Abstract
The suprachiasmatic nucleus of the brain is the circadian center, relaying rhythmic environmental and behavioral information to peripheral tissues to control circadian physiology. As such, central clock dysfunction can alter systemic homeostasis to consequently impair peripheral physiology in a manner that is secondary to circadian malfunction. To determine the impact of circadian clock function in organ transplantation and dissect the influence of intrinsic tissue clocks versus extrinsic clocks, we implemented a blood vessel grafting approach to surgically assemble a chimeric mouse that was part wild-type (WT) and part circadian clock mutant. Arterial isografts from donor WT mice that had been anastamosed to common carotid arteries of recipient WT mice (WT:WT) exhibited no pathology in this syngeneic transplant strategy. Similarly, when WT grafts were anastamosed to mice with disrupted circadian clocks, the structural features of the WT grafts immersed in the milieu of circadian malfunction were normal and absent of lesions, comparable to WT:WT grafts. In contrast, aortic grafts from Bmal1 knockout (KO) or Period-2,3 double-KO mice transplanted into littermate control WT mice developed robust arteriosclerotic disease. These lesions observed in donor grafts of Bmal1-KO were associated with up-regulation in T-cell receptors, macrophages, and infiltrating cells in the vascular grafts, but were independent of hemodynamics and B and T cell-mediated immunity. These data demonstrate the significance of intrinsic tissue clocks as an autonomous influence in experimental models of arteriosclerotic disease, which may have implications with regard to the influence of circadian clock function in organ transplantation.
Keywords: vascular, remodeling, rejection, RAG-KO
Circadian rhythms are governed by a central clock, located in the suprachiasmatic nucleus (SCN) in the hypothalamus of the brain, to control central rhythmic functions such as sleep/wake cycles and locomotor function (1). The SCN, and its impact on locomotor function, in many ways acts as the conductor to organize a symphony of peripheral rhythms (2). Global circadian clock gene disruption changes systemic homeostasis by influencing locomotor function (3, 4), metabolism (5, 6), and blood pressure (7). Thus, central disorders of circadian rhythm may impinge on general physiology to cause secondary impairments in peripheral tissues. Indeed, the presence of circadian clock components in the periphery has demonstrated that there are tissue-specific functions of the circadian clock, corroborated by tissue-specific transgenic clock mutants (8, 9) and genetic rescue studies (10). However, even tissue-specific strategies of genetic disruption are vulnerable to off-target effects (9, 11). Recent data have demonstrated an important influence of global clock disruption in the progression of chronic disease (12–15). With central and tissue-specific clock disruption impairing overall physiology so pervasively, the direct function of intrinsic tissue clocks remains unclear in long-term pathology.
Results
WT:WT Arterial Isografts Are Absent of Disease and Exhibit Structural Adaptation Accompanied by Intact Vascular and Circadian Responses.
