Abstract
Under feeding conditions, the incretin hormone GLP-1 promotes pancreatic islet viability by triggering the cAMP pathway in beta cells. Increases in PKA activity stimulate the phosphorylation of CREB, which in turn enhances beta cell survival by upregulating IRS2 expression. Although sustained GLP-1 action appears important for its salutary effects on islet function, the transient nature of CREB activation has pointed to the involvement of additional nuclear factors in this process. Following the acute induction of CREB-regulated genes, cAMP triggers a second delayed phase of gene expression that proceeds via the HIF transcription factor. Increases in cAMP promote the accumulation of HIF1α in beta cells by activating the mTOR pathway. As exposure to rapamycin disrupts GLP-1 effects on beta cell viability, these results demonstrate how a pathway associated with tumor growth also mediates salutary effects of an incretin hormone on pancreatic islet function.
Keywords: islet cell, insulin
The incretin hormone GLP-1 enhances islet cell survival through induction of the cAMP pathway in beta cells (1, 2). In turn, cAMP signaling stimulates the phosphorylation and activation of CREB, leading to increases in gene expression that promote beta cell viability. Disruption of CREB activity, through transgenic expression of dominant negative CREB inhibitors in beta cells, leads to hyperglycemia and diabetes, due in part to decreases in pancreatic islet mass and to consequent reductions in circulating insulin concentrations (3, 4).
CREB has been found to promote islet cell survival by upregulating insulin receptor substrate 2 (IRS2) gene expression and thereby activating the Ser/Thr kinase AKT. IRS2-AKT signaling appears critical for GLP-1 action; pancreatic islet cells from mice with a knockout of the IRS2 gene are resistant to growth promoting effects of GLP-1 agonist (5). Conversely, IRS2 overexpression in islet cells appears sufficient to improve islet mass and glucose homeostasis in a mouse model of diabetes (6).
GLP-1 stimulates the expression of IRS2 and other genes in part via the PKA-mediated phosphorylation of CREB at Ser133 (7). CREB promotes target gene expression with burst-attenuation kinetics; rates of transcription peak within 1 h and decrease to near baseline levels after 4 h, paralleling the phosphorylation and subsequent dephosphorylation of CREB. By contrast with the transient kinetics of CREB activation, however, sustained GLP-1 action appears important for its salutary effects on islet function (8), pointing to the involvement of additional transcriptional regulators in this process.
Here we show that GLP-1 promotes two phases of cAMP-dependent gene expression in beta cells: an acute CREB-mediated phase and a delayed phase that proceeds via the hypoxia-inducible factor (HIF). GLP-1 was found to increase HIF activity through induction of the IRS2-AKT pathway and through the subsequent activation of the Ser/Thr kinase mTOR, a central regulator of cell growth and proliferation (9, 10). Based on its ability to stimulate oxidative stress defense and metabolic programs that enhance beta cell viability, we propose that HIF mediates long-term effects of GLP-1 on pancreatic islet function.
Results
cAMP Stimulates CREB and HIF Pathways in β Cells.
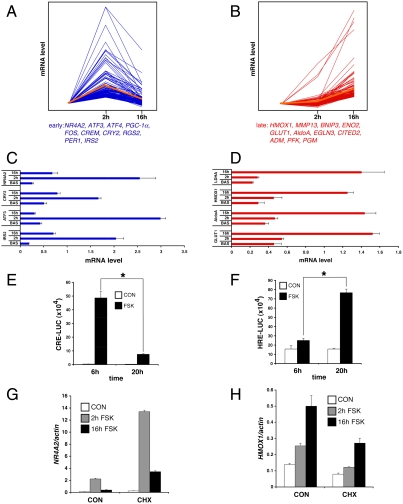
We performed gene profiling studies to evaluate effects of the GLP-1 -cAMP pathway in INS-1 insulinoma cells. Short-term (2 h) exposure to the cAMP agonist Forskolin (FSK) up-regulated a number of CREB target genes that contain a conserved cAMP response element (CRE) and that are CREB-occupied in vivo (IRS2, RGS2, PGC1, NR4A2) (11); these were down-regulated after prolonged (16 h) treatment with FSK (Fig. 1A and Fig. S1).
