Abstract
Stress has been identified as a major causal factor for many mental disorders. However, our knowledge about the chain of molecular and cellular events translating stress experience into altered behavior is still rather scant. Here, we have characterized a murine ortholog of the putative tumor suppressor gene DRR1 as a unique stress-induced protein in brain. It binds to actin, promotes bundling and stabilization of actin filaments, and impacts on actin-dependent neurite outgrowth. Endogenous DRR1 localizes to some, but not all, synapses, with preference for the presynaptic region. Hippocampal virus-mediated enhancement of DRR1 expression reduced spine density, diminished the probability of synaptic glutamate release, and altered cognitive performance. DRR1 emerges as a protein to link stress with actin dynamics, which in addition is able to act on synaptic function and cognition.
Keywords: actin dynamics, stress physiology, stress regulation, synaptic plasticity, TU3A
Stressful situations evoke a plethora of molecular and cellular processes used by the organism to optimize biological fitness. However, they can also trigger deleterious effects on the brain (1). Because excessive or prolonged stress exposure has been associated with the development of a variety of diseases, including affective disorders, major research efforts aim at deciphering protective factors and those predisposing to development of stress-associated diseases.
Molecular and cellular correlates of stress-induced cognitive (mal)adaptation (2) have been localized mainly to the hippocampal formation and include changes in neuronal network dynamics and neuronal architecture (3). One of the most consistently observed effects following repeated stress exposure is dendritic remodeling of CA3 hippocampal pyramidal neurons, including a reduction in synapse number (4). Although reorganization of cytoskeletal proteins, in particular modulation of actin dynamics, has been recognized as an important process influencing synaptic function (5), molecular players translating stressful environmental stimuli into adaptive changes in synaptic plasticity and neuronal functioning remain largely unknown.
We recently identified the murine ortholog of DRR1 (down-regulated in renal cell carcinoma 1, also known as TU3A and Fam107A), a gene described originally as a tumor suppressor gene (6), as a putative stress-regulated gene in the brain (7). Here we present DRR1 as a stress-responsive protein that binds to and remodels actin. In addition, DRR1 has the potency to impact on neuroplasticity and synaptic function and to modulate hippocampus-dependent cognitive processes.
Results
Up-Regulation of the Tumor Suppressor DRR1 in the Brain by Stress and Glucocorticoids.
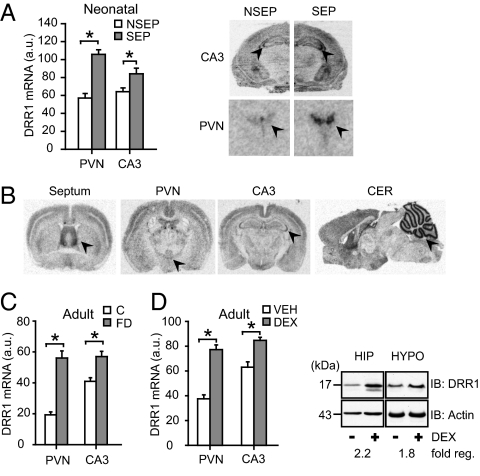
A microarray study indicated that DRR1 is a strongly up-regulated gene in the neonatal mouse brain in response to the robust stressor maternal separation (7). We first validated its stress dependency in the brain by in situ hybridization. Following 24 h of maternal separation, DRR1 mRNA expression was significantly increased in the hypothalamic paraventricular nucleus (PVN) and the CA3 region of the hippocampus (Fig. 1A). In the adult mouse brain, we observed a strong basal expression in the septum, the neocortex, the CA3 region of the hippocampus, and the cerebellum on mRNA (Fig. 1B) and protein level (Fig. S1 A–C). Stress responsiveness of DRR1 in the PVN and in the CA3 region was found also in adult mice by applying 24 h of food deprivation (Fig. 1C), a stressor that is comparable in duration and intensity to the maternal separation paradigm.
Fig. 1.