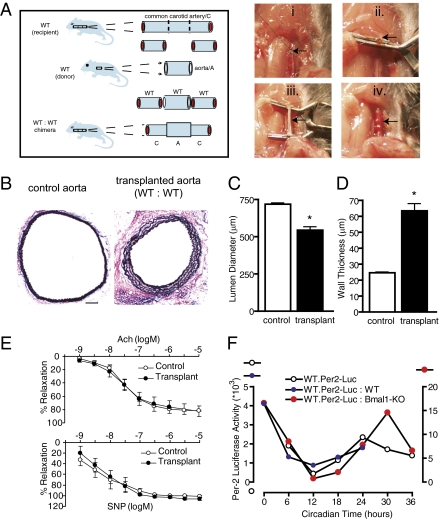
To determine the relationship between extrinsic and intrinsic tissue-specific circadian clocks, we implemented an organ transplant approach in mice using arterial grafting (Fig. 1A). Transplant models in animals have traditionally been used to examine organ rejection using donor organs/tissues from animals of a particular strain or species to a recipient animal of a different strain (allograft) (16) or species (xenograft) (17). Here we implement a grafting strategy between mice of identical strain (isograft) and sex (male) to assess tissue-extrinsic and -intrinsic influences of circadian clock gene mutation, a transplant model that exhibits limited immune and tissue responses (18). Aortae from wild-type (WT) mice were used as donors and anastamosed to the common carotid artery of recipient, same-strain WT mice (WT donor to WT recipient transplants; WT:WT), to intentionally induce remodeling in this “size-mismatch” model of isograft transplantation. In its native environment, the size of the control aorta (determined as lumen diameter) was 718 ± 9.38 μm. Four weeks after heterotopic transplantation (aorta displaced from natural origin emanating from the heart and intercalated within the common carotid artery), the aorta changed its dimensions, becoming smaller through a process called inward remodeling (Fig. 1B). The aortic diameter narrowed to 544 ± 24.4 μm (Fig. 1C), and it also exhibited an increase in wall thickness (Fig. 1D). The grafted aorta was viable, because endothelium-dependent relaxation and smooth muscle cell-dependent relaxation (Fig. 1E) were comparable to control aortae, and ultrasound imaging revealed patent grafts that sustained blood flow to downstream vascular branches (Fig. S1). Thus, the donor/transplanted aorta altered its structure as adaptation to the flow dynamics in the common carotid artery (vascular remodeling). Furthermore, circadian rhythmicity was retained in the grafted arteries. By using mice transgenic for a fusion protein containing the Period-2 circadian clock component and the luciferase reporter (WT.Per2-Luc) (19), donor aortae from WT.Per2-Luc mice were transplanted into WT mice demonstrating a circadian variation in gene expression, 4 wk postimplantation, that was comparable in pattern to naïve aortae from the WT.Per2-Luc mice (Fig. 1F).
Fig. 1.
Heterotopic arterial isografts exhibit adaptive structural adaptation in WT:WT transplant conditions, accompanied by intact vascular and circadian responses. (A) The transplant model was performed by transecting (arrow) the common carotid artery at its midpoint (i), placing cuffs (arrow) on each end of the transected common carotid artery (ii), sleeving and securing the aorta (arrow) over the cuffs (iii), and then reestablishing blood flow in the graft (arrow) by releasing clamps (iv). (B) Van Gieson staining of the native aorta (Left) versus WT aortic grafts placed in WT mice (Right) reveals enhanced collagen staining (red) and expansion of the media delineated by the interelastic lamina (black) in the transplants. (Bar: 100 μm). (C and D) Lumen diameter (C) and wall thickness (D) were calculated through morphometric analysis of common carotid artery cross sections (Materials and Methods; n = 8, WT; n = 8, WT:WT; *P < 0.05). (E) Aortic grafts were isolated after 4 wk and placed in an organ bath, and concentration-response curves to acetylcholine (Ach) and sodium nitroprusside (SNP) were determined as an assay for vascular function (n = 5, WT; n = 5, WT:WT; *P < 0.05). (F) Aorta from Per-2 luciferase transgenic mice were grafted into either WT mice (WT.Per2-Luc:WT) or Bmal1-KO mice (WT.Per2-Luc:Bmal1-KO) for 4 wk, at which time the grafts were excised and placed in DMEM absent growth factors at 37 °C in an oxygenated incubator in darkness. Equal aortic segments of the grafts and also native untransplanted aorta (WT.Per-2Luc) were sampled at 6-h intervals for up to 36 h and quantified by standard bioluminescence using automated luciferin dispensation.
Global Circadian Clock Mutation Does Not Confer Disease to WT Arteries.