Fig. 1.
Serial Induction of CREB and HIF pathways by cAMP in beta cells. (A and B) Profiles of mRNA accumulation for early (A) and late (B) cAMP inducible genes in INS-1 insulinoma cells exposed to FSK for 2 or 16 h, respectively. Representative genes up-regulated in response to FSK indicated below for each group. (C and D) Q-PCR analysis of early (C) and late (D) cAMP responsive genes in primary mouse islets exposed to FSK for 2 or 16 h. (E and F) Transient assay of CREB (E) and HIF (F) reporter activity in INS-1 cells exposed to FSK for 6 or 20 h. (G and H) Effect of cycloheximide (CHX) on mRNA amounts for early (NR4A2) and late (HMOX1) cAMP response genes in INS-1 cells. (*; P < 0.05). Data are means ± s.d.
We detected a second set of genes that were expressed at low levels at 2 h but increased after 16 h exposure to FSK, when CREB target genes are down-regulated (Fig. 1B and Fig. S1). Many of these late response genes correspond to hypoxia-inducible genes by GO analysis; they code for proteins that promote glucose uptake and glycolysis (GLUT1, AldoA, TPI) as well as oxidative stress defense (HMOX1). Similar to its effects in INS-1 cells, exposure to FSK stimulated two waves of gene expression in cultured mouse pancreatic islets, with CREB target genes predominating at early times followed by hypoxia-inducible genes at later times (Fig. 1 C and D).
Based on these profiles, we suspected that cAMP mediates induction of two distinct transcription pathways in beta cells. Supporting this notion, exposure to FSK increased the activity of a CRE-Luc reporter in INS-1 cells after 6 h but less so after 20 h (Fig. 1E). By contrast, the activity of a hypoxia-inducible factor (HIF) reporter (HRE-luc) was low at early times (6 h) but increased after 20 h (Fig. 1F). Consistent with a role for cAMP in this process, exposure to phospho-diesterase inhibitor IBMX also increased HRE-luc reporter activity in INS-1 cells (Fig. S2). The effects of FSK and IBMX on HIF activity appear to be PKA-dependent because exposure to the PKA antagonist H89 blocked induction of the HRE-luc reporter by FSK as well as IBMX (Fig. S2).
We imagined that the two phases of cAMP inducible transcription in beta cells may have different requirements for new protein synthesis. Treatment with the protein synthesis inhibitor cycloheximide (CHX) has been found to increase CREB target gene expression (12), for example. By contrast with these effects on CREB-dependent transcription, exposure to CHX disrupted the induction of late phase genes like HMOX1 in response to FSK (Fig. 1 G and H).
GLP-1 Stimulates HIF Activity via Induction of mTOR.
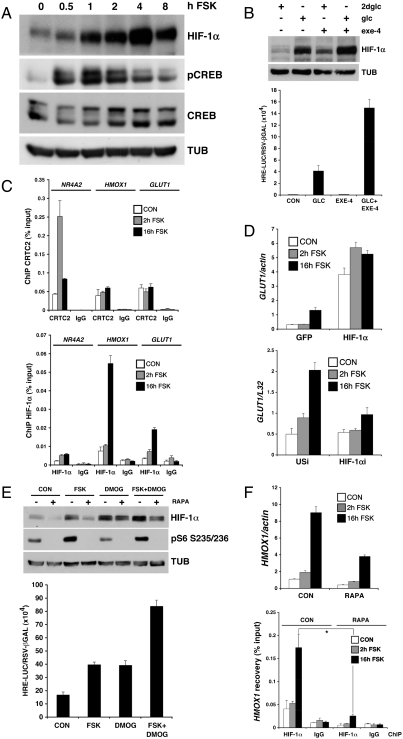
Originally identified as an oxygen-sensing heterodimer that contains an unstable alpha subunit and a constitutively expressed beta subunit, HIF induces metabolic reprogramming in response to hypoxia and growth factor signaling (13). Hypoxia has been shown to increase HIF1α stability, whereas growth factors appear to increase the translation of HIF1α mRNA (14). Exposure of INS-1 cells to FSK increased HIF1α protein but not mRNA levels to maximal levels after 4–8 h (Fig. 2A and Fig. S1). Similar to FSK, prolonged exposure to GLP-1 agonist (Exendin-4) also increased HIF1α protein accumulation and HRE-luc reporter activity in INS-1 cells, particularly under high glucose (20 mM) conditions, when GLP-1 is active (Fig. 2B).
