Abstract
Although extracellular control of canonical Wnt signaling is crucial for tissue homeostasis, the role of the extracellular microenvironment in modulating this signaling pathway is largely unknown. In the present study, we show that a member of the small leucine-rich proteoglycan family, biglycan, enhances canonical Wnt signaling by mediating Wnt function via its core protein. Immunoprecipitation analysis revealed that biglycan interacts with both the canonical Wnt ligand Wnt3a and the Wnt coreceptor low-density lipoprotein receptor-related protein 6 (LRP6), possibly via the formation of a trimeric complex. Biglycan-deficient cells treated with exogenous Wnt3a had less Wnt3a retained in cell layers compared with WT cells. Furthermore, the Wnt-induced levels of LRP6 phosphorylation and expression of several Wnt target genes were blunted in biglycan-deficient cells. Both recombinant biglycan proteoglycan and biglycan core protein increased Wnt-induced β-catenin/T cell-specific factor–mediated transcriptional activity, and this activity was completely inhibited by Dickkopf 1. Interestingly, recombinant biglycan was able to rescue impaired Wnt signaling caused by a previously described missense mutation in the extracellular domain of human LRP6 (R611C). Furthermore, biglycan's modulation of canonical Wnt signaling affected the functional activities of osteoprogenitor cells, including the RUNX2-mediated transcriptional activity and calcium deposition. Use of a transplant system and a fracture healing model revealed that expression of Wnt-induced secreted protein 1 was decreased in bone formed by biglycan-deficient cells, further suggesting reduced Wnt signaling in vivo. We propose that biglycan may serve as a reservoir for Wnt in the pericellular space and modulate Wnt availability for activation of the canonical Wnt pathway.
Keywords: mineralization, regeneration, skeleton
Canonical Wnt signaling regulates diverse biological processes during development and tissue homeostasis (1). Furthermore, defective Wnt signaling plays major roles in diseases such as cancer (2) and osteoporosis (3). The extracellular control of Wnt signaling is dependent on both the extracellular storage of Wnt proteins and the secretion of Wnt antagonists, such as secreted frizzled-related proteins, Wnt inhibitory factor 1, sclerostin, and Dickkopf (Dkk) family members (1). In addition to binding signaling molecules, matrix components also can be actively involved in modulating the activation of Wnt/β-catenin signaling. Activation of the canonical pathway starts by binding of Wnt to the frizzled receptor and its coreceptor low-density lipoprotein receptor-related protein 6 (LRP6) or its close relative LRP5. This binding results in LRP6 phosphorylation and activation and recruitment of the Axin complex, which is composed of the scaffolding protein Axin, the tumor-suppressor protein adenomatous polyposis coli, casein kinase 1, and glycogen synthase kinase 3, to the receptors. This complex is responsible for the constitutive proteasome-mediated degradation of cytoplasmic β-catenin protein in the absence of a Wnt signal. However, on activation of the Wnt/β-catenin pathway, β-catenin is stabilized within the cytosol and then translocated to the nucleus, where it accumulates and activates lymphoid enhancer-binding factor/T cell-specific factor (TCF)-mediated gene transcription. The extracellular microenvironment that regulates this pathway by modulating the activity of Wnt proteins and/or their antagonists remains largely unknown. In this regard, several different proteoglycans and/or their glycosaminoglycan chain components that are abundantly present at the cell surface have been reported to stimulate the Wnt/β-catenin pathway. These include free heparan sulfate (4), heparin chains (5–7), and heparan sulfate proteoglycans, such as glypican-3 (8) and syndecan-1 (9).