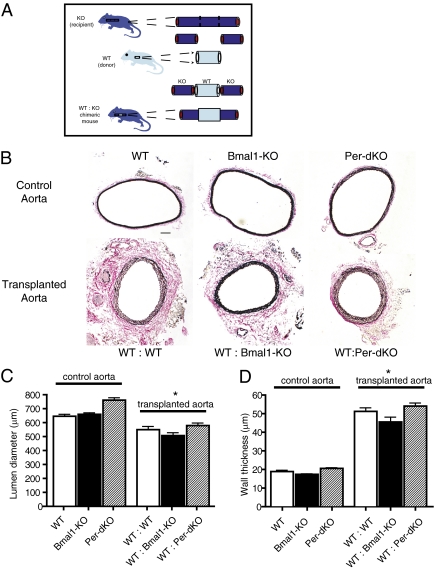
The Bmal1 and Period genes are critical to circadian rhythm because mice deficient in these components exhibit impaired locomotor rhythm (3, 20). To determine the effects of global circadian clock gene disruption on tissues with intact clocks, we created surgical chimeras of mice (Fig. 2A) by grafting aortae from littermate control WT mice (donors) into recipient mice with either disruption of Bmal1 [Bmal1 knockout (Bmal1-KO)] or Period gene isoforms [Per-double knockout (Per-dKO)]. Comparisons of the aorta in naïve, untransplanted conditions revealed no structural differences (Fig. 2B Upper) with regard to lumen diameter (Fig. 2C) or wall thickness (Fig. 2D) among WT, Bmal1-KO, and Per-dKO mice. Surprisingly, in transplanted blood vessels, there were no differences in the structural adaptation of grafts when littermate control WT mice were grafted into circadian clock knockout mice (Fig. 2B Lower). Although lumen diameter decreased (Fig. 2C) and wall thickness increased (Fig. 2D) relative to control aorta, vascular remodeling was comparable among WT:WT, WT:Bmal1-KO, and WT:Per-dKO graft transplants. There were no notable features of disease despite exposure for 4 wk in the milieu of global, extrinsic clock dysfunction. Indeed, robust circadian clock oscillation was preserved in WT aortic grafts placed in Bmal1-KO mice (Fig. 1F), providing further evidence of the intrinsic function and autonomy of peripheral clocks.
Fig. 2.
Global circadian clock mutation does not confer disease to WT arteries. (A) Genetic chimeras of mice were made by anastamosing donor aortic grafts from WT mice (littermate control) to the circulation of either recipient Bmal1-KO or Per-dKO mice (KO). (B) Van Gieson staining revealed comparable inward remodeling among groups in representative aortic graft cross-sections. (Bar: 100 μm.) (C and D) Although there was a significant change in the structural architecture of aortic grafts versus the native aorta, there was no difference in increment of structural adaptation among the WT:WT, WT:Bmal1-KO, and WT:Per-dKO indexed as lumen diameter (C) and wall thickness (D) (n = 10, WT; n = 10, Bmal1-KO; n = 5, Per-dKO; n = 10, WT:WT; n = 10, WT:Bmal1-KO; n = 5, WT:Per-dKO; *P < 0.05 versus corresponding control aorta).
Local Vascular Clock Dysfunction Confers Transplant Arteriosclerosis.