Fig. 2.
GLP-1 stimulates HIF activity via induction of mTOR. (A) Immunoblots showing time course of HIF1α and phospho (Ser133) CREB accumulation in INS-1 cells exposed to FSK. (B) Effect of GLP-1 agonist Exendin-4 and 20 mM glucose or 2-deoxy glucose (2dglc), alone and in combination, on HIF1α protein amounts (top) and on HRE-luciferase reporter activity (bottom) in INS-1 cells. (C) Chromatin immunoprecipitation (ChIP) assay showing effect of FSK on recruitment of the CREB coactivator CRTC2 (top) or HIF1α (bottom) to early (NR4A2) and late (HMOX1, GLUT1) cAMP responsive genes in INS-1 cells. (D) Effect of HIF1α overexpression (top) or RNAi-mediated depletion (bottom) on GLUT1 mRNA amounts in INS-1 cells exposed to FSK for 2 or 16 h. (E) Effect of FSK and DMOG, alone or together, on HIF1α and phospho-S6 protein amounts (top) and on HRE-luc reporter activity (bottom) in INS-1 cells. Exposure to rapamycin indicated. (F) Effect of rapamycin on HMOX1 mRNA amounts (top) and on recruitment of HIF1α to the HMOX1 promoter (bottom) in INS-1 cells exposed to FSK.
By contrast with the delayed induction of HIF, FSK exposure promptly increased CREB phosphorylation after only 1 h, returning to near baseline after 4 h (Fig. 2A). Indeed, short-term treatment also enhanced recruitment of the CREB coactivator CRTC2 (15) to early (NR4A2) but not late phase (HMOX1, GLUT1) genes by chromatin immunoprecipitation (ChIP) assay (Fig. 2C). Conversely, prolonged exposure to FSK increased the occupancy of HIF1α over late (HMOX1 and GLUT1) but not early (NR4A2) promoters.
We wondered whether the slow accumulation of HIF1α protein accounts for the delayed kinetics of HIF target gene expression in INS-1 cells. Supporting this idea, overexpression of HIF1α up-regulated HIF target gene expression even in the absence of cAMP agonist, while RNAi-mediated knockdown of HIF1α blocked it (Fig. 2D). Collectively, these studies indicate that activation of the cAMP pathway by GLP-1 triggers early and late phases of gene expression, which are coordinated by CREB and HIF, respectively.
Based on the ability for mTOR to stimulate HIF1α translation in response to nutrient and growth factor signals (14), we examined whether cAMP activates this pathway in beta cells. Exposure of INS-1 cells to FSK increased the phosphorylation of the ribosomal protein S6, a downstream target of the mTORC1 complex, which contains mTOR, mLST8, PRAS40, and Raptor (Fig. 2E). Consistent with the proposed role of mTORC1, FSK treatment increased the activity of a HIF1α-luc translational reporter (16) containing the 5′UTR of HIF1α (Fig. S3); these effects were blocked by cotreatment with the mTORC1 inhibitor rapamycin.