The canonical Wnt signaling pathway appears to be particularly important for bone biology. Mutations in either LRP5 or LRP6 or their downstream signaling targets have been associated with several bone-related diseases (10). Wnt signaling has been proposed to control bone mass through diverse mechanisms, including renewal of stem cells (11), stimulation of preosteoblast replication (12), and enhancement of osteoblast activity (13). Wnt signaling in mature osteoblasts has been shown to control bone resorption by regulating the expression and secretion of the osteoclastogenesis inhibitor osteoprotegerin (14). Despite the significant progress made over the past decade, our understanding of the role of the extracellular microenvironment in modulating Wnt/β-catenin signaling in bone remains limited. In this regard, we are particularly interested in one of the members of the small leucine-rich proteoglycan family, biglycan (15), because of its pronounced expression in bone, where it is concentrated in the pericellular space (16). Previous studies in our laboratory have shown that mice deficient in biglycan develop age-related osteopenia resulting from a bone formation defect involving a reduced number of osteoblasts at the bone surface (17). In addition, expression of BGN (located on the X chromosome) may be related to stature in humans, given that patients with Turner syndrome (XO) have short stature and low levels of BGN, whereas patients with supernumerary sex chromosomes have increased limb length and high levels of BGN (18). In vitro studies revealed that biglycan's modulation of growth factor activities, including both TGF-β (19) and bone morphogenetic protein 2/4 (20) signaling in osteoprogenitor cells, likely contributes to the mechanism through which biglycan affects bone formation. Although biglycan and canonical Wnt signaling are both associated with skeletal tissues, whether this proteoglycan plays a direct role in modulating this signaling pathway is not known. In the present study, we used bone as a model system to identify a novel player, biglycan, in the extracellular microenvironment that modulates canonical Wnt signaling.
Results
Biglycan Interacts with Wnt3a and LRP6 via Its Core Protein.
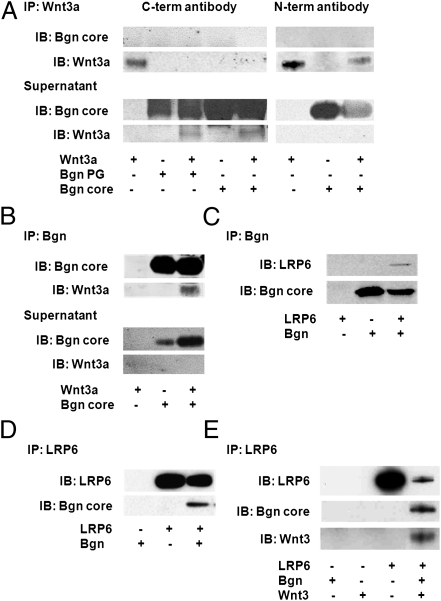
Given that biglycan was previously reported to bind growth factors, such as TGF-β, we first assessed whether biglycan could directly interact with Wnt3a, a prototypical, well-characterized activator of the β-catenin-dependent canonical pathway (21). Immunoprecipitation of mixtures of recombinant human biglycan (with and without glycanation) with recombinant human Wnt3a using antibodies specific to the C terminus of Wnt3a pulled down Wnt3a only in the absence of biglycan (Fig. 1A, Left). This result suggests that biglycan blocks the ability of the antibodies against Wnt3a to pull down Wnt3a, thus indirectly suggesting that biglycan interacts with Wnt3a at the C terminus. This suggestion is further supported by the finding that antibodies to the N-terminal region of Wnt3a pulled down Wnt3a from the mixture of recombinant biglycan (nonglycanated) and Wnt3a (Fig. 1A, Right). Because biglycan was not found in the pull-down of Wnt3a, we assumed that the antibodies against Wnt3a affected the interaction between biglycan and Wnt3a. To confirm a direct interaction between biglycan core proteins and Wnt3a, we immunoprecipitated mixtures of recombinant human biglycan (nonglycanated) with recombinant human Wnt3a using antibodies specific to biglycan, followed by immunoblotting with biglycan- and Wnt3a-specific antibodies. We found that biglycan core proteins directly interacted with Wnt3a (Fig. 1B).
Fig. 1.
Biglycan interacts with Wnt3a and LRP6 via its core protein. (A and B) Immunoblotting with biglycan or Wnt3a antibodies of immunoprecipitates (IPs) collected using biglycan and Wnt3a antibodies after mixing of recombinant human biglycan (with and without glycanation) with recombinant human Wnt3a protein. (A) IP using antibodies to the C terminus of Wnt3a (Left) or with antibodies to the N-terminal region of Wnt3a (Right). (B) IP using antibodies to Bgn. (C and D) IPs of cell lysates of HEK-293T cells overexpressing V5-tagged human biglycan and V5-tagged human LRP6 using antibodies for biglycan (C) or LRP6 (D), followed by immunoblotting of pull-down lysates with V5-specific antibodies. (E) IPs of cell lysates of HEK-293T cells overexpressing V5-tagged human biglycan, V5-tagged human LRP6, and HA-tagged human/mouse Wnt3 using antibodies for LRP6, followed by immunoblotting of pull-down lysates with V5- and HA-specific antibodies.
