Abstract
The mouse protocadherin (Pcdh) -α, -β, and -γ gene clusters encode more than 50 protein isoforms, the combinatorial expression of which generates vast single-cell diversity in the brain. At present, the mechanisms by which this diversity is expressed are not understood. Here we show that two transcriptional enhancer elements, HS5-1 and HS7, play a critical role in Pcdhα gene expression in mice. We show that the HS5-1 element functions as an enhancer in neurons and a silencer in nonneuronal cells. The enhancer activity correlates with the binding of zinc finger DNA binding protein CTCF to the target promoters, and the silencer activity requires the binding of the REST/NRSF repressor complex in nonneuronal cells. Thus, the HS5-1 element functions as a neuron-specific enhancer and nonneuronal cell repressor. In contrast, the HS7 element functions as a Pcdhα cluster-wide transcription enhancer element. These studies reveal a complex organization of regulatory elements required for generating single cell Pcdh diversity.
Keywords: chromatin, stochastic gene expression, transcription repression
The mammalian brain contains an estimated 1015 synapses that form with high precision between specific groups of neurons (1). Currently the molecular mechanisms underlying this enormous complexity and specificity of neural circuit assembly are poorly understood, but one model for its establishment is based on the “chemoaffinity hypothesis” and its “area code hypothesis” extension (2–4). According to this view, otherwise identical neurons acquire unique molecular identities via differential expression of cell-surface molecules, allowing them to distinguish self from nonself and thus establish proper neural circuitry. Recent studies of the Drosophila neuronal adhesion protein, Dscam1, have provided important insights into the generation of cell-surface diversity in the nervous system, and its importance for accurate neural circuit assembly (4). Alternative splicing of Dscam1 pre-mRNA leads to generation of ∼38,000 unique protein isoforms. Homophilic interactions between individual Dscam isoforms on the surface of neurons leads to repulsion between sister dendrites and axons, establishing neuronal self-recognition and avoidance, processes essential for the assembly of the nervous system (5–8). In contrast to Drosophila, mouse and human Dscam genes do not generate significant diversity (9, 10). The mechanism by which unique neuronal identities are established in mammals is unknown.
The clustered protocadherins (Pcdhs) are attractive candidates for generating cell-surface diversity in vertebrates (11). Pcdhs are related to the classic cadherin adhesion proteins, and are diverse single-pass transmembrane proteins present at synaptic junctions and more generally on the cell surface (11, 12). In mammals, more than 50 isoforms are encoded by three contiguous gene clusters (Pcdhα, Pcdhβ, and Pcdhγ). In mice, the Pcdhα and -γ clusters contain 14 and 22 variable exons, respectively, each of which encodes the entire extracellular domain, the transmembrane domain, and a portion of the cytoplasmic domain of a single isoform. The 3′ end of these two clusters also contains three constant exons that encode the remainder of the α- or γ-cytoplasmic domain. A single variable exon is spliced to its respective constant exons to generate a complete mRNA. A subset of the α- and γ-variable exons, termed the C-type exons, are phylogenetically distinct and contain unique promoter motifs (11). The mouse Pcdhβ cluster encodes an additional 22 isoforms and, in contrast to the α- and γ-clusters, each Pcdhβ protein is encoded entirely by a single variable exon and has only a short intracellular domain (11).
A combination of alternative splicing and stochastic promoter choice in the Pcdhα and -γ clusters generates an enormous cell surface diversity of Pcdhs in individual neurons (13, 14). Previous studies in our laboratory demonstrated that each variable exon is preceded by its own promoter, and that promoter choice determines splice site selection (13). Single-cell RT-PCR studies of mouse Purkinje cells have shown that one to three non–C-type variable exons in Pcdhα and Pcdhγ are expressed stochastically from each allele in each cell (15, 16). In contrast, the expression of the C-type variable exons in the Pcdhα and Pcdhγ clusters is biallelic and uniform. Thus, by expressing distinct subsets of Pcdhα, -β, and -γ isoforms, individual neurons are endowed with highly diverse cell-surface identities. This stochastic and combinatorial pattern of Pcdh gene expression can potentially generate over 14,820 unique combinations of isoforms. If Pcdhβ follows a similar expression pattern, there is potential for 3 × 106 distinct combinations. The mechanisms of Pcdh gene regulation required for the generation of such a vast repertoire of cell-surface identities are not understood.