Exposure to rapamycin also disrupted S6 phosphorylation and HIF1α accumulation in INS-1 cells exposed to FSK (Fig. 2E). By contrast, triggering of the hypoxia pathway with the prolyl hydroxylase inhibitor Dimethyloxalyl Glycine (DMOG) increased HIF1α but not phospho-S6 protein amounts in INS-1 cells; and rapamycin did not attenuate DMOG-induced HIF1α accumulation (Fig. 2E). Rather, costimulation with DMOG and FSK increased HRE-luc reporter activity additively, suggesting that cAMP and hypoxia pathways stimulate HIF1α via distinct mechanisms (Fig. 2E).
We tested whether the cAMP-dependent induction of late phase genes in islet cells proceeds via mTORC1. Consistent with its inhibitory effects on HIF1α accumulation, exposure to rapamycin blocked the recruitment of HIF1α to the HMOX1 promoter by ChIP assay of INS-1 cells (Fig. 2F). Correspondingly, rapamycin also attenuated the FSK-dependent upregulation of HMOX1 mRNA, but it had no effect on the induction of CREB target genes such as NR4A2 (Fig. S3). These results suggest that mTOR mediates the effects of cAMP on induction of late but not early phase genes in INS-1 cells.
Although cAMP functions as a senescence signal in most cells, it promotes the growth and proliferation of a small subset of endocrine cells (17, 18), prompting us to test whether the effects of cAMP on HIF activity are cell context-dependent. By contrast with its stimulatory effects in INS-1 cells, exposure to FSK had no effect on HIF1α accumulation or HRE-luc reporter activation in cultured primary hepatocytes (Fig. S4). Arguing against general differences in HIF1α inducibility, exposure to DMOG triggered HIF1α accumulation and HRE reporter activation comparably in hepatocytes and INS-1 cells (Fig. S4). Taken together, these results indicate that the effects of cAMP on the HIF pathway are indeed cell-type restricted.
cAMP Promotes mTOR Activation via Induction of IRS2-AKT Signaling.
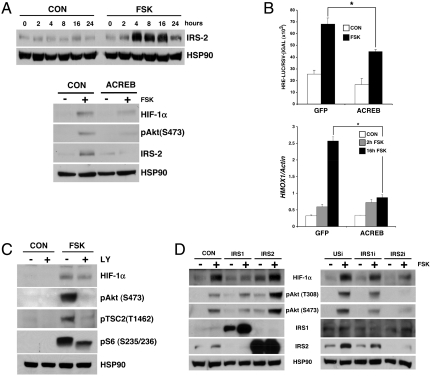
Realizing that the Ser/Thr kinase AKT stimulates mTOR activity and that CREB up-regulates IRS2 expression in beta cells (3), we wondered whether cAMP enhances mTOR activity in part via induction of the IRS2-AKT pathway. Exposure to FSK triggered IRS2 accumulation as well as AKT activation in INS-1 cells (Fig. 3A). Disrupting CREB activity, by overexpression of the CREB inhibitor A-CREB, blocked IRS2-AKT pathway activation and HIF1α accumulation (Fig. 3A). Correspondingly, A-CREB overexpression also disrupted HRE-luc reporter activation and HIF target gene induction in cells exposed to FSK (Fig. 3B).
Fig. 3.
cAMP stimulates mTOR via CREB-dependent increases in IRS2-AKT signaling. (A) Top: Immunoblot showing IRS2 protein amounts in INS-1 cells exposed to FSK. Bottom: Immunoblot showing effect of dominant negative A-CREB expression on IRS2-AKT signaling and HIF1α accumulation in INS-1 cells. (B) Effect of A-CREB expression on HRE-luc activity (top) and HMOX1 mRNA levels (bottom) in INS-1 cells exposed to FSK (*, P < 0.01. Data are means ± s.d.). (C) Immunoblot showing effects of FSK on AKT activation and TSC2 phosphorylation in INS-1 cells exposed to FSK for 16 h. Effects of PI3-kinase inhibitor LY294002 (LY) indicated. (D) Immunoblots showing effects of IRS1 and IRS2 overexpression (left) or RNAi-mediated knockdown (right) on AKT activation and HIF1α accumulation in INS-1 cells. Exposure to FSK indicated.