Because Wnt3a was previously reported to bind and activate canonical Wnt signaling through the LRP6 receptor (22), we hypothesized that biglycan also could interact with this receptor to locally enhance Wnt function. Immunoprecipitation of cell lysates of HEK-293T cells overexpressing V5-tagged human biglycan and V5-tagged human LRP6 using antibodies specific for biglycan or LRP6, followed by immunoblotting of pull-down lysates with V5-specific antibodies, revealed that biglycan indeed was bound to LRP6 (Fig. 1 C and D). Furthermore, we found that immunoprecipitation of cell lysates using antibodies specific for LRP6 resulted in the pull-down of LRP6, biglycan, and Wnt3 when cells overexpressed HA-tagged human/mouse Wnt3 in addition to V5-tagged human biglycan and V5-tagged human LRP6 (Fig. 1E). The possible formation of a trimeric complex of these proteins suggests a role for biglycan in the presentation of Wnt to the LRP6 receptor, thereby modulating the canonical Wnt pathway.
Biglycan-Deficient Calvarial Cells Retain Less Wnt3a in Cell Layers and Have Reduced Wnt-Induced Phosphorylation of LRP6 and β-Catenin/TCF-Mediated Transcriptional Activity.
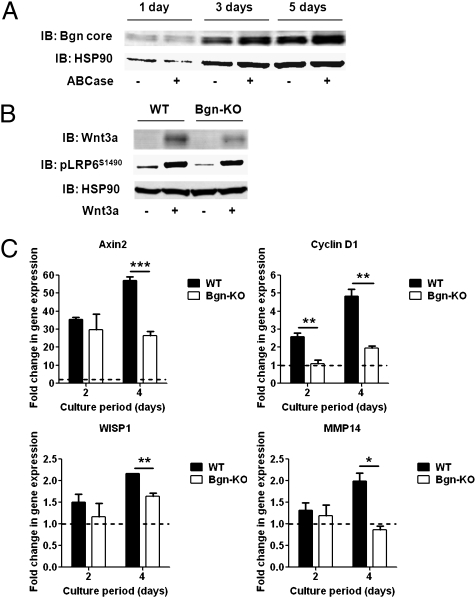
Immunoblot analysis revealed that cells obtained from calvaria of newborn WT mice had increased levels of biglycan synthesis with time in culture (Fig. 2A). Treatment of lysate proteins with chondroitinase ABC resulted in increased detection of biglycan core proteins, indicating synthesis of both glycanated and nonglycanated biglycan core proteins by the calvarial cells (Fig. 2A). Because biglycan directly interacts with Wnt3a, we next determined whether levels of Wnt3a retained in the calvarial cell layers and their surrounding matrix were decreased by the absence of biglycan. Immunoblotting revealed that Wnt3a was decreased by ∼60% in the cell layers of biglycan-deficient cells (Fig. 2B), suggesting that the proteoglycan may serve to retain Wnt3a at the cell surface/pericellular environment. We then determined whether reduced levels of Wnt3a in the calvarial cell layer in the absence of biglycan affected the phosphorylation of LRP6 at S1490 (pLRP6S1490), an event that is required for full induction of β-catenin activity (23). Immunoblotting showed that the Wnt3a-induced pLRP6S1490 appeared less in biglycan-deficient cells compared with WT cells (Fig. 2B), suggesting reduced activation of the canonical Wnt pathway in the absence of biglycan.
Fig. 2.
Biglycan-deficient calvarial cells retain less Wnt3a in cell layers and demonstrate both reduced Wnt-induced phosphorylation of LRP6 and reduced β-catenin/TCF-mediated transcriptional activity. (A) Immunoblot analysis for biglycan core protein after treatment of an equal volume of lysate proteins obtained from WT calvarial cell layers with chondroitinase ABC. (B) Immunoblot analysis for Wnt3a and phosphorylation of LRP6 (pLRP6S1490) on protein extracts obtained from WT and biglycan-deficient calvarial cells after treatment with Wnt3a. (C) Relative expression levels of Wnt target genes measured by real-time RT-PCR in WT and biglycan-deficient calvarial cells after treatment with Wnt3a. Values represent mean relative expression ± SD (n = 3), shown as Wnt3a-induced fold change in gene expression. Gene expression was normalized to untreated controls at each time point (indicated by the dotted line). *P < 0.05; **P < 0.01; ***P < 0.001.