In an effort to gain insight into the mechanisms governing the stochastic combinatorial expression of Pcdhs, we previously conducted a systematic search for regulatory sequences in the Pcdhα cluster using evolutionary sequence conservation and DNase I hypersensitivity (17). We identified two neuron-specific transcriptional enhancers, HS5-1 and HS7, and demonstrated that HS5-1 is required for maximum levels of total Pcdhα expression in ES cell-derived neurons (17). Here we present a detailed characterization of the effects of HS5-1 and HS7 deletions on Pcdhα expression in the mouse nervous system. Deleting the HS5-1 enhancer in neuronal tissues affects Pcdhα transcription negatively—but not uniformly—across the cluster. The general trend is a decrease of the effect of the HS5-1 deletion with increasing distance between promoter and enhancer. An exception to this trend is PcdhαC2, which is the only isoform whose expression was not affected by the HS5-1 deletion, and which is at the 3′-most end of the cluster. A striking feature of Pcdhα expression in the HS5-1 KO mice is a sudden decrease in expression between Pcdhα5 and -6, creating a group of moderately affected (Pcdhα1–5) and a group of strongly affected (Pcdhα6–12) isoforms.
We show that the zinc finger DNA binding protein CTCF binds to Pcdhα promoters in vivo, and that the strongly affected isoforms, but not the moderately affected isoforms, have reduced CTCF binding in the HS5-1 knockout (KO). Deletion of the HS7 enhancer causes a more moderate and uniform negative effect on Pcdhα expression. We conclude that the regulation of Pcdhα expression in the nervous system involves a complex network of enhancer-promoter interactions that includes, but is not exclusive to the HS5-1 and HS7 enhancers. Analysis of the HS5-1 deletion in nonneuronal tissues revealed an additional regulatory role of the enhancer. In nonneuronal tissues HS5-1 is involved in repression of Pcdhα expression via a neuron-restrictive silencer element (NRSE)/neuron-restrictive silencer factor (NRSF) mechanism, thus establishing neuronal specificity of Pcdhα expression.
Results
Deletion of the HS5-1 Enhancer Has a Differential Effect on Individual Pcdhα Isoform Expression in Neuronal Tissues.
To determine the role of the HS5-1 enhancer in Pcdhα gene expression, we used qRT-PCR to characterize the levels of individual Pcdhα isoforms in HS5-1 KO homozygous mice compared with wild-type 129SV/Jae mice. Total mRNA was extracted from several neuronal tissues: whole brain, cortex, cerebellum, hippocampus, and olfactory bulb.
In neuronal tissues obtained from P9 mice, deletion of HS5-1 affected Pcdhα expression negatively, but the effect was not uniform across the cluster (Fig. 1). Although the 5′ end of the cluster was only moderately affected, expression of isoforms in the 3′ end of the cluster was substantially diminished in the HS5-1 deletion mice. An exception to this trend was PcdhαC2, which is located at the 3′-most end of the cluster, but was not affected by the HS5-1 enhancer deletion. The decrease in Pcdhα expression across the cluster was not gradual, as would be expected were it only a function of distance from the enhancer element. An abrupt difference in the effect of the enhancer element was observed between isoforms Pcdhα5 and Pcdhα6. In HS5-1 KO whole brain, Pcdhα1 to -5 were expressed at levels 60% to 80% that of wild-type, but the expression of Pcdhα6 and -7 dropped to about 15% that of wild-type. Removing HS5-1 had the strongest effect on the 3′-most non–C-type variable isoforms Pcdhα10, -11, and -12, with the deletion mice having more than 10-fold decrease in expression. Pcdhα8 and -9 in mutant mice were expressed at 20% to 40% of the wild-type animals in all brain regions, except for hippocampus, where Pcdhα8 was not strongly affected by the enhancer deletion.
Fig. 1.
HS5-1 deletion differentially affects Pcdhα isoform expression in neuronal tissues. Total mRNA was extracted from the whole brain, cortex, cerebellum, hippocampus, and olfactory bulb of HS5-1 KO homozygous mice and 129Sv/Jae wild-type mice at P9. Expression of individual Pcdhα isoforms was analyzed by qRT-PCR and normalized to Rps17. Each bar represents the average of at least three replicates and error bars represent SEM. Quantification of constant exon 3 (con3) represents total Pcdhα levels. *P < 0.05 comparing KO to wild-type expression using a two-tailed, unpaired Student t test.
Although PcdhαC2 was not affected by the HS5-1 deletion, the transcript level of the C-type variable isoform PcdhαC1 decreased approximately threefold compared with wild-type in HS5-1 KO mice, indicating that αC1 expression is also regulated by HS5-1.
The effect of the HS5-1 deletion on Pcdhα expression followed the same general trend in all brain regions that were analyzed, with small differences in magnitude. No effect was observed on the expression of Pcdhβ or -γ isoforms.
CTCF Binding to Pcdhα Promoters Correlates with Expression.