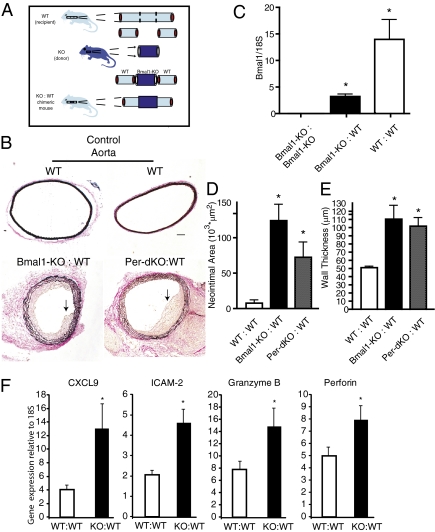
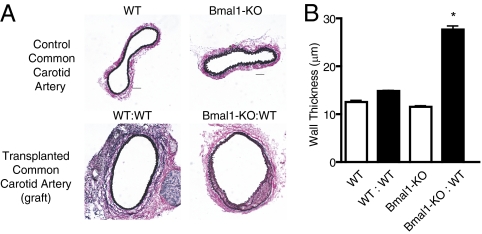
The converse studies revealed a striking and remarkable difference in response. When circadian clock gene mutant aortae were transplanted into WT littermates (Fig. 3A), the grafted vessels with tissue-intrinsic dysfunctional clocks exhibited a robust pathological response with perivasculitis and intimal hyperplasia (Fig. 3B) characteristic of transplant arteriosclerosis (21). To determine whether host cells were infiltrating the graft, Bmal1-KO grafts transplanted for 2 wk were analyzed for Bmal1 expression by RT-PCR, given that the presence of undisrupted Bmal1 mRNA in Bmal1-KO mice would necessarily be derived from the recipient WT mouse. Indeed, WT:WT grafts exhibited a strong signal for Bmal1, whereas Bmal1-KO grafts transplanted in Bmal1-KO mice (Bmal1-KO:Bmal1-KO) exhibited no detectable expression (Fig. 3C). However, Bmal1 expression was strong in Bmal1-KO grafts (Fig. 3C) isolated from WT mice (Bmal1-KO:WT), providing evidence of host cell infiltration of the donor graft. Established endpoints of transplant arteriosclerotic disease (22) including lesion development in the form intimal hyperplasia (Fig. 3D) and wall thickening (Fig. 3E), were observed in both Bmal1-KO:WT and Per-dKO:WT transplants due to an increased hyperplastic response in the circadian clock-disrupted arteries, as evidenced by increased proliferating cell nuclear antigen staining (Fig. S2). As occurred when the aorta was anastamosed to the common carotid artery in the size-mismatch approach, a pathological response was also observed when size-matched vessels were transplanted from Bmal1-KO to WT mice (common carotid artery to common carotid artery; Fig. 4). Common carotid artery grafts from Bmal1-KO homotopically grafted into the common carotid artery of WT mice exhibited robust wall thickening, whereas WT:WT common carotid grafts were indistinguishable from naïve common carotid arteries (Fig. S3), suggesting that the hemodynamic stress caused by the size mismatch was not in itself responsible for the arteriosclerotic response.
Fig. 3.
Local dysfunction in the vascular clock mediates transplant arteriosclerosis. (A) Donor aortic segments from either Bmnal1-KO or Per-dKO mice were grafted to littermate control WT mice. (B) Van Gieson staining revealed the development of intraluminal lesions (black arrows) in Bmal1-KO:WT and Per-dKO:WT aortic grafts. (Bar: 100 μm.) (C) RNA was isolated from aortic grafts harvested 2 wk after transplantation and analyzed by quantitative RT-PCR by the ΔΔCt method. Bmal1 message was absent in Bmal1-KO:Bmal1:KO grafts (KO:KO), whereas Bmal1 expression was present in WT:WT transplants, using a primer strategy that was designed to not amplify in the Bmal1-KO. Bmal1-KO grafts that were in WT hosts expressed Bmal1, providing evidence of infiltrating WT host cells in the arterial graft of the Bmal1-KO. *P < 0.05; Student's unpaired t test. (D and E) Quantitative analysis of grafts confirmed the development of a prominent lesion/neointima (D) and an increase in wall thickness (E) in KO:WT transplants (n = 10, WT:WT; n = 10, Bmal1-KO:WT; n = 5, Per-dKO:WT; *P < 0.05). (F) RNA was further screened by RT-PCR for markers of which were found to be up-regulated in the Bmal1-KO:WT grafts (KO:WT) (n = 10, WT:WT; n = 9, KO:WT; *P < 0.05; Student's unpaired t test).
Fig. 4.
