Abstract
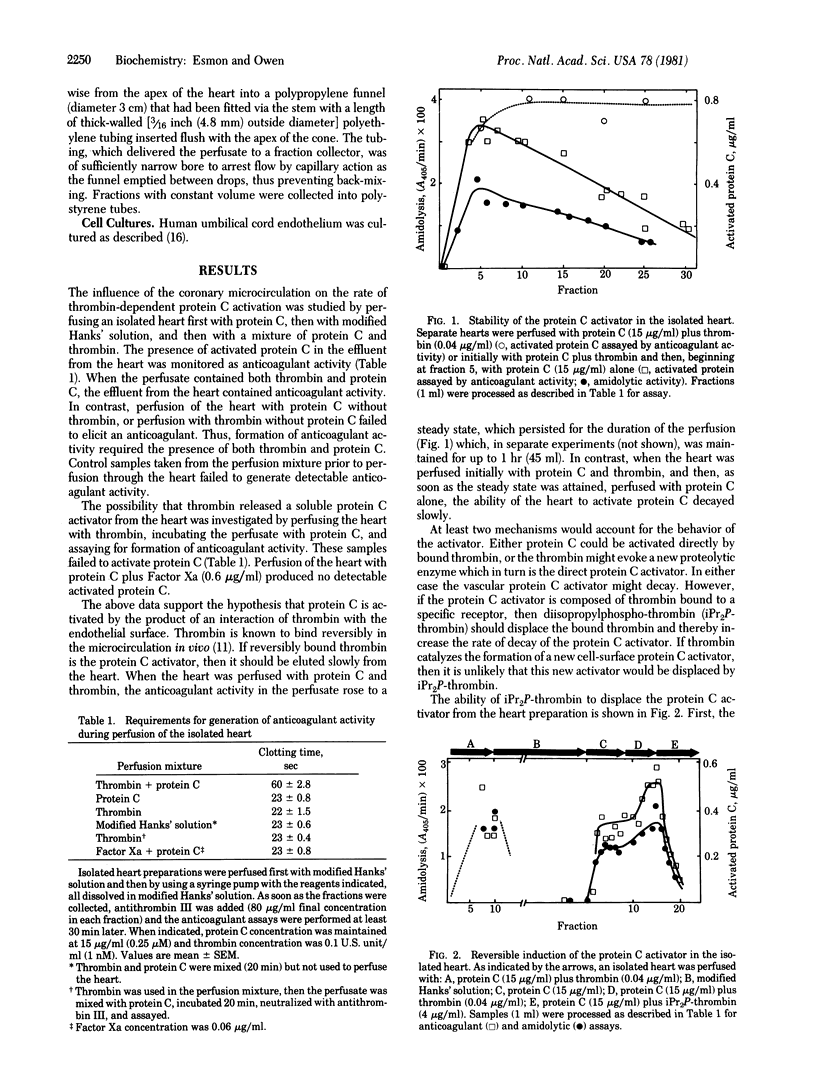
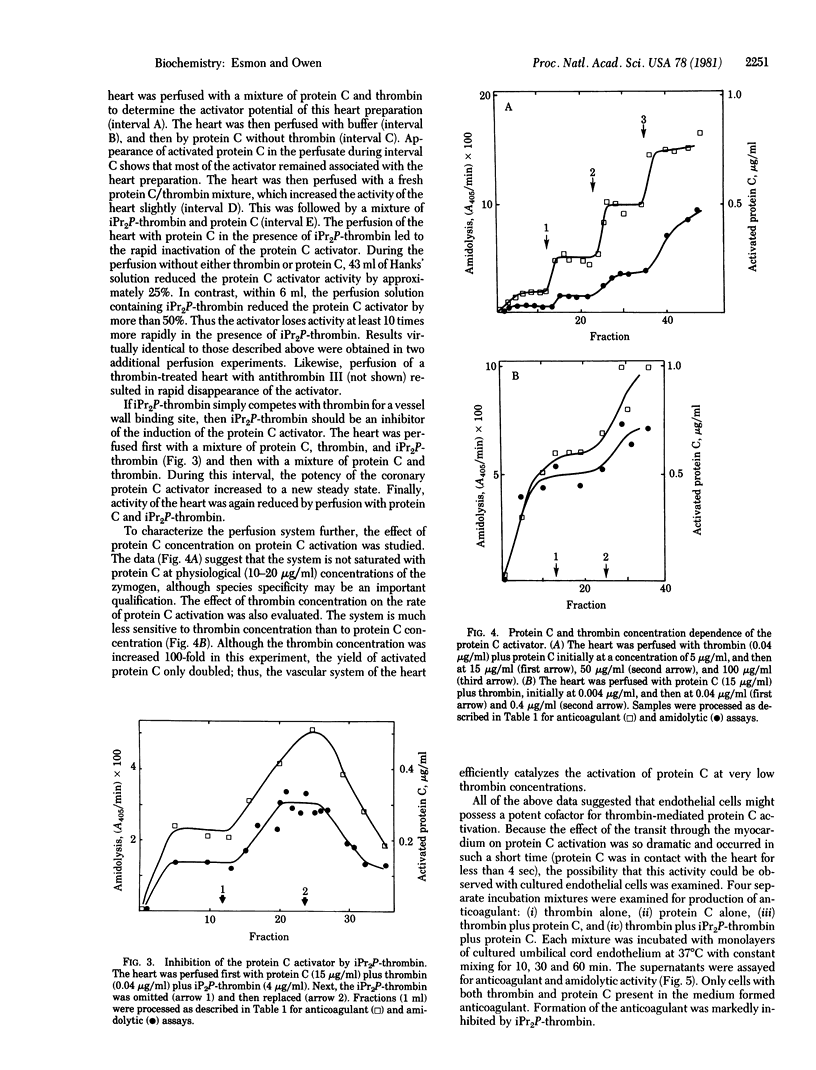
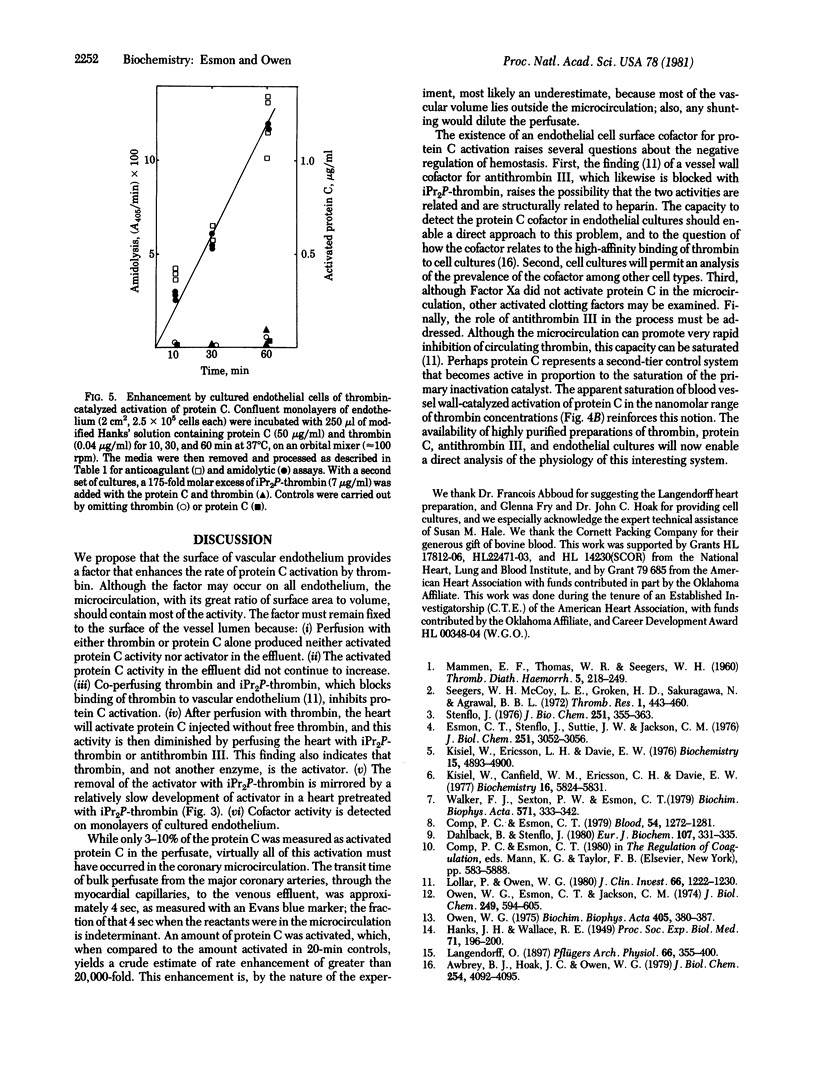
Perfusion of the myocardium with protein C in the presence of thrombin (EC 3.4.21.5) elicits a potent anticoagulant activity, which is identified as activated protein C on the basis of synthetic substrate hydrolysis and anticoagulant properties. The rate of activated protein C formation during the transit through the myocardium is at least 20,000 times that of thrombin-catalyzed activation of protein C in the perfusion solution. The capacity of the heart to activate protein C is maintained for at least 1 hr when thrombin is present in the perfusate, but decays (half-life approximately 30 min) once thrombin is omitted. Addition of diisopropyl-phospho-thrombin increases this decay rate more than 10-fold. Coperfusing diisopropylphospho-thrombin with active thrombin lowers the amount of protein C activation in the myocardium. Cultured monolayers of human endothelium enhance the rate of thrombin-catalyzed protein C activation. As with myocardium, the activation