Expression of DRR1 in distinct brain areas and GR-dependent stress induction. (A) DRR1 mRNA expression is significantly increased in PVN (T14 = 6.746; P = 0.0001) and hippocampal CA3 region (T14 = 2.399; P = 0.031) after maternal separation (SEP) at postnatal day 9. (B) Autoradiographs of DRR1 mRNA expression in adult mouse brain. Arrowheads indicate (from left to right) septum, PVN, CA3, and cerebellum. (C) Food deprivation (FD) in adult mice led to up-regulation of DRR1 mRNA after 24 h in PVN (T13 = 7.070; P = 0.0001) and CA3 (T14 = 3.905; P = 0.002). (D) The GR agonist dexamethasone (DEX) led 8 h after injection to increased expression of DRR1 mRNA in PVN (T10 = 7.931; P = 0.0001) and CA3 (T10 = 4.348; P = 0.001), verified on the protein level (blots).
To test whether stress increases DRR1 expression via glucocorticoids, which constitute the end point of the prevailing physiological stress hormone cascade, we treated adult animals with the synthetic and selective glucocorticoid receptor (GR) agonist dexamethasone. This resulted in a significant increase of DRR1 expression in the PVN and in the CA3 region 8 h after injection compared with vehicle-treated controls on the level of mRNA and protein (Fig. 1D). Moreover, treatment with the GR antagonist RU486 completely abolished induction of DRR1 in the PVN during both postnatal maternal separation and food deprivation in adult animals (Fig. S1D).
GR may target DRR1 either indirectly via impacting on other transcription factors or directly via binding to glucocorticoid response elements (GREs) in regulatory regions of the gene. We identified conserved GREs and tested the three with the highest conservation, located in the promoter, intron 1, and 3′ UTR of DRR1, for binding to recombinant GR. All three GREs indeed specifically bind to the receptor in vitro (Fig. S1E), indicating their functionality.
DRR1 Binds to, Stabilizes, and Bundles F-Actin.
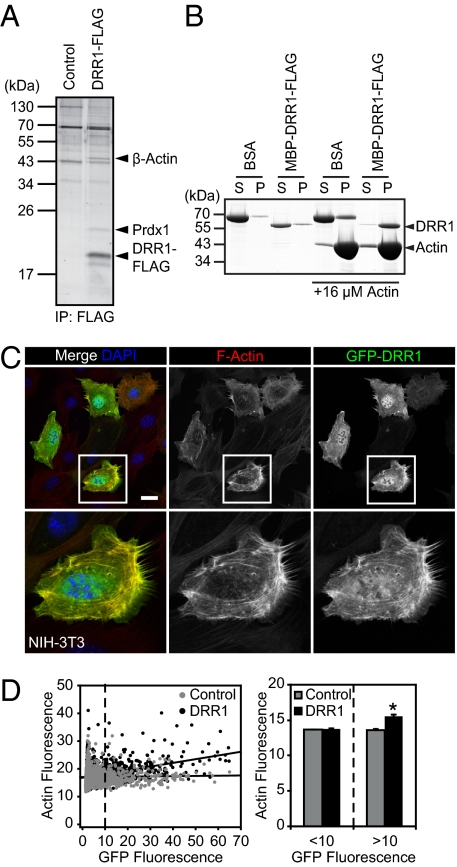
The DRR1 protein is highly conserved within vertebrates and features a yet-uncharacterized domain (Fig. S2A), but its molecular actions are unknown. We first identified the protein interaction partners of DRR1 as a route to eventually unravel its molecular functions. DRR1-FLAG was overexpressed in HEK-293 cells and immunoadsorbed, and among the coprecipitated proteins we identified peroxiredoxin-1 (Prdx1) and β-actin using mass spectrometry (Fig. 2A). Western blot analysis verified the interactions of DRR1 with Prdx1 and β-actin in vitro (Fig. S2B). In addition, interaction with actin was confirmed by cosedimentation with F-actin (Fig. 2B), and further coimmunoprecipitation experiments indicated that GFP-DRR1 interacted with actin indistinguishably from FLAG-DRR1 (Fig. S2B). Evaluation of protein interaction in vivo by expression of GFP-tagged DRR1 in NIH 3T3 cells and immunocytochemistry revealed no colocalization with Prdx1 (Fig. S2C). However, DRR1 strongly colocalized with F-actin (Fig. 2C; correlation coefficient 0.872; details in Fig. S2 D and E). Moreover, enhanced DRR1 expression was correlated with increased F-actin–rich structures (Fig. 2D).
Fig. 2.