We next assessed whether biglycan could modulate the canonical Wnt pathway at the transcriptional level. Because the crux of the canonical mechanism is known to be the nuclear accumulation of β-catenin (24), we analyzed whether Wnt3a could induce a translocation of β-catenin from the cytoplasm to the nucleus in WT and biglycan-deficient calvarial cells. Immunofluorescence analysis showed that Wnt3a induced a nuclear localization of β-catenin in both WT and biglycan-deficient cell types (Fig. S1), indicating activation of the canonical Wnt pathway. However, there was no clear difference between the cell types in the number of cells demonstrating Wnt-induced translocation of β-catenin. In the nucleus, β-catenin complexes with TCF/lymphoid enhancer-binding factor transcription factors, thereby regulating the expression of genes controlling proliferation and cell cycle progression (24). Real-time RT-PCR revealed that the Wnt3a-induced expression of several Wnt target genes encoding Axin2, Cyclin D1, WISP1, and MMP14 was blunted in the absence of biglycan, with a 25–60% reduction after 4 d of Wnt3a treatment (Fig. 2C).
Both Biglycan Proteoglycan and Biglycan Core Protein Enhance Wnt-Induced β-Catenin/TCF-Mediated Transcriptional Activity via LRP6, and This Activity Is Inhibited by Dkk1.
To further elucidate the mechanism by which biglycan affects canonical Wnt signaling, we analyzed the effect of recombinant biglycan (25) on Wnt signaling in a β-catenin reporter cell line (HEK-293T cells stably transfected with the SuperTOPflash reporter plasmid, in which several TCF4-binding repeats drive firefly luciferase expression) (26). Recombinant biglycan alone had no effect on the reporter activity, but in the presence of increasing amounts of Wnt3a, both recombinant biglycan proteoglycan and biglycan core proteins increased the Wnt-induced β-catenin reporter activity in the cells overexpressing LRP6-WT (Fig. 3A). Biglycan core protein was more effective than biglycan proteoglycan in enhancing Wnt signaling, indicating that the functional sites likely reside in the core protein, and that the glycosaminoglycan chains possibly interfere with biglycan's effect on Wnt signaling. Recombinant biglycan proteoglycan and biglycan core proteins also increased the Wnt-induced β-catenin reporter activity in cells overexpressing LRP6 mutant (LRP6-MT; in which all serine/threonine residues in all PPPS/TP motifs were replaced with alanine) (Fig. 3B), which most likely is mediated by endogenously expressed LRP5/6 in these cells.
Fig. 3.
Both biglycan proteoglycan and biglycan core protein enhance Wnt-induced β-catenin/TCF-mediated transcriptional activity via LRP6, and this activity is inhibited by Dkk1. (A) Effect of biglycan proteoglycan (PG) and biglycan core protein on Wnt3a-induced reporter activity in β-catenin reporter cells overexpressing LRP6-WT or inactive LRP6-MT. Values represent mean relative luminescence ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001, control (no biglycan) vs. biglycan treatment. (B) Effect of biglycan PG and biglycan core protein on Wnt3a-induced reporter activity in β-catenin reporter cells overexpressing LRP6-MT. Values represent mean relative luminescence ± SD (n = 3). *P < 0.05; **P < 0.01, control (no biglycan) vs. biglycan treatment. (C) Effect of Dkk1 on Wnt3a-induced reporter activity in β-catenin reporter cells in the presence of biglycan core protein. Values represent mean relative luminescence ± SD (n = 3). *P < 0.05, +Wnt3a/−biglycan core vs. +Wnt3a/+biglycan core at 0 and 10 ng/mL Dkk1. (D) Effect of LiCl-induced β-catenin reporter activity in β-catenin reporter cells in the presence of biglycan core protein. NaCl was used for control purposes. Values represent mean relative luminescence ± SD (n = 3).
Considering our finding that biglycan affected the Wnt-associated signaling cascade but had no effect on its own, we speculated that the mechanism by which biglycan enhances Wnt signaling involves direct mediation of Wnt function. To test this hypothesis, we assessed whether the Wnt signaling antagonist Dkk1, which is known to bind LRP6 with high affinity and prevent formation of the Frizzled-Wnt-LRP6 complex in response to Wnts (27), could inhibit biglycan's effect on Wnt signaling. Recombinant Dkk1 dose-dependently inhibited Wnt3a-induced β-catenin reporter activity, including biglycan-enhanced activity (Fig. 3C), indicating that biglycan directly mediates Wnt function.