Vasculopathy is independent of size-mismatch grafting. (A) Donor common carotid artery segments from either WT or Bmal1-KO mice were grafted to littermate control WT mice. Wall thickness was increased in Bmal1-KO:WT common carotid artery grafts, whereas WT:WT homotopic grafts were indistinguishable from naïve common carotid arteries. (Bar: 50 μm.) (B) Although there was no difference in lumen diameter groups, a significant increase in wall thickness was observed in Bmal1-KO:WT carotid grafts (n = 8, WT:WT; n = 8, Bmal1-KO:WT; *P < 0.001).
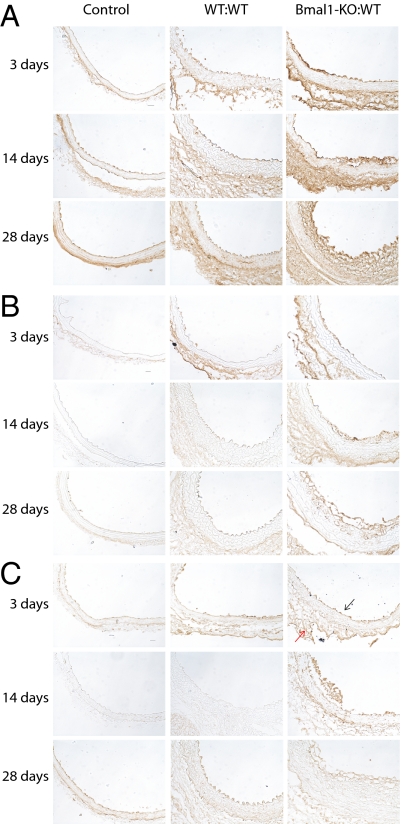
Macrophage Infiltration and T-Cell Receptor Activation Are Selectively Induced in Bmal1-KO to WT Transplants.
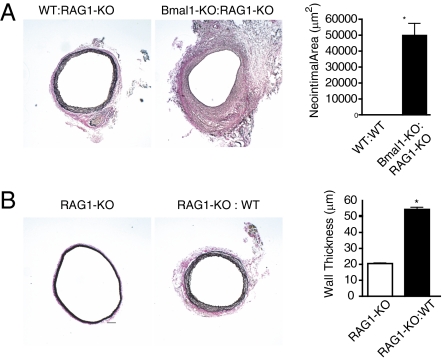
Evidence for an inflammatory and immune response was apparent within the vasculature of Bmal1-KO mice grafted into WT mice. Quantitative PCR revealed up-regulation of the chemokine CXCL-9 that is involved in the graft arteriosclerotic response (23) and the inflammatory adhesion molecule ICAM-2 that has been associated with T-cell clearance (24), both of which exhibit a circadian rhythm in vascular tissue (25). In addition, granzyme B and perforin were up-regulated in Bmal1-KO:WT grafts that are degradatory enzymes released by cytotoxic T cells (Fig. 3F). Moreover, immunohistochemistry revealed that Bmal1-KO:WT grafts exhibited macrophage accumulation that was evident within 3 d of grafting and sustained to 28 d later in both endothelium and adventitia of the aorta (Fig. 5 A and B). Macrophage accumulation was further accompanied by sensitization of the endothelium to T cells (Fig. 5C). However, B- and T-cell infiltration did not influence the response, because transplant arteriosclerosis persisted in Bmal1-KO aortic grafts placed in recipient recombination-activating gene knockout (RAG-1-KO) mice (Fig. 6A and Fig. S4 A and B), which lack mature B and T cell lymphocytes (26). The effect was specific to Bmal1 disruption, as RAG-KO transplants grafted into WT mice exhibited no transplant arteriosclerosis (Fig. 6B and Fig. S4C). These data demonstrate a significant and intrinsic tissue function of circadian clocks to condition transplant arteriosclerosis.
Fig. 5.