DRR1 binds to and increases F-actin in cells. (A) Coimmunoprecipitation of proteins associated with DRR1-FLAG expressed in HEK-293 cells. (B) Pelletation assays of F-actin with DRR1 revealed binding of recombinant DRR1 to F-actin. S, supernatant; P, pellet. (C) Colocalization of DRR1 with F-actin and increase of F-actin–rich areas (Center panels, visualized with phalloidin) in NIH 3T3 cells overexpressing GFP-DRR1. (Scale, 25 μm.) (D) Correlation plot: actin and DRR1-GFP, actin and GFP. DRR1 overexpression in NIH 3T3 cells significantly increased F-actin [DRR1-GFP: 2,747 cells counted (12.67% with GFP > 10, R2 = 0.1341); GFP: 2,828 cells counted (9.34% with GFP > 10, R2 = 0.0005)]. (Right) Average increase of actin fluorescence in cells with detectable DRR1 transfection (>10).
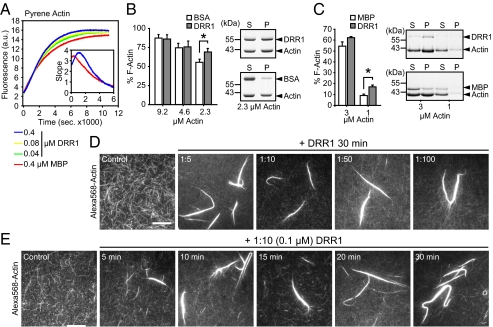
The apparent increase in F-actin in GFP-DRR1 overexpressing cells prompted us to assess a potential influence of DRR1 on actin polymerization. We first investigated the ability of DRR1 to influence polymerization of globular actin to F-actin. DRR1 was expressed and purified as fusion protein to maltose-binding protein (MBP) (Fig. S3A). Interaction of MBP-DRR1-FLAG with actin was verified in lysates from HEK-293 cells (Fig. S3B). We followed the kinetics of filament assembly via the increase in fluorescence of pyrene-labeled actin upon polymerization. Addition of DRR1 somewhat prolongs the lag phase of actin polymerization at certain concentrations (Fig. 3A, Inset). It also leads to a moderate increase in fluorescence toward the end of the reaction in a dose-dependent manner (Fig. 3A). The effect size of increased F-actin is difficult to assess in this readout because we cannot exclude the possibility that enhanced light scattering due to the formation of actin bundles has some influence on the overall fluorescence measurement. DRR1 had no effect on pyrene-actin fluorescence during the 10-min incubation before starting the polymerization.
Fig. 3.
DRR1 affects F-actin assembly in vitro. (A) DRR1 affects actin filament formation time- and dose-dependently (4 μM actin). (Inset) Slope at the beginning of the reaction. (B and C) DRR1 stabilizes preformed F-actin. Percentage of F-actin in the breakdown sedimentation assay represents the fraction of total actin found in the pellet. P, pellet; S, supernatant. (B) F-actin was diluted to the indicated concentrations in the presence of 1.6 μM DRR1 (at 2.3 μM actin, *P = 0.009, n = 5). (C) F-actin was incubated with 1:10 molar ratio DRR1 before diluting to the indicated concentration (at 1 μM actin, *P = 0.0084, n = 4). (D and E) DRR1 induces actin bundles. F-actin, partially labeled with AlexaFluor-568, was incubated with DRR1, either for 30 min at the indicated molar ratios of Actin:DRR1 (D) or at a 1:10 molar ratio for the indicated times (E) before analysis by TIRF microscopy. (Scale, 10 μm.)
Because the net amount of F-actin represents equilibrium of the assembly and disassembly reactions, we investigated whether DRR1 stabilizes F-actin filaments. Preformed actin filaments were diluted to induce breakdown of filaments. In the presence of DRR1, the net amount of F-actin was higher than in the control at certain concentrations, suggesting a stabilizing influence of DRR1 on actin filaments (2.3 and 1 μM actin; Fig. 3 B and C and Fig. S3C).
Microscope analysis of the structures produced in the pyrene–actin assay (Fig. 3A) indicated bundling activity of DRR1 (Fig. S3D). Similar structures were observed when Alexa568-labeled actin and total internal reflection fluorescence (TIRF) microscopy was used (Fig. S3E). To investigate this more thoroughly, we analyzed the effect of adding DRR1 protein to already formed F-actin under various conditions. This setting more closely resembles the in vivo situation, where DRR1 is up-regulated after stress with substantial amