permuting the regression residuals of a second predictor (e.g., co-occurrence).
Supplementary Material
Acknowledgments
We are grateful to J. Diller for allowing us to use her private field station (Panguana, Peru), for sharing her home and experience to Moro's family, and for providing field assistance to K. Siu-Ting. We thank L. M. Arenas for invaluable help with the figures. This study was primarily supported by Austrian Science Foundation Grant FWF-P18811 (to W.H.). Research permits were provided by the National Institute of Natural Resources of Peru (Permit 078-2003).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.J.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104773108/-/DCSupplemental.
References
- 1.Jones G. Acoustic signals and speciation: The roles of natural and sexual selection in the evolution of cryptic species. Adv Stud Behav. 1997;26:317–354. [Google Scholar]
- 2.Ryan MJ, Rand AS. Sexual selection and signal evolution: The ghost of biases past. Philos Trans R Soc Lond B Biol Sci. 1993;340:187–195. [Google Scholar]
- 3.Hunter ML, Krebs JR. Geographical variation in the song of the great tit (Parus major) in relation to ecological factors. J Anim Ecol. 1979;48:759–785. [Google Scholar]
- 4.Irwin DE. Song variation in an avian ring species. Evolution. 2000;54:998–1010. doi: 10.1111/j.0014-3820.2000.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 5.Welch AM, Semlitsch RD, Gerhardt HC. Call duration as an indicator of genetic quality in male gray tree frogs. Science. 1998;280:1928–1930. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- 6.Grether GF. Carotenoid limitation and mate preference evolution: A test of the indicator hypothesis in guppies (Poecilia reticulata) Evolution. 2000;54:1712–1724. doi: 10.1111/j.0014-3820.2000.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 7.Endler JA, Houde AE. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution. 1995;49:456–468. doi: 10.1111/j.1558-5646.1995.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 8.Godin J-GJ, Dugatkin LA. Variability and repeatability of female mating preference in the guppy. Anim Behav. 1995;49:1427–1433. [Google Scholar]
- 9.Narins P, Feng A. Hearing and sound communication in amphibians: Prologue and prognostication. In: Narins PM, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians, Springer Handbook of Auditory Research. New York: Springer; 2007. pp. 1–11. [Google Scholar]
- 10.Ryan MJ, Phelps SM, Rand AS. How evolutionary history shapes recognition mechanisms. Trends Cogn Sci. 2001;5:143–148. doi: 10.1016/s1364-6613(00)01616-8. [DOI] [PubMed] [Google Scholar]
- 11.Endler JA, Westcott DA, Madden JR, Robson T. Animal visual systems and the evolution of color patterns: Sensory processing illuminates signal evolution. Evolution. 2005;59:1795–1818. doi: 10.1554/04-669.1. [DOI] [PubMed] [Google Scholar]
- 12.Höbel G, Gerhardt HC. Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea) Evolution. 2003;57:894–904. doi: 10.1111/j.0014-3820.2003.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago: Univ of Chicago Press; 2002. p. xi. [Google Scholar]
- 14.Gerhardt HC. Acoustic properties used in call recognition by frogs and toads. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley; 1988. pp. 455–483. [Google Scholar]
- 15.Gerhardt HC. Conducting playback experiments and interpreting their results. In: McGregor PK, editor. Playback and Studies of Animal Communication. New York: Plenum; 1992. pp. 59–77. [Google Scholar]
- 16.Ryan MJ, Rand AS. Species recognition and sexual selection as a unitary problem in animal communication. Evolution. 1993;47:647–657. doi: 10.1111/j.1558-5646.1993.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 17.Enquist M, Arak A. Selection of exaggerated male traits by female aesthetic senses. Nature. 1993;361:446–448. doi: 10.1038/361446a0. [DOI] [PubMed] [Google Scholar]
- 18.Ryan MJ, Rand W, Hurd PL, Phelps SM, Rand AS. Generalization in response to mate recognition signals. Am Nat. 2003;161:380–394. doi: 10.1086/367588. [DOI] [PubMed] [Google Scholar]
- 19.Narins PM, Zelick R. The effects of noise on auditory processing and behavior in amphibians. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley; 1988. pp. 511–536. [Google Scholar]
- 20.Grether GF, Losin N, Anderson CN, Okamoto K. The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol Rev Camb Philos Soc. 2009;84:617–635. doi: 10.1111/j.1469-185X.2009.00089.x. [DOI] [PubMed] [Google Scholar]
- 21.Luther D. The influence of the acoustic community on songs of birds in a neotropical rain forest. Behav Ecol. 2009;20:864–871. [Google Scholar]
- 22.Hebets E, Papaj D. Complex signal function: Developing a framework of testable hypotheses. Behav Ecol Sociobiol. 2005;57:197–214. [Google Scholar]
- 23.Duellman WE, Pyles RE. Acoustic resource partitioning in anuran communities. Copeia. 1983;1983:639–649. [Google Scholar]