CTCF is a ubiquitously expressed DNA binding protein that can bind to insulator elements, and mediate the formation of DNA looping between enhancers and promoters. Genome-wide mapping of CTCF binding in nonneuronal cells identified sites in the vicinity of clustered protocadherin promoters (18, 19). To investigate the role of CTCF in regulating Pcdh expression, we analyzed CTCF binding to the Pcdhα cluster in vivo by ChIP experiments on whole-brain tissue from HS5-1 KO and wild-type mice. We first analyzed binding to promoters of the constitutively active C-type isoforms and the HS5-1 enhancer (Fig. 2A). ChIP of CTCF resulted in strong enrichment at the promoter of PcdhαC1 and at the HS5-1 enhancer. In contrast, we observed very weak signal at the PcdhαC2 promoter. Neither C-type promoter was significantly affected by the HS5-1 deletion. ChIP of CTCF resulted in weak enrichment at the promoters of the alternatively expressed Pcdhα isoforms, but for all of these sites the signal was significantly higher than the signal at each of the negative control sites (Fig. 2B). This weak enrichment is consistent with binding of CTCF at active promoters within a heterogeneous population of cells. To test whether CTCF binding correlates with expression of alternative promoters, we performed ChIP on liver chromatin from the same mice. Pcdhα genes are expressed at very low or undetectable levels in the liver (20). The weak CTCF ChIP signal observed at non–C-type promoters was significantly higher than the signal observed at each site in corresponding liver ChIP reactions (Fig. S1). These results clearly show a direct correlation between HS5-1 enhancer activity, promoter activity and binding of CTCF.
Fig. 2.
CTCF binding at Pcdhα promoters correlates with expression. qPCR analysis of CTCF ChIP experiments performed on chromatin from whole brain of HS5-1 KO homozygous or 129Sv/Jae wild-type mice. Specific PCR primers were used to assay the quantity of DNA recovered in each immunoprecipitation and this quantity is expressed relative to the quantity detected in an input chromatin control. (A) Analysis of CTCF binding at the promoters of the C-type Pcdhα isoforms. Sites A and B are known intergenic CTCF sites (38). Neg A and Neg B are intergenic sites on chromosome 7 without CTCF motifs; −2.6kb αc1 is a site 2.6-kb upstream of the Pcdhαc1 transcription start site. Each bar represents the average of three or four experiments and error bars represent SEM. (B) Analysis of CTCF binding at the promoters of alternatively expressed Pcdhα isoforms. Negative control site data are the same as in A. *P < 0.05 and **P < 0.01 using a two-tailed, unpaired Student t test. N.D. indicates not determined.
The Pcdhα6 to -12 isoforms, the expression of which was strongly decreased by the HS5-1 deletion, showed a concomitant decrease in promoter CTCF binding (Fig. 2B). Interestingly, isoforms Pcdhα1 to -5, the levels of which were mildly but reproducibly decreased in the HS5-1 KO, exhibited unchanged or slightly increased promoter CTCF binding. These results imply that the mechanism of HS5-1 regulation differs between isoforms Pcdhα1 to -5 and Pcdhα6 to -12. For example, the two sets of isoforms could be part of distinct chromatin domains.
Deletion of the HS7 Enhancer Decreases the Expression of Pcdhα Isoforms, Including PcdhαC2, in a Subset of Brain Tissues.
To study the effect of the HS7 enhancer deletion on Pcdhα expression, RNA was extracted from homozygous HS7 KO and wild-type mice and analyzed as described for the HS5-1 KO mouse.
Deletion of the HS7 enhancer displayed the strongest effect in whole brain and cerebellum, and affected Pcdhα expression more moderately and evenly across the cluster compared with the HS5-1 deletion (Fig. 3). Pcdhα gene expression in other brain regions was not strongly affected (Fig. S2), implying that the HS7 enhancer is not uniformly active across the mouse brain. Although the effect of the HS7 KO on Pcdhα expression was relatively even across the cluster, a general decrease with increasing distance between the enhancer and promoter was observed.
Fig. 3.
HS7 deletion negatively affects the expression of Pcdhαs, including PcdhαC2, in a subset of brain tissues. Total mRNA was extracted from the whole brain, cerebellum, cortex, hippocampus, and olfactory bulb of HS7 KO homozygous mice and 129Sv/Jae wild-type mice. Data for cortex, hippocampus, and olfactory bulb are included in SI Materials and Methods. Expression of individual Pcdhα isoforms was analyzed by qRT-PCR and normalized to Rps17. Each bar represents the average of at least three replicates and error bars represent SEM. Quantification of constant exon 3 (con3) represents total Pcdhα levels. *P < 0.05 comparing KO to wild-type expression using a two-tailed, unpaired Student t test.