To test whether biglycan modulates extracellular Wnt function, we next examined intracellular activation of the Wnt pathway in β-catenin reporter cells in the presence or absence of biglycan core proteins by use of lithium chloride (LiCl), an inhibitor of glycogen synthase kinase-3β, whose function is to target β-catenin for destruction (28). Inhibition of glycogen synthase kinase-3β prevents the constitutive proteasome-mediated degradation of cytoplasmic β-catenin, resulting in the accumulation and nuclear translocation of β-catenin, where it induces β-catenin/TCF-mediated transcriptional activity. Recombinant biglycan core proteins had no effect on the dose-dependent LiCl-induced β-catenin reporter activity (Fig. 3D), confirming that biglycan mediates Wnt signaling in the extracellular environment and does not affect intracellular events.
Biglycan Core Protein Rescues Impaired Wnt Signaling Caused by a Missense Mutation in the Extracellular Domain of Human LRP6 (R611C).
A missense mutation in the extracellular domain of LRP6 was previously linked to a family with early coronary disease and osteoporosis in humans (29). This missense mutation, which substitutes cysteine for arginine (R611C) at a highly conserved residue of an epidermal growth factor-like domain (Fig. 4A), was shown to impair Wnt signaling in vitro. Because recombinant biglycan was able to enhance Wnt signaling mediated by LRP6, we hypothesized that biglycan could rescue defective Wnt signaling caused by the LRP6-R611C mutation. Overexpression of LRP6-R611C in β-catenin reporter cells resulted in an ∼30–65% reduction in β-catenin reporter activity (depending on the Wnt3a concentration) compared with cells overexpressing LRP6-WT (Fig. 4 B and C). Recombinant biglycan core protein increased the Wnt3a-induced reporter activity in a dose-dependent manner (Fig. 4B), but just to a limited extent in cells expressing only endogenous LRP6 (indicated by LRP6-MT). Immunoblotting (using antibodies to V5-fusion peptide) showed comparable expression levels of LRP6-R611C and LRP6-WT in the β-catenin reporter cells. Furthermore, immunoprecipitation using antibodies to biglycan (Fig. 4D) and LRP6 (Fig. 4E), followed by detection with V5-specific antibodies, revealed that biglycan's interaction with LRP6 was not affected by the R611C missense mutation in the LRP6 receptor.
Fig. 4.
Biglycan core protein rescues impaired β-catenin/TCF-mediated transcriptional activity caused by the R611C missense mutation in LRP6 and stimulates Wnt-induced RUNX2 transcriptional activity. (A) Location of the R611C mutation in LRP6. (B) Effect of biglycan core protein on Wnt3a-induced β-catenin reporter activity in β-catenin reporter cells overexpressing LRP6-WT or LRP6-R611C. Values represent the mean relative luminescence ± SD (n = 3). *P < 0.05; **P < 0.01, LRP6-R611C (no biglycan) vs. LRP6-R611C (biglycan-treated) and LRP6-WT (no biglycan). (C) Wnt3a-induced β-catenin reporter activity in cells expressing only endogenous LRP6 (indicated by LRP6-MT) compared with cells overexpressing LRP6-WT or LRP6-R611C. Values represent mean relative luminescence ± SD (n = 3). *P < 0.05; **P < 0.01. (D and E) Immunodetection of the V5 tag (fused with biglycan, LRP6-WT, and LRP6-R611C) of IPs collected using biglycan (D) or LRP6 (E) antibodies after harvesting of β-catenin reporter cells overexpressing biglycan, LRP6-WT, LRP6-R611C, or a combination of these. (F) Effect of biglycan core protein on Wnt3a-induced p6OSE2-luc reporter activity in MC3T3-E1 cells. Values represent mean relative luminescence ± SD (n = 6). **P < 0.01.
Given the finding that biglycan enhanced Wnt-mediated signaling in cells, we assessed whether biglycan would affect Wnt-induced osteogenic commitment of osteoprogenitor cells. Recombinant biglycan core protein stimulated the Wnt-induced reporter activity driven by RUNX2-responsive promoter sequences in murine preosteoblast MC3T3-E1 cells overexpressing LRP6-WT (Fig. 4F).