- 24.Hödl W. Call differences and calling site segregation in anuran species from central Amazonian floating meadows. Oecologia. 1977;28:351–363. doi: 10.1007/BF00345990. [DOI] [PubMed] [Google Scholar]
- 25.Wollerman L, Wiley H. Possibilities for error during communication by neotropical frogs in a complex acoustic environment. Behav Ecol Sociobiol. 2002;52:465–473. [Google Scholar]
- 26.Páez VP, Bock BC, Rand AS. Inhibition of evoked calling of Dendrobates pumilio due to acoustic interference from cicada calling. Biotropica. 1993;25:242–245. [Google Scholar]
- 27.Luther DA. Signaller: Receiver coordination and the timing of communication in Amazonian birds. Biol Lett. 2008;4:651–654. doi: 10.1098/rsbl.2008.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong S, Parada H, Narins PM. Heterospecific acoustic interference: Effects on calling in the frog Oophaga pumilio in Nicaragua. Biotropica. 2009;41:74–80. doi: 10.1111/j.1744-7429.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amézquita A, et al. Masking interference and the evolution of the acoustic communication system in the Amazonian dendrobatid frog Allobates femoralis. Evolution. 2006;60:1874–1887. [PubMed] [Google Scholar]
- 30.Ryan MJ. Constraints and patterns in the evolution of anuran acoustic communication. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley; 1988. pp. 637–677. [Google Scholar]
- 31.Gerhardt HC. The evolution of vocalization in frogs and toads. Annu Rev Ecol Syst. 1994;25:293–324. [Google Scholar]
- 32.Roithmair ME. Territoriality and male mating success in the dart-poison frog, Epipedobates femoralis (Dendrobatidae, Anura) Ethology. 1992;92:331–343. [Google Scholar]
- 33.Hödl W, Amézquita A, Narins PM. The role of call frequency and the auditory papillae in phonotactic behavior in male Dart-poison frogs Epipedobates femoralis (Dendrobatidae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:823–829. doi: 10.1007/s00359-004-0536-1. [DOI] [PubMed] [Google Scholar]
- 34.Capranica RR, Moffat JM. Neurobehavioral correlates of sound communication in anurans. In: Ewert JP, Capranica RR, Ingle D, editors. Advances in Vertebrate Neuroethology. New York: Plenum; 1983. pp. 701–730. [Google Scholar]
- 35.Gottsberger B, Gruber E. Temporal partitioning of reproductive activity in a neotropical anuran community. J Trop Ecol. 2004;20:271–280. [Google Scholar]
- 36.Sueur J. Cicada acoustic communication: Potential sound partitioning in a multispecies community from Mexico (Hemiptera: Cicadomorpha: Cicadidae) Biol J Linn Soc Lond. 2002;75:379–394. [Google Scholar]
- 37.Witte K. How cricket frog females deal with a noisy world: Habitat-related differences in auditory tuning. Behav Ecol. 2005;16:571–579. [Google Scholar]
- 38.ten Cate C, Rowe C. Biases in signal evolution: Learning makes a difference. Trends Ecol Evol. 2007;22:380–387. doi: 10.1016/j.tree.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Gerhardt HC. Female choice in treefrogs: Static and dynamic acoustic criteria. Anim Behav. 1991;42:615–636. [Google Scholar]
- 40.Erdtmann L, Amézquita A. Differential evolution of advertisement call traits in dart-poison frogs (Anura: Dendrobatidae) Ethology. 2009;115:801–811. [Google Scholar]
- 41.Gerhardt HC. Reproductive character displacement of female mate choice in the grey treefrog, Hyla chrysoscelis. Anim Behav. 1994;47:959–969. [Google Scholar]
- 42.Bernal X, Rand AS, Ryan MJ. Sex differences in response to nonconspecific advertisement calls: Receiver permissiveness in male and female túngara frogs. Anim Behav. 2007;73:955–964. [Google Scholar]
- 43.Aichinger M. Annual activity patterns of anurans in a seasonal neotropical environment. Oecologia. 1987;71:583–592. doi: 10.1007/BF00379302. [DOI] [PubMed] [Google Scholar]
- 44.Amézquita A, Castellanos L, Hödl W. Auditory matching of male Epipedobates femoralis (Anura: Dendrobatidae) under field conditions. Anim Behav. 2005;70:1377–1386. [Google Scholar]
- 45.Charif RA, Clark CW, Fristrup KM. Raven 1.2 User's Manual. Ithaca, NY: Cornell Laboratory of Ornithology; 2004. [Google Scholar]
- 46.Cocroft RB, Ryan MJ. Patterns of advertisement call evolution in toads and chorus frogs. Anim Behav. 1995;49:283–303. [Google Scholar]
- 47.Weary D, Weisman R. SoundEdit v. 2.0.3. Anim Behav. 1993;45:417–418. [Google Scholar]
- 48.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. p. xvii. [Google Scholar]
- 49.Crawley MJ. The R Book. Chichester, UK: Wiley; 2007. p. viii. [Google Scholar]
- 50.Potter DM. A permutation test for inference in logistic regression with small- and moderate-sized data sets. Stat Med. 2005;24:693–708. doi: 10.1002/sim.1931. [DOI] [PubMed] [Google Scholar]
- 51.Werft W, Benner A. glperm: A permutation of regressor residuals test for inference in generalized linear models. The R Journal. 2010;2:39–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.