Macrophage infiltration and T-cell receptor activation are selectively induced in Bmal1-KO to WT transplants. Aortic transplants were assessed at 3, 7, and 28 d after transplant for markers of rejection including the macrophage markers CD68 (A) and F4/80 (B) and the T-cell receptor antigen CD3 (C) by immunohistochemistry. (Bar: 20 μm.) Positive staining was evident in the endothelium (black arrow) and adventitia (red arrow) of Bmal1-KO:WT aortic grafts.
Fig. 6.
Transplant arteriosclerosis in Bmal1-KO grafts persists in RAG1-KO recipient mice. (A) Donor aortic segments from either WT or Bmal1-KO mice were grafted to recipient recombination-activating gene knockout (RAG-1-KO) mice. Robust intraluminal lesions (Left) persisted in Bmal1-KO:RAG-1-KO aortic grafts. (Bar: 100 μm.) There was a significant difference in increment of structural adaptation between the WT:RAG-1-KO and Bmal1-KO RAG-1-KO, indexed as neointimal area (Right) (n = 6, WT; n = 6, Bmal1-KO; n = 6, WT:RAG-1-KO; n = 6, Bmal1-KO:RAG-1-KO; *P < 0.05). (B) To test whether lesion formation was generic to other knockout mice, donor aortic segments from RAG-1-KO mice were grafted to recipient WT mice. RAG-KO grafts exhibited no evidence of lesion formation (Left), but exhibited the typical increase in wall thickness (Right) caused by heterotopic placement of the aorta to the common carotid artery (n = 6, RAG-1-KO; n = 6, RAG-1-KO:WT; *P < 0.05). (Bar: 100 μm.)
Discussion
The circadian clock exerts a significant influence on systemic homeostasis. Ex vivo, peripheral tissues lose circadian rhythm over time more quickly than SCN explants, suggesting that the central clock entrains circadian oscillators in the periphery (2). Locomotor rhythm (3, 27–29), glucose and lipid metabolism (5, 6, 8, 14), and blood pressure (9, 30–32) are altered in mice with global circadian clock gene mutation, which has an array of secondary and indirect influences in the periphery. In the present studies, WT arteries, having an intact clock, were not affected when chronically immersed in a systemic milieu of global circadian clock dysfunction. Moreover, RAG-KO grafts transplanted into WT mice also exhibited no signs of arteriosclerosis, suggesting that neither the transplant procedure nor indiscriminate gene disruption, per se, were sufficient to induce vasculopathy. Only when circadian clock gene mutation was intrinsic to the grafted blood vessels were pathological responses incurred. Although it is surprising that global clock dysfunction did not impair the local tissue response, the observation further emphasizes the intrinsic importance of peripheral clocks in long-term tonicity that may (when perturbed) contribute to disease. Indeed, with regard to tissue-specific and intrinsic circadian gene oscillation, it is known that discrete peripheral tissues types can maintain rhythm after SCN lesion (33), whereas genetic rescue of the central clock selectively restores peripheral circadian clock oscillations and physiology (10), perhaps reflecting tissue-specific differences in circadian clock dynamics (34). The present observations demonstrate the intrinsic significance of tissue clocks in chronic pathology and suggest that peripheral disease related to central circadian dysfunction may not be a simple secondary consequence, but may also relate to direct peripheral tissue circadian clock dysfunction.
Numerous mechanisms have been implicated in transplant arteriosclerosis, including perivascular inflammation (21), humoral immunity (35), and vascular cell activation (36). Circadian rhythms are an important influence in these facets of defense (37, 38) and are additionally implicated in conditioning responses during human organ transplants. In humans, aberrations in the circadian rhythm in human blood pressure have been described after kidney transplants (39, 40). Moreover, recent human studies suggest that the time of day conditions the success in liver transplant patients, with nighttime operative start times being prone to worse outcome (41). Our data directly implicate the circadian clock mechanism to condition transplant arteriosclerosis, buttressed by the congruence of phenotype in mice mutated in both activators (Bmal1) and repressors of the circadian clock (Per) and underscoring the significance of the oscillatory function per se of this unique signaling mechanism (34). Further, the arteriosclerotic response conferred by the circadian clock in grafts was also independent of hemodynamics and B and T cell-mediated immunity, demonstrating the tissue-intrinsic significance of the circadian clock in transplant vasculopathy.