Biglycan-Deficient Cells Have Reduced Wnt-Induced Mineralization and Show Less Wnt-Induced Secreted Protein 1 Expression in Bone Formed in Transplants and During Fracture Healing.
We further addressed the possibility that biglycan's modulation of canonical Wnt signaling could be involved in controlling functional activities of osteoprogenitor cells. To directly assess the Wnt-induced effects on osteogenic differentiation of bone marrow stromal cells (BMSCs), we analyzed the levels of calcium deposition by WT and biglycan-deficient cells in cultures. Alizarin red staining revealed that biglycan-deficient BMSCs had less Wnt-induced calcium deposition after 21 d of culture and demonstrated no significant increase in response to exogenously added Wnt3a (Fig. 5A). To assess whether biglycan also modulates canonical Wnt signaling in osteoprogenitor cells in an in vivo context, we seeded WT and biglycan-deficient BMSCs in gelatin sponges, transplanted them s.c. into immunocompromised mice, and harvested them after 6 wk. Micro-CT analysis revealed significantly lower trabecular-like bone formation and bone mineral density in the biglycan-deficient transplants (Fig. 5B). H&E-stained sections through the center of the transplants confirmed the reduced levels of trabecular structures in the absence of biglycan. Interestingly, the biglycan-deficient transplants had marrow spaces that contained less fat but increased numbers of hematopoietic cells (Fig. 5C), similar to what is found in biglycan-deficient mice (17). Immunostaining for Wnt-induced secreted protein 1 (WISP1), a protein known to be up-regulated by Wnt (30), revealed lower WISP1 expression levels in the bone of transplants in the absence of biglycan (Fig. 5D), suggesting reduced Wnt signaling in biglycan-deficient osteoprogenitor cells. To further address biglycan's effect on canonical Wnt signaling in vivo, we analyzed WISP1 expression levels during bone formation in a fracture healing model, given that WISP1 was previously shown to be expressed during skeletal development and fracture repair (31). WISP1 expression was reduced in woven bone in the callus area of femurs obtained from biglycan-deficient mice at 14 d postfracture (Fig. 5 E and F). Moreover, WISP1 gene expression levels were significantly decreased in callus areas of femurs of biglycan-deficient mice at 7 d postfracture (Fig. 5G). These data indicate that biglycan's modulation of canonical Wnt signaling might be involved in the osteogenic differentiation of osteoprogenitor cells, which may ultimately affect the bone formation process.
Fig. 5.
Biglycan-deficient cells have reduced Wnt-induced mineralization in cultures and show less WISP1 expression in trabecular structures formed in transplants and during fracture healing. (A) Wnt-induced calcium deposition assessed by alizarin red staining of WT and biglycan-deficient BMSCs. Values represent mean relative alizarin red staining ± SD (n = 3). *P < 0.05; **P < 0.01. (B) Micro-CT images of WT (Upper) and biglycan-deficient (Lower) transplants and quantitative assessment of the bone mineral density of trabecular structures. (C) H&E staining of sections of WT (Upper) and biglycan-deficient (Lower) transplants. (D) Immunostaining for WISP1 on sections through the center of WT (Upper) and biglycan-deficient (Lower) transplants. Arrows indicate positive staining in the trabecular-like bone formed in the transplants. (E) H&E staining of sections of WT (Upper) and biglycan-deficient (Lower) femurs at 14 d postfracture. White arrows indicate the fracture area. (F) Immunostaining for WISP1 on sections through WT (Upper) and biglycan-deficient (Lower) femurs at 14 d postfracture showing WISP1 expression surrounding the woven bone formed in the callus area. (G) Relative WISP1 gene expression levels measured by real-time RT-PCR in WT and biglycan-deficient callus areas of femurs at 7 d postfracture. Values represent mean relative expression ± SD (n = 4). *P < 0.05.
Discussion
The present study describes a mechanism by which biglycan in the extracellular microenvironment contributes to the control of the canonical Wnt signaling pathway. We have identified that this small leucine-rich proteoglycan is a modulator of Wnt signaling through a direct interaction of its core protein with Wnt ligand and its coreceptor, LRP6. In the absence of biglycan, less Wnt was retained in cell layers, apparently resulting in reduced activation of the Wnt/β-catenin pathway. Recombinant biglycan's enhanced Wnt-induced signaling through LRP6 was mediated by its core protein. The enhanced Wnt-induced signaling could be completely inhibited by the extracellular Wnt inhibitor Dkk1, confirming biglycan's extracellular role in mediating Wnt function. Although we used bone as a model system in this study, our findings may have important implications for two reasons, given biglycan's wide tissue distribution (15, 16, 32). First, biglycan is likely to play a physiological role in the control of Wnt/β-catenin signaling. Second, biglycan has potential as a valuable biological agent in the treatment of Wnt-related diseases.