rate is inhibited by including diisopropylphospho-thrombin in the medium. It is proposed that the surface of vascular endothelium provides a cofactor that enhances the rate of protein C activation by thrombin.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awbrey B. J., Hoak J. C., Owen W. G. Binding of human thrombin to cultured human endothelial cells. J Biol Chem. 1979 May 25;254(10):4092–4095. [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Activated protein C inhibits platelet prothrombin-converting activity. Blood. 1979 Dec;54(6):1272–1281. [PubMed] [Google Scholar]
- Dahlbäck B., Stenflo J. Inhibitory effect of activated protein C on activation of prothrombin by platelet-bound factor Xa. Eur J Biochem. 1980 Jun;107(2):331–335. doi: 10.1111/j.1432-1033.1980.tb06033.x. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Stenflo J., Suttie J. W. A new vitamin K-dependent protein. A phospholipid-binding zymogen of a serine esterase. J Biol Chem. 1976 May 25;251(10):3052–3056. [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Ericsson L. H., Davie E. W. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976 Nov 2;15(22):4893–4900. doi: 10.1021/bi00667a022. [DOI] [PubMed] [Google Scholar]
- Lollar P., Owen W. G. Clearance of thrombin from circulation in rabbits by high-affinity binding sites on endothelium. Possible role in the inactivation of thrombin by antithrombin III. J Clin Invest. 1980 Dec;66(6):1222–1230. doi: 10.1172/JCI109973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAMMEN E. F., THOMAS W. R., SEEGERS W. H. Activation of purified prothrombin to autoprothrombin I or autoprothrombin II (platelet cofactor II or autoprothrombin II-A). Thromb Diath Haemorrh. 1960 Dec 15;5:218–249. [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. I. Characterization of the reaction products formed during the activation of bovine prothrombin. J Biol Chem. 1974 Jan 25;249(2):594–605. [PubMed] [Google Scholar]
- Owen W. G. Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta. 1975 Oct 20;405(2):380–387. doi: 10.1016/0005-2795(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355–363. [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]