Interestingly, although the HS5-1 deletion led to no change in PcdhαC2 expression, removing HS7 led to a statistically significant twofold decrease in the levels of PcdhαC2 in cerebellum and whole brain. Thus, HS7 appears to be directly involved in the regulation of PcdhαC2 expression.
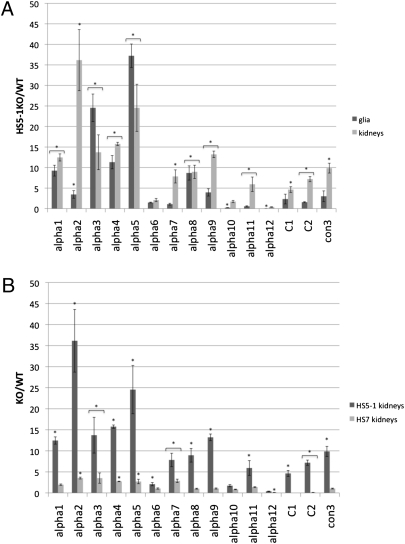
Deletion of the HS5-1 Enhancer Increases Pcdhα Expression in Glia and Kidney Cells.
We examined the effect of the HS5-1 enhancer deletion on Pcdhα gene expression in primary kidney cells and cultured glia to study the role of HS5-1 in nonneuronal tissues. In wild-type animals, Pcdhα mRNAs are barely detectable in these tissues (20, 21). Surprisingly, deletion of the HS5-1 enhancer led to a dramatic increase of Pcdhα expression in both kidney and glial cells (Fig. 4A). The observed effect was strongest for isoforms Pcdhα1 to -5, where transcription levels were about 10- to 30-fold higher in the KO animals. Most isoforms on the 3′ end of the cluster also showed a substantial, but less pronounced increase in expression (2- to 10-fold). The levels of Pcdhα12 in kidneys and glia and of Pcdhα10 and -11 in glia only were decreased in the HS5-1 deletion. Deletion of HS7 did not lead to a substantial increase in Pcdhα expression in kidneys (Fig. 4B).
Fig. 4.
HS5-1 deletion leads to increase of Pcdhα expression in glia and kidneys. (A) Total mRNA was extracted from cultured glia and kidneys of HS5-1 KO homozygous or 129Sv/Jae wild-type mice. Expression of individual Pcdhα isoforms was analyzed by qRT-PCR and normalized to Rps17. Each bar represents the average of at least three replicates and error bars represent SEM. (B) HS7 deletion does not lead to substantial increase of Pcdhα expression in kidneys. Expression of individual Pcdhα isoforms was analyzed by qPCR as described in Fig. 4A. Each bar represents the average of at least three replicates and error bars represent SEM. Quantification of constant exon 3 (con3) represents total Pcdhα levels. *P < 0.05 comparing KO to wild-type expression using a two-tailed, unpaired Student t test.
Activity of a Noncanonical Split NRSE Site in Established Kidney and Neuroblastoma Cell Lines.
NRSF is a DNA binding protein that recognizes a 21-bp consensus motif NRSE and recruits corepressor complexes that inhibit transcription of neuron-specific genes in nonneuronal tissues (22–24). Tan et al. identified numerous NRSE motifs in the fugu Pcdh cluster, and demonstrated that these motifs could repress expression of reporter genes (25). They also identified sequences matching NRSE motifs in the mammalian Pcdh cluster variable exons and the HS5-1 enhancer. We determined whether NRSF binds to these sites by analyzing ChIPseq data from eight different cell lines, made available by the ENCODE project (26), including a human glioblastoma line (U87) and a human neuroblastoma line (SK-N-SH). NRSF binding was not observed to any of the predicted Pcdhα variable exon NRSEs. However, we observed strong NRSF binding to the NRSE within HS5-1 in all eight cell lines (Fig. 5 A and B). Much weaker, but reproducible NRSE binding was present at several other locations within the Pcdhα cluster, particularly in H1 embryonic stem cells. Nearly all of these sites correspond to an NRSE half-site found within the coding sequence of every non–C-type Pcdhα exon (Fig. S3 A and B). There is also an NRSF binding site detected ∼2 kb downstream from HS7 that corresponds to a cryptic NRSE sequence, but this binding was observed only in ES cells (Fig. S3C). This weak or nonexistent binding at HS7 is consistent with the absence of significant effects from the deletion of the HS7 enhancer in nonneuronal cells.
Fig. 5.
NRSE site in HS5-1 represses nonneuronal Pcdhα4 promoter activity in Luciferase reporter assays. (A and B) Plot of NRSF ChIPseq signal at Pcdhα12 coding sequence NRSE (A) and HS5-1 NRSE (B) for eight human cell lines. Scaling of ChIPseq signal varies between cell lines but is the same at both sites. The position of the NRSE is indicated. The lower portion of each panel shows a plot of sequence conservation among mammals (Phastcons). (C) Diagram of the HS5-1 enhancer element indicating constructs used in reporter assays. The location of the NRSE sequence is highlighted. The plot below the figure indicates degree of sequence conservation among mammals. (D and E) Plot of luciferase reporter activity of Pcdhα4 promoter alone and in constructs bearing variants of HS5-1 constructs in TCMK1 mouse kidney cells (n = 3) (D) or undifferentiated CAD cells (n = 5) (E). Expression of each vector is compared with expression from the Pcdhα4 promoter alone, which is set to 1. Error bars represent SEM. *P < 0.05, **P < 0.01 in two-tailed, unpaired Student t test.
To determine whether the NRSE site in the HS5-1 enhancer is involved in Pcdhα repression in nonneuronal cells, we generated a series of luciferase reporter constructs using the Pcdhα4 promoter. These constructs contained HS5-1 located downstream from the firefly luciferase reporter gene. We compared constructs with a wild-type HS5-1 to constructs with a truncated HS5-1 (contains NRSE), deleted NRSE, and no enhancer (Fig. 5C). We transfected the luciferase reporter constructs in mouse TCMK1 kidney and CAD neuroblastoma cell lines.
In TCMK1 cells, full-length HS5-1 (bearing the NRSE) strongly repressed Pcdhα4 promoter activity (Fig. 5D). In the presence of the enhancer, luciferase activity was reduced over 13-fold compared with the Pcdhα4 promoter alone. Deletion of the NRSE site in HS5-1 fully reversed the repressive effect. A reporter construct containing a truncated HS5-1, which includes the NRSE site, had a strong repressive effect, indistinguishable from the wild-type full-length HS5-1, with luciferase activity over 10-fold reduced compared with the Pcdhα4 promoter alone. These results indicate that HS5-1 represses Pcdhα4 promoter activity in TCMK1 cells, and that the NRSE site is required for this repression.
In the mouse neuroblastoma cell line CAD, HS5-1 did not repress Pcdhα4 promoter activity as indicated by the comparable luciferase activity of reporters containing either the Pcdhα4 promoter alone or with the HS5-1 enhancer (Fig. 5E). Deleting the NRSE site caused a moderate (less than twofold), but reproducible increase in luciferase activity. This moderate, NRSE-dependent repression of HS5-1 enhancer activity may be because of low level or heterogeneous expression of NRSF in CAD cells, as has been observed for other neuroblastoma cell lines (27).
Taken together, these observations show that the NRSE site in HS5-1 functions as a repressor in the nonneuronal TCMK1 kidney cells but not in the neuroblastoma CAD cells.
Discussion
Activation of Neuronal Pcdhα Expression by the HS5-1 Enhancer Correlates with CTCF Binding to the Affected Promoters.
Deletion of the HS5-1 enhancer led to a significant decrease in Pcdhα transcription in the nervous system, but the affect was not uniform across the cluster. The levels of Pcdhα6 to -12 and C1 transcription decreased 2- to >100-fold, but the decreases in Pcdhα1 to -5 transcription were less than twofold (Fig. 1). Independent studies by Yokota et al. showed the same overall effect of the HS5-1 deletion in the whole brain of P21 mice (28). Although the general trend observed in both studies was a decrease of the effect of the HS5-1 deletion with increasing distance between promoter and enhancer, Pcdhα8 and -9 were affected less than the flanking isoforms, and an abrupt decrease in the affect was observed between Pcdhα5 and -6. Thus, it appears that the Pcdhα cluster is organized into at least two regulatory domains, one that is strongly dependent on the HS5-1 enhancer (Pcdhα6–12) and the other that is not (Pcdhα1–5).
We show that CTCF binds to Pcdhα promoters and the HS5-1 enhancer in vivo, and that reductions in Pcdhα expression in the HS5-1 KO correlate with reductions in CTCF binding to the strongly affected promoters. Based on the binding pattern of CTCF and previous studies with other genes (18, 29), it is likely that CTCF mediates the interaction between the HS5-1 enhancer and the promoters it regulates. Consistent with this proposal, binding of CTCF to the promoters of Pcdhα6 to -12, the isoforms most strongly affected by the HS5-1 deletion, was significantly diminished in the HS5-1 KO mice (Fig. 2). However, the ChIP studies also revealed a surprising result. Isoforms Pcdhα1 to -5 had a moderate, but reproducible decrease in expression in the HS5-1 deletion, but we did not observe a corresponding decrease in CTCF binding. Based on these observations, we propose that Pcdhα6 to -12 are regulated via CTCF-mediated interactions between the HS5-1 enhancer and the Pcdhα6 to -12 promoters. The promoters of Pcdhα1 to -5, on the other hand, do not participate in CTCF-mediated contacts with HS5-1, but may interact through CTCF with an additional, currently unidentified regulatory element. HS5-1 may affect Pcdhα1 to -5 either directly via a CTCF-independent mechanism or indirectly by participating in the formation of a higher-order chromatin structure, consisting of a number of enhancer elements and promoter regions.