Although the precise mechanism through which biglycan enhances Wnt/β-catenin signaling is not yet clear, we expect that biglycan's core protein plays a crucial role in this process by mediating Wnt function through the formation of a possible trimeric complex with both Wnt and LRP6. This supposition is supported by our findings that biglycan alone had no effect on Wnt/β-catenin signaling, and that the extracellular Wnt inhibitor Dkk1 could completely inhibit biglycan's effect on Wnt signaling. Possible mechanisms through which biglycan affects the canonical Wnt pathway through LRP6 include modulation of Wnt's availability to bind to the coreceptor complex of LRP6 and Frizzled proteins and/or participation in the formation of this receptor complex. Further studies are needed to elucidate this mechanism in more detail. In light of biglycan's ability to mediate Wnt3a function, it is tempting to speculate that biglycan also may bind and mediate the function of other canonical (such as Wnt1) or noncanonical Wnt proteins and/or Wnt antagonists. This possibility is intriguing, given that other Wnt-binding proteoglycans, such as glypicans, are able to bind several Wnt ligands (5, 33, 34). Biglycan also may modulate Wnt signaling not only through LRP6, but also via other Wnt coreceptors, such as LRP5, Derailed/RYK, ROR, Frizzled, and FRL1/crypto; however, attributing such potential roles for biglycan remains speculative at this point.
The role of the extracellular microenvironment in regulating Wnt signaling is likely to be highly specific to maintain tissue homeostasis. In this respect, closely related matrix proteins may have differential effects on this signaling pathway. These differential effects are illustrated by the previously reported finding that decorin, a closely related family member of biglycan (15), also affects β-catenin signaling (35). But decorin functions by interacting with the Met receptor, which, in contrast to biglycan, leads to decreased β-catenin/TCF-mediated transcriptional activity through suppression of intracellular levels of β-catenin in tumor cells. Although increased decorin expression levels in the absence of biglycan in osteoprogenitor cells has been reported (20), total β-catenin levels were not affected in these cells, making a substantial role for decorin in β-catenin signaling in these cells unlikely.
Recombinant human biglycan (rhBGN) could be a potentially interesting therapeutic agent in treating Wnt-related diseases by locally enhancing canonical Wnt signaling. Systemically delivered rhBGN was reported to reduce muscle pathology in an mdx mouse model of Duchenne muscular dystrophy (36). RhBGN was shown to be well tolerated in animals dosed for as long as 3 mo, indicating its potential value for therapy. Although the present study only highlights a role for rhBGN in enhancing Wnt/β-catenin signaling in cells in culture, we propose that rhBGN may exert similar effects on Wnt activities in cells in vivo. This information may lead to new treatment modalities for Wnt-related diseases. In this respect, it is of crucial importance to assess the contribution of defective Wnt signaling to the characteristic features of the disease. For example, a previously characterized family harboring a missense mutation in LRP6 substituting cysteine for arginine at a highly conserved residue of an epidermal growth factor-like domain (R611C), which has been linked to impaired Wnt signaling in vitro (29). Additional studies by that group revealed that the mechanisms by which the mutation imparts its effects are likely to be Wnt-independent, at least in part. The mechanisms of hypercholesterolemia in LRP6-R611C mutation carriers likely involve altered receptor affinity for LDL and its subcellular localization, along with reduced cellular LDL clearance (37). Furthermore, atherosclerosis in these mutation carriers may be due to enhanced vascular smooth muscle cell proliferation in response to PDGF (38). Thus, this may indicate the involvement of signaling pathways other than Wnt in the contribution of this mutation to osteoporosis, as was uncovered in hypercholesterolemia and atherosclerosis defects. However, we cannot rule out the possibility that a Wnt-mediated mechanism that affects the proliferation or apoptosis of osteoprogenitor cells may be involved in the effects of this mutation.
In conclusion, we have identified biglycan as component in the extracellular microenvironment that modulates canonical Wnt signaling. Our findings shed new light on the thus-far limited knowledge of the tight extracellular control of signaling pathways in tissue homeostasis. Furthermore, understanding the mechanisms through which matrix proteins modulate canonical Wnt signaling may lead to new therapeutic approaches for the targeting and cure of Wnt-related diseases.
Materials and Methods
The LRP6-R611C mutant construct was generated by site-directed mutagenesis of the WT LRP6 sequence using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent) in accordance with the manufacturer's instructions. The following oligonucleotides were used to generate the mutant construct: forward, 5′-ctcagggcctttgctgtgcttgc-3′; reverse, 5′-gcaagcacagcaaaggccctgag-3′.
Details regarding animals, cell culture, plasmids, DNA transfections and reporter assays, immunoprecipitation, immunoblotting and immunofluorescence analysis, quantitative RT-PCR, primer sets (Table S1), in vitro calcium accumulation, in vivo osteogenesis assay, the fracture healing model, and statistical analysis are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Li Li, Azusa Maeda, Emily Pinnow, and Brian Sworder, Craniofacial and Skeletal Diseases Branch, National Institute for Dental and Craniofacial Research, for technical assistance. This research was supported in part by the Division of Intramural Research, National Institute for Dental and Craniofacial Research, of the Intramural Research Program of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110629108/-/DCSupplemental.
References
- 1.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 3.Patel MS, Karsenty G. Regulation of bone formation and vision by LRP5. N Engl J Med. 2002;346:1572–1574. doi: 10.1056/NEJM200205163462011. [DOI] [PubMed] [Google Scholar]
- 4.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 5.Ai X, et al. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombres M, et al. Heparin activates Wnt signaling for neuronal morphogenesis. J Cell Physiol. 2008;216:805–815. doi: 10.1002/jcp.21465. [DOI] [PubMed] [Google Scholar]
- 7.Ling L, et al. Synergism between Wnt3a and heparin enhances osteogenesis via a phosphoinositide 3-kinase/Akt/RUNX2 pathway. J Biol Chem. 2010;285:26233–26244. doi: 10.1074/jbc.M110.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 9.Alexander CM, et al. Syndecan-1 is required for Wnt-1–induced mammary tumorigenesis in mice. Nat Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 10.Williams BO, Insogna KL. Where Wnts went: The exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 12.Kato M, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodine PV, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 14.Glass DA, 2nd, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Fisher LW, Termine JD, Young MF. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989;264:4571–4576. [PubMed] [Google Scholar]
- 16.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- 17.Xu T, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 18.Papp C, et al. Prenatal diagnosis of Turner syndrome: Report on 69 cases. J Ultrasound Med. 2006;25:711–717. doi: 10.7863/jum.2006.25.6.711. [DOI] [PubMed] [Google Scholar]
- 19.Chen XD, Shi S, Xu T, Robey PG, Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- 20.Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4–induced osteoblast differentiation. FASEB J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu H, et al. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- 22.Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol. 2003;23:5825–5835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf J, Palmby TR, Gavard J, Williams BO, Gutkind JS. Multiple PPPS/TP motifs act in a combinatorial fashion to transduce Wnt signaling through LRP6. FEBS Lett. 2008;582:255–261. doi: 10.1016/j.febslet.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hocking AM, Strugnell RA, Ramamurthy P, McQuillan DJ. Eukaryotic expression of recombinant biglycan: Post-translational processing and the importance of secondary structure for biological activity. J Biol Chem. 1996;271:19571–19577. [PubMed] [Google Scholar]
- 26.Xu Q, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand–receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 27.Semënov MV, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 28.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani A, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1– and β-catenin–responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- 31.French DM, et al. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004;165:855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegrowski Y, Pillarisetti J, Danielson KG, Suzuki S, Iozzo RV. The murine biglycan: Complete cDNA cloning, genomic organization, promoter function, and expression. Genomics. 1995;30:8–17. doi: 10.1006/geno.1995.0002. [DOI] [PubMed] [Google Scholar]
- 33.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 34.De Cat B, et al. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625–635. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldoni S, et al. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amenta AR, et al. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci USA. 2011;108:762–767. doi: 10.1073/pnas.1013067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, et al. Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ Res. 2008;103:1280–1288. doi: 10.1161/CIRCRESAHA.108.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keramati AR, et al. Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases, PDGF-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2011;108:1914–1918. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.