AKT has been found to enhance mTOR activity by phosphorylating the GTPase activating protein TSC2, an inhibitor of the small G protein Rheb, an mTOR activator (9). Amounts of phospho (Thr 1462) TSC2 were up-regulated in INS-1 cells exposed to FSK (Fig. 3C); these effects were blocked when AKT activity was inhibited by coincubation with the PI3 kinase inhibitor LY294002 (LY). The phosphorylation of TSC2 in INS-1 cells appears important for the subsequent induction of mTORC1 by FSK because treatment with LY inhibitor also reduced phospho-S6 protein amounts in cells exposed to FSK (Fig. 3C). LY inhibitor had more modest effects on HIF1α accumulation, however, suggesting the presence of additional regulatory contributions in this setting.
If IRS2 regulates mTOR activity, then altering cellular IRS2 protein amounts should correspondingly modulate HIF1α protein levels. Overexpression of IRS2 but not IRS1 in INS-1 cells potentiated AKT activity and HIF1α accumulation (Fig. 3D). Conversely, RNAi-mediated knockdown of IRS2 decreased effects of FSK on the upregulation of AKT and HIF1α. By contrast, IRS1 knockdown, albeit less efficient, did not interfere with AKT or HIF1α. Collectively, these results indicate that the acute activation of the IRS2-AKT pathway by CREB is required for subsequent induction of the HIF pathway by mTOR in response to cAMP signaling.
mTOR-HIF Pathway Mediates GLP-1 Effects on β Cell Viability.
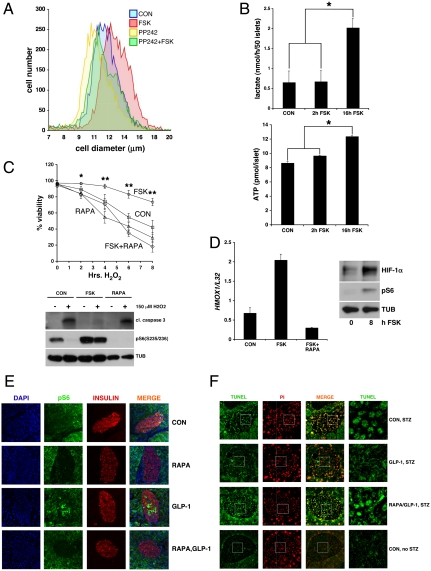
Realizing that cAMP stimulates mTOR activity and that mTOR regulates beta cell size (19, 20), we considered whether cAMP promotes beta cell growth via this pathway. Supporting this notion, FSK treatment increased the average diameter of INS-1 cells (Fig. 4A); coincubation with a direct inhibitor of mTOR (PP242) blocked effects of FSK on cell size and on S6 phosphorylation as well as HIF1α induction (Fig. 4A and Fig. S5).
Fig. 4.
The mTOR-HIF pathway mediates effects of GLP-1 on islet cell viability. (A) Effect of FSK on cell size in INS-1 cells exposed to FSK. Effect of mTOR inhibitor PP242 shown. (B) Effect of short-term (2 h) or long-term (16 h) FSK treatment on glycolytic flux, measured by lactate production (top) and on ATP generation (bottom) in primary cultured islets. (*; P < 0.05. Data are means ± s.d.) (C) Effects of FSK pretreatment (16 h) on INS-1 cell survival following exposure to oxidative stress (150 μM H2O2). Cell viability monitored by trypan blue exclusion (top) and by immunoblot for cleaved caspase-3 protein amounts (bottom). Effect of rapamycin indicated. (*, P < 0.05; **, P < 0.01. Data are means ± s.d.) (D) Left: Effect of rapamycin on HMOX1 mRNA amounts in pancreatic islets exposed to FSK. Right: Immunoblot showing effect of FSK on HIF1α and phospho-S6 protein amounts in primary cultures of mouse pancreatic islets. (E and F) Immunohistochemical analysis showing effects of hepatic Ad-GLP-1 expression on S6 phosphorylation (E) and STZ-induced apoptosis (F) in pancreatic islets of ad libitum fed mice. Administration of rapamycin indicated.