A salient feature of our approach is the use of experimental transplantation to dissect global effects from local tissue influences in gene function. The ability to examine gene dysfunction in a finite and local segment of tissue limits the systemic disturbances induced by gene disruption. Indeed, even tissue-specific deletion strategies exhibit complex secondary effects, with disadvantages including a lack of appropriate cell specificity and genetic compensation, which are both magnified during development of the organism. For example, blood vessel (endothelial cell)-specific deletion of Bmal1 has an unexpected, albeit subtle, effect on locomotor activity (9), which may be a consequence of altered endothelial signaling (12, 42, 43) (that is the summation of an impairment across the entire vascular network) while endothelial-specific targeting in itself as influences on circulating progenitor cells (11). These data demonstrate the significance of tissue circadian clocks as an autonomous influence in experimental models of arteriosclerotic disease, which may have implications with regard to the influence of circadian clock function in organ transplantation.
Materials and Methods
See SI Materials and Methods for detailed descriptions.
Mice.
All animal studies were performed according to protocols approved by the Georgia Health Sciences University Institutional Committee for Use and Care of Laboratory Animals. Male, littermate control WT , Period-2 Luciferase transgenic (Per-Luc), Bmal1-KO (Jackson Laboratories), RAG1-KO (Jackson Laboratories), and Per-2,3 double-knockout (Per-dKO) mice were housed under standard 12-h light/dark conditions. For syngeneic (isograft) transplant studies, littermate controls were used in Bmal1-KO (congenic C57BL/6J) and Period isoform knockout mice, respectively; only male mice were used as donors and recipients.
Transplant Model.
We implemented two models of arterial grafting, a size-mismatch (heterotopic) and size-match (homotopic) approach. To stimulate remodeling, we intentionally performed heterotopic transplants (aorta to common carotid artery) as described (18, 19). To test whether the response was cause by heterotopic placement or size mismatch of the artery, we also implemented a homotopic, size-match approach, where common carotid artery was grafted to common carotid artery, which caused no remodeling in control blood vessels. Grafts were harvested 4 wk after transplant and analyzed as described in SI Materials and Methods.
Histomorphometry.
Four weeks after transplantation, mice were anesthetized, exsanguinated, and perfused via the left ventricle with physiologic saline. Mice were subsequently perfusion fixed with neutral buffered formalin. Both control, native aortae in the thoracic cavity and grafted aortae were carefully excised and postfixed either overnight for morphometric studies or immediately embedded in frozen medium for cryotome processing. Morphometric analysis of the large arteries was performed by using video microscopy as described. Perimeter (p) of the vessel lumen was taken as the circumference (C) of a circle, and lumen diameter (D) was determined from the equation D = C/p, assuming that the vessel cross-sections were circular in vivo. To determine thrombus area, the internal elastic lamina (IEL) and patent lumen were circumscribed to derive a radius (R) value from the formula R = 2C/p, and then IEL area and luminal area (A) were calculated by using the formula A = пR2. Lesion/neointimal area was derived from the difference of IEL area and lumen area.
Statistical Analysis.
The significance of differences was assessed by two-way ANOVA to test for all sources of variation unpaired t tests as indicated. Concentration response curves of vascular function were analyzed by t tests, repeated-measures two-way, and one-way ANOVA with a Bonferonni correction. Differences were considered significant when P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. David Weaver for providing Period isoform transgenic mice. This work was supported in part by National Institutes of Health Grant HL089576 (to R.D.R.) and American Recovery and Reinvestment Act Grant Supplement HL089576-01A1S1 (to R.D.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112998108/-/DCSupplemental.