Single-cell studies of Pcdhα expression in Purkinje neurons (15, 16) may support the existence of an additional, unidentified enhancer element. Close examination of single cell expression data shows that one-half to one-third of the cells express more than two non–C-type variable isoforms. If there are two Pcdhα1 to -12 isoforms expressed on one or both chromosomes, the simultaneous expression of the two isoforms might be regulated via a chromosome looping architecture that involves two separate contacts of HS5-1. Alternatively or in addition, a second, as yet unidentified, enhancer may regulate the Pcdhα1 to -5 isoforms.
A puzzling feature of the expression of the Pcdhα gene cluster is that unlike Pcdhα1 to -12, which are expressed independently on the paternal and maternal chromosomes (monoallelic), PcdhαC1 and PcdhαC2 are expressed from both chromosomes (biallelic) (15, 16). We demonstrate that PcdhαC1, but not PcdhαC2, requires the HS5-1 enhancer for expression. Our analysis of CTCF binding clarifies this relationship. We show that the PcdhαC1 promoter and HS5-1 are both bound by CTCF, whereas the promoter of PcdhαC2 is not.
It is not clear how the HS5-1 enhancer can stochastically activate one of the Pcdhα1 to -12 isoforms independently on the two chromosomes, and regulate uniform expression of PcdhαC1 on both chromosomes. We note that, although PcdhαC1 expression was reduced more than threefold in the HS5-1 deletion, significant reduction in CTCF binding at the PcdhαC1 promoter in HS5-1 KO mice was not observed. In contrast to PcdhαC1, we found that CTCF binding was lost from the promoters of the strongly affected isoforms. This finding indicates that regulation of CTCF binding at the PcdhαC1 promoter is distinct from regulation of CTCF binding at non–C-type promoters. Taken together, these findings provide additional evidence for the existence of discrete chromatin domains and a complex organization of enhancer-promoter interactions in the Pcdhα gene cluster.
In contrast to HS5-1, we show that the HS7 enhancer is required for maximal expression of both the CTCF-dependent and independent Pcdhα promoters. The HS7 enhancer deletion affected Pcdhα expression most strongly in the cerebellum, and the observed effect was more moderate and uniformly distributed than that of the HS5-1 deletion (Fig. 3). Pcdhα expression in the cerebellum decreased two- to fivefold compared with wild-type in the HS7 deletion mice. Deleting the HS7 enhancer led to a reproducible twofold decrease in PcdhαC2 expression, the only isoform not affected by the HS5-1 deletion. This finding suggests that HS7 regulates Pcdhα expression by a CTCF-independent mechanism. The negative effect of the HS7 deletion on the rest of the Pcdhα cluster might be because of disruption of a higher-order chromatin structure that is essential for proper Pcdhα expression.
Deleting HS5-1 and HS7 individually revealed that both enhancers are required for maximum levels of Pcdhα gene expression. However, the differential response of individual Pcdhα isoforms to the enhancer deletions clearly indicate that additional enhancers are likely to be required for maximum levels of transcription. We have searched for additional enhancer elements in the cluster, but none have been identified. However, preliminary carbon copy chromosome conformation capture studies suggest the involvement of longer-range regulatory elements in the expression of the Pcdhα cluster. For example, potential regulatory elements in the Pcdhγ cluster appear to engage in long-range interactions with regions in the α-cluster. In addition, recent studies by Yokota et al. identified a Pcdhβ cluster control region located downstream from the Pcdhγ cluster and demonstrated that the cluster control region is required for maximum-level expression of Pcdhβ in mice (28). Their work demonstrates the presence of regulatory elements in the Pcdh cluster that act over a distance of over 320 kb. Thus, we conclude that the regulation of Pcdhα expression involves a complex network of enhancer-promoter interactions that includes, but is not limited to the HS5-1 and HS7 enhancers.
HS5-1 Enhancer Represses Pcdhα Expression in Nonneuronal Cells via a Noncanonical Split NRSE Sequence.