HIF has been shown to promote cell growth by stimulating glycolysis and by decreasing mitochondrial oxidative metabolism (13). This metabolic shift—referred to as the Warburg effect—is thought to promote the accumulation of biomass associated with tumor growth while reducing reactive oxygen species that often accompany increases in metabolic activity (21). We wondered whether triggering of the cAMP pathway promotes islet viability in part by reprogramming the metabolic activity of beta cells. Consistent with this idea, exposure of primary cultured islets to FSK increased lactate accumulation and secretion, measures of glycolysis, and HIF activity in islets (22); as a result, FSK treatment also led to increases in intracellular ATP concentrations (Fig. 4B).
Having seen that HIF stimulates the expression of stress defense genes, we tested whether activation of the cAMP pathway protects beta cells against oxidative stress. Treatment of INS-1 cells with hydrogen peroxide increased beta cell death by trypan blue exclusion and by measurement of cleaved caspase 3 amounts (Fig. 4C). Preincubation with FSK protected against cell death; these effects were reversed when mTORC1 activity was inhibited with rapamycin. In keeping with these results in INS-1 cells, exposure of cultured primary islets to FSK also increased HIF1α accumulation and HMOX1 expression in primary cultures of pancreatic islets; these effects were blocked by coincubation with rapamycin (Fig. 4D).
We examined whether GLP-1 also regulates the mTOR pathway in vivo. Adenoviral expression of GLP-1 (Ad-GLP-1) in liver increased circulating levels of GLP-1, leading to corresponding decreases in circulating glucose concentrations relative to control (Ad-GFP) mice (Figs. S6 and S7). Phospho-S6 staining—nearly undetectable in control mice—was substantially up-regulated in pancreatic beta cells from mice expressing Ad-GLP-1 (Fig. 4E). The effects of Ad-GLP-1 on S6 phosphorylation appear mTOR-dependent because intraperitoneal (IP) injection of rapamycin blocked GLP-1-dependent increases in phospho-S6 staining (Fig. 4E).
Based on the ability for Ad-GLP-1 to stimulate the mTOR pathway in vivo, we tested whether mTOR-HIF induction protects against the development of diabetes following administration of streptozotocin (STZ). Although absent from islets of control mice, beta cell apoptosis increased markedly in STZ-treated animals, leading to increases in circulating blood glucose concentrations (Fig. 4F and Fig. S7). Remarkably, Ad-GLP-1 expression blocked STZ-dependent increases in beta cell apoptosis and circulating glucose concentrations; these effects were reversed when rapamycin was coadministered (Fig. 4F and Fig. S7). Taken together, these experiments indicate that GLP-1 enhances islet function through activation of the mTOR-HIF pathway.
Discussion
Current evidence indicates that HIF exerts a supportive role in islet function, although the mechanisms by which HIF activity are modulated in beta cells have not been addressed. Pancreatic islet expression of HIFβ/ARNT is decreased in human diabetes, for example (23). Moreover, mice with a knockout of HIF-1α in islets display glucose intolerance and beta cell dysfunction, due in part to the decreased expression of glycolytic genes (24). Indeed, glycolytic flux has been found to play a key role in beta cell proliferation, providing a direct link between this HIF-regulated pathway and the maintenance of islet mass (25). The degree of HIF activation appears critical, however; unbridled HIF induction in mice with a knockout of the E3 ligase Von Hippel-Lindau (VHL) leads to persistent defects in glucose-stimulated insulin secretion (26).