References
- 1.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 3.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitaterna MH, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudic RD, Fulton DJ. Pressed for time: The circadian clock and hypertension. J Appl Physiol. 2009;107:1328–1338. doi: 10.1152/japplphysiol.00661.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westgate EJ, et al. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 10.McDearmon EL, et al. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science. 2006;314:1304–1308. doi: 10.1126/science.1132430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 12.Anea CB, et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunger MK, et al. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- 14.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudic RD, et al. COX-2-derived prostacyclin modulates vascular remodeling. Circ Res. 2005;96:1240–1247. doi: 10.1161/01.RES.0000170888.11669.28. [DOI] [PubMed] [Google Scholar]
- 17.Tereb DA, et al. Human T cells infiltrate and injure pig coronary artery grafts with activated but not quiescent endothelium in immunodeficient mouse hosts. Transplantation. 2001;71:1622–1630. doi: 10.1097/00007890-200106150-00023. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich H, et al. Mouse model of transplant arteriosclerosis: Role of intercellular adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2000;20:343–352. doi: 10.1161/01.atv.20.2.343. [DOI] [PubMed] [Google Scholar]
- 19.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng B, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 21.Hillebrands JL, et al. Origin of neointimal endothelium and alpha-actin-positive smooth muscle cells in transplant arteriosclerosis. J Clin Invest. 2001;107:1411–1422. doi: 10.1172/JCI10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 23.Hancock WW, et al. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter JC, Hall A. Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB J. 2009;23:492–502. doi: 10.1096/fj.08-115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudic RD, et al. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- 26.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 27.Cermakian N, Monaco L, Pando MP, Dierich A, Sassone-Corsi P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 2001;20:3967–3974. doi: 10.1093/emboj/20.15.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley CA, et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 29.van der Horst GT, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 30.Curtis AM, et al. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol. 2005;566:213–224. doi: 10.1113/jphysiol.2005.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanida M, et al. Autonomic and cardiovascular responses to scent stimulation are altered in cry KO mice. Neurosci Lett. 2007;413:177–182. doi: 10.1016/j.neulet.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 33.Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudic RD. Time is of the essence: vascular implications of the circadian clock. Circulation. 2009;120:1714–1721. doi: 10.1161/CIRCULATIONAHA.109.853002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi C, et al. Immunologic basis of transplant-associated arteriosclerosis. Proc Natl Acad Sci USA. 1996;93:4051–4056. doi: 10.1073/pnas.93.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell RN, Libby P. Vascular remodeling in transplant vasculopathy. Circ Res. 2007;100:967–978. doi: 10.1161/01.RES.0000261982.76892.09. [DOI] [PubMed] [Google Scholar]
- 37.Castanon-Cervantes O, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fildes JE, Yonan N, Keevil BG. Melatonin—a pleiotropic molecule involved in pathophysiological processes following organ transplantation. Immunology. 2009;127:443–449. doi: 10.1111/j.1365-2567.2009.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumgart P, et al. Blood pressure elevation during the night in chronic renal failure, hemodialysis and after renal-transplantation. Nephron. 1991;57:293–298. doi: 10.1159/000186278. [DOI] [PubMed] [Google Scholar]
- 40.McGregor DO, Olsson C, Lynn KL. Autonomic dysfunction and ambulatory blood pressure in renal transplant recipients. Transplantation. 2001;71:1277–1281. doi: 10.1097/00007890-200105150-00016. [DOI] [PubMed] [Google Scholar]
- 41.Lonze BE, et al. Operative start times and complications after liver transplantation. Am J Transplant. 2010;10:1842–1849. doi: 10.1111/j.1600-6143.2010.03177.x. [DOI] [PubMed] [Google Scholar]
- 42.Viswambharan H, et al. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 43.Wang CY, et al. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–2173. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.