Deletion of the HS5-1 enhancer led to a 20- to 30-fold increase in the expression of a subset of Pcdhα isoforms in kidney cells and cultured glia (Fig. 4A). In contrast, expression of Pcdhα in wild-type kidneys and glia is barely detectable. Thus, HS5-1 appears to serve a dual function as a Pcdhα enhancer in neuronal tissues and as a Pcdhα repressor in nonneuronal tissues. Interestingly, the HS5-1 sequence contains an NRSE binding site (30). Our analysis of ChIPseq data from the ENCODE project demonstrates NRSF binding to the HS5-1 NRSE site in several human cell lines. NRSF is a transcriptional repressor, whose role in silencing neuronal genes in differentiated nonneuronal tissues is well established (31–34). REST acts in conjunction with its corepressors CoREST and mSin3 to recruit histone deacetylases and additional silencing machinery to the NRSE, leading to the repression of neuronal-specific genes in nonneuronal cells (22, 31, 32, 35). In contrast, deletion of the HS7 enhancer, which does not contain an NRSE site, did not lead to a substantial increase in Pcdhα expression in kidneys (Fig. 4B). Thus, HS5-1, but not HS7, appears to be involved in NRSF-mediated silencing of Pcdhα genes in nonneuronal tissues.
We tested this hypothesis using luciferase reporter constructs in the mouse nonneuronal TCMK1 kidney cell line and in the mouse neuroblastoma CAD cell line (Fig. 5 C–E). In TCMK1 cells, the HS5-1 enhancer containing the NRSE site led to strong repression of Pcdhα4 promoter activity, reducing luciferase levels more than 10-fold. The repressive effect of HS5-1 was lost upon deletion of the NRSE site. A truncated HS5-1 that contains the NRSE site alone was capable of repressing Pcdhα4 promoter activity. The luciferase reporter studies in undifferentiated neuroblastoma CAD cells did not point to a strong repressive activity of HS5-1 in that cell line (Fig. 5E). Deletion of the NRSE site led to a twofold promoter activation, which could be a result of the heterogeneous or low-level expression of NRSF in CAD cells. From these results we conclude that HS5-1 has a strong NRSF-dependent repressive effect on Pcdhα expression in the nonneuronal kidney TCMK1 cell line, but not in the neuron-like CAD cells.
This NRSF-mediated silencing extends across the entire Pcdhα cluster, including isoforms that were only moderately affected by deletion of HS5-1 in neurons. Even PcdhαC2, which was not affected at all by deletion of HS5-1 in neuronal tissue, was up-regulated in nonneuronal cells. One possible reason for this is that HS5-1 interacts with different variable isoform promoters in nonneuronal cells. Alternatively, HS5-1 may interact with other enhancer elements that act on these isoforms and maintain them in an inactive state.
Studies of the Drosophila Dscam genes have elegantly demonstrated the importance of single-cell diversity of cell-surface proteins in neural circuit assembly (36). In this case, the diversity is generated by stochastic alternative pre-mRNA splicing of an extraordinarily complex pre-mRNA. The unique genomic organization of the vertebrate Pcdh genes (11), the combination of promoter choice and alternative splicing required for Pcdh expression (13), and stochastic monoallelic expression (15, 37) generate enormous cell-surface diversity. There is mounting evidence for a role of this diversity in neuronal connectivity (4). Here we characterize the function of key Pcdhα regulatory elements in vivo. These studies reveal a complex organization of promoters and enhancers, provide evidence for an important role for CTCF, and suggest that additional regulatory elements and mechanisms are required to generate Pcdh diversity.
Materials and Methods
Generation of KO Mice.
Targeting constructs were prepared as described in SI Materials and Methods. ES cell targeting and mouse chimera production are described in SI Materials and Methods.
Cell Line and Glial Cultures.
Cell lines and glia were cultured as described in SI Materials and Methods.
RNA Extraction, Reverse Transcription, qPCR, and Chromatin Immunoprecipitation.
RNA was extracted as described in SI Materials and Methods. Reverse transcription, qPCR and ChIP were done as described in SI Materials and Methods.
High-Throughput Sequencing Data Analysis.
Analysis details can be found in SI Materials and Methods.
Transient Reporter Assays.
Reporter constructs were made as described in SI Materials and Methods. Further details can be found in SI Materials and Methods. See Table S1 for primer sequences.
Supplementary Material
Acknowledgments
We thank Monica Carrasco, Noam Rudnick, and Nicole Follmer for expert technical and data analysis assistance; Sean Buchanan, Stefanie Schalm, and Scott Ribich for critical reading of the manuscript; and Prof. Nicole Francis for helpful discussions and for generously hosting P.K. in her laboratory. This work was supported by National Institutes of Health Grant R01NS043915 (to T.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114357108/-/DCSupplemental.