Our results indicate that GLP-1 promotes islet viability through the upregulation of HIF1α. GLP-1 stimulates HIF1α accumulation via the cAMP-mediated induction of the mTOR pathway in beta cells. cAMP appears to trigger mTOR activation in part via the CREB-mediated induction of IRS2-AKT signaling. Additional regulatory inputs appear likely, as the effects of cAMP on mTOR activity are cell-type dependent. Although the underlying mechanism remains unclear, we imagine that PKA may regulate additional mTOR regulators that are selectively expressed in beta cells and perhaps other endocrine cell types. The identification of these factors should provide further insight into the mechanisms by which GLP-1 promotes pancreatic islet viability.
Materials and Methods
Full details of methods are in SI Text.
Immunoblot and Immunoprecipitation.
Immunoblots were performed as described (27). For immunoprecipitations, cell lysates were incubated with primary antibody and 25 μl of a 50% slurry of protein G agarose beads for 4 h with rotation at 4 °C. Immunoprecipitates were washed with lysis buffer and denatured by boiling in 20 μl SDS sample buffer.
Immunofluorescence.
Whole pancreases were fixed in 4% paraformaldehyde, fresh-frozen, and cryosectioned. Sections were incubated with primary antibody overnight and with fluorophore conjugated secondary antibody for 1 h. Sections were mounted with Vectashield mounting medium containing DAPI (Vector Labs) and analyzed in a Zeiss LSM 710 Laser Scanning Confocal Microscope. TUNEL staining on paraffin embedded pancreatic sections was performed with the ApoBrdU DNA Fragmentation Assay Kit (BioVision; K401-60).
Chromatin Immunoprecipitation.
INS-1 cells were plated on 15 cm dishes and near-confluent cells were exposed to forskolin as specified. Chromatin immunoprecipitation with HIF-1α and CRTC2 antisera was performed as described (28). Oligonucleotides used for ChIP analysis are listed in Table S1.
Cell Culture and Transfection.
INS-1 insulinoma cells were cultured in RPMI 1640 (Mediatech) with 10% heat inactivated fetal bovine serum (Sigma), 2 mM glutamine (Mediatech), 1 mM sodium pyruvate (Mediatech), 100 μg/mL penicillin-streptomycin (Mediatech) and 0.05 mM β-mercaptoethanol. HEK 293T cells were cultured in DMEM (Mediatech) with 10% fetal bovine serum and 100 μg/ml penicillin-streptomycin. Forskolin (10 μM), LY294002 (50 μM), and DMOG (1 mM) were added to cells as indicated. Cells were exposed to rapamycin (50 nM) or to cycloheximide (100 μg/mL) overnight. For glucose and Exendin-4 treatments, INS-1 cells were glucose and serum starved for 1 h, and stimulated with glucose or 2-deoxyglucose (20 mM) with or without Exendin-4 (10 nM). HEK 293T cells were transfected with polyethylenimine (PEI) and INS-1 cells with Lipofectamine2000 (Invitrogen; 11668-019). For HIF-1α translational reporter experiment, INS-1 cells were transfected with pLUXHIF1α5’UTR or pLUX control vector (generous gift of John Blenis). After 24 h, cells were exposed to rapamycin overnight where indicated. After forskolin treatment, cells were harvested for Renilla/firefly luciferase activity. Normalized luciferase activities are shown.
Plasmids and DNA Manipulations.
Site-directed mutagenesis was performed with Pfu Turbo polymerase (Stratagene). For adenovirus construction, cDNAs and short hairpin sequences were subcloned in the pAdTRACK vector. Complete viral vectors were generated by homologous recombination with the AdEASY vector as described (12).
Real-Time Quantitative PCR.
mRNA levels were quantified by Q-PCR analysis. Oligonucleotides were designed to target rat gene sequences and are listed in Table S2.
Isolation of Primary Islets.
Pancreatic islets were isolated as described (3). Primary islets were cultured in complete RPMI medium for 1–2 d to recover from the isolation.
Lactate, ATP, and Oxidative Stress Measurements.
Lactate release was measured using a lactate assay kit from Eton Bioscience (1200012002). Intraislet ATP concentration was measured using the ATP Bioluminescence Assay Kit HS II (Roche, 11 699 709 001). For viability measurements, INS-1 cells were treated with forskolin and rapamycin for 16 h. After exposure to 150 μM hydrogen peroxide, cells were trypsinized and harvested, and viability was assessed by trypan blue dye exclusion.
Cell Size Determination.
For cell size measurements, INS-1 cells were plated on 6 cm dishes, treated as indicated, trypsinized, and resuspended in PBS. Cell diameter was measured using a particle size counter.
Gene Profiling Experiment.
Gene profiling experiments were performed on total RNA from INS-1 cells using an Affymetrix Rat Gene Array as previously described (11). Microarray data were deposited in the NCBI Gene Expression Ominbus (GEO) and are accessible through the GEO series accession number (GSE31736).
Adenoviral GLP-1 Delivery and Streptozotocin-Induced Diabetes.
Ad-GLP1 virus was provided by G. Parsons (29). GLP-1 expressing or control (Ad-GFP) virus was delivered to male C57BL/6J mice (the Jackson Laboratory; 000664) by tail vein injection. Streptozotocin was injected at 100 mg/kg in citrate buffer once a day for three consecutive days. Rapamycin was injected every other day at 10 mg/kg body in PBS containing 5% Tween80 and 5% PEG400.
Supplementary Material
Acknowledgments.
We thank Reuben Shaw for helpful discussions, John Blenis for gift of HIF translation vector, and Geoffrey Parsons for gift of Ad-GLP-1 virus. We also thank Matt Zones for help with cell size studies. This study was supported by the Juvenile Diabetes Research Foundation, National Institutes of Health grants (R01-DK049777 and R01-DK083834), The Keickhefer Foundation, The Clayton Foundation for Medical Research, The Leona M. and Harry B. Helmsley Charitable Trust, and by Charles Brandes.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114228108/-/DCSupplemental.
References
- 1.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 3.Jhala US, et al. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–80. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inada A, et al. Overexpression of inducible cyclic AMP early repressor inhibits transactivation of genes and cell proliferation in pancreatic beta cells. Mol Cell Biol. 2004;24:2831–41. doi: 10.1128/MCB.24.7.2831-2841.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S, et al. Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem. 2006;281:1159–68. doi: 10.1074/jbc.M508307200. [DOI] [PubMed] [Google Scholar]
- 6.Norquay LD, et al. Insulin receptor substrate-2 in beta-cells decreases diabetes in nonobese diabetic mice. Endocrinology. 2009;150:4531–40. doi: 10.1210/en.2009-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altarejos JY, Montminy M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–51. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buse JB, et al. DURATION-1: Exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33:1255–61. doi: 10.2337/dc09-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–18. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA. 2005;102:4459–64. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. HIF-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–6. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani G, et al. Effect of cyclic adenosine 3′,5′-monophosphate/protein kinase a pathway on markers of cell proliferation in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2005;90:6721–4. doi: 10.1210/jc.2005-0977. [DOI] [PubMed] [Google Scholar]
- 18.Ringel MD, Schwindinger WF, Levine MA. Clinical implications of genetic defects in G proteins. The molecular basis of McCune-Albright syndrome and Albright hereditary osteodystrophy. Medicine (Baltimore) 1996;75:171–84. doi: 10.1097/00005792-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Ruvinsky I, et al. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granot Z, et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009;10:296–308. doi: 10.1016/j.cmet.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehetner J, et al. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev. 2008;22:3135–46. doi: 10.1101/gad.496908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunton JE, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–49. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Cheng K, et al. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120:2171–83. doi: 10.1172/JCI35846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porat S, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–9. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Cantley J, et al. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119:125–35. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319:1402–5. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- 28.Ravnskjaer K, et al. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. EMBO J. 2007;26:2880–9. doi: 10.1038/sj.emboj.7601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons GB, et al. Ectopic expression of glucagon-like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007;14:38–48. doi: 10.1038/sj.gt.3302842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.