References
- 1.Jontes JD, Phillips GR. Selective stabilization and synaptic specificity: A new cell-biological model. Trends Neurosci. 2006;29:186–191. doi: 10.1016/j.tins.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Dreyer WJ. The area code hypothesis revisited: Olfactory receptors and other related transmembrane receptors may function as the last digits in a cell surface code for assembling embryos. Proc Natl Acad Sci USA. 1998;95:9072–9077. doi: 10.1073/pnas.95.16.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipursky SL, Sanes JR. Chemoaffinity revisited: Dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Schmucker D. Molecular diversity of Dscam: Recognition of molecular identity in neuronal wiring. Nat Rev Neurosci. 2007;8:915–920. doi: 10.1038/nrn2256. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro L. Self-recognition at the atomic level: Understanding the astonishing molecular diversity of homophilic Dscams. Neuron. 2007;56:10–13. doi: 10.1016/j.neuron.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Soba P, et al. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wojtowicz WM, et al. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwala KL, Ganesh S, Amano K, Suzuki T, Yamakawa K. DSCAM, a highly conserved gene in mammals, expressed in differentiating mouse brain. Biochem Biophys Res Commun. 2001;281:697–705. doi: 10.1006/bbrc.2001.4420. [DOI] [PubMed] [Google Scholar]
- 10.Agarwala KL, et al. Dscam is associated with axonal and dendritic features of neuronal cells. J Neurosci Res. 2001;66:337–346. doi: 10.1002/jnr.1226. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 12.Noonan JP, et al. Extensive linkage disequilibrium, a common 16.7-kilobase deletion, and evidence of balancing selection in the human protocadherin alpha cluster. Am J Hum Genet. 2003;72:621–635. doi: 10.1086/368060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasic B, et al. Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Mol Cell. 2002;10:21–33. doi: 10.1016/s1097-2765(02)00578-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Su H, Bradley A. Molecular mechanisms governing Pcdh-gamma gene expression: Evidence for a multiple promoter and cis-alternative splicing model. Genes Dev. 2002;16:1890–1905. doi: 10.1101/gad.1004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esumi S, et al. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko R, et al. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. J Biol Chem. 2006;281:30551–30560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- 17.Ribich SA, Tasic B, Maniatis T. Identification of long-range regulatory elements in the protocadherin-alpha gene cluster. Proc Natl Acad Sci USA. 2006;103:19719–19724. doi: 10.1073/pnas.0609445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handoko L, et al. CTCF-mediated functional chromatin interactome in pluripotent cells. Nat Genet. 2011;43:630–638. doi: 10.1038/ng.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TH, et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallosso AR, et al. Frequent long-range epigenetic silencing of protocadherin gene clusters on chromosome 5q31 in Wilms’ tumor. PLoS Genet. 2009;5:e1000745. doi: 10.1371/journal.pgen.1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohmura N, et al. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 22.Andrés ME, et al. CoREST: A functional corepressor required for regulation of neural-specific gene expression. Proc Natl Acad Sci USA. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraner SD, Chong JA, Tsay HJ, Mandel G. Silencing the type II sodium channel gene: A model for neural-specific gene regulation. Neuron. 1992;9:37–44. doi: 10.1016/0896-6273(92)90218-3. [DOI] [PubMed] [Google Scholar]
- 24.Mori N, Schoenherr C, Vandenbergh DJ, Anderson DJ. A common silencer element in the SCG10 and type II Na+ channel genes binds a factor present in nonneuronal cells but not in neuronal cells. Neuron. 1992;9:45–54. doi: 10.1016/0896-6273(92)90219-4. [DOI] [PubMed] [Google Scholar]
- 25.Tan YP, et al. Regulation of protocadherin gene expression by multiple neuron-restrictive silencer elements scattered in the gene cluster. Nucleic Acids Res. 2010;38:4985–4997. doi: 10.1093/nar/gkq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birney E, et al. ENCODE Project Consortium NISC Comparative Sequencing Program Baylor College of Medicine Human Genome Sequencing Center Washington University Genome Sequencing Center Broad Institute Children's Hospital Oakland Research Institute Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mucha M, et al. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J Neurosci. 2010;30:13235–13245. doi: 10.1523/JNEUROSCI.1981-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokota S, et al. Identification of the cluster control region for the Protocadherin-{beta} genes located beyond the Protocadherin-{gamma} cluster. J Biol Chem. 2011;286:31885–31895. doi: 10.1074/jbc.M111.245605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips JE, Corces VG. CTCF: Master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 31.Ballas N, et al. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 32.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Chong JA, et al. REST: A mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 34.Schoenherr CJ, Anderson DJ. Silencing is golden: Negative regulation in the control of neuronal gene transcription. Curr Opin Neurobiol. 1995;5:566–571. doi: 10.1016/0959-4388(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko R, et al. Allelic gene regulation of Pcdh-alpha and -gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. J Biol Chem. 2006;281:30551–30560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- 38.Wendt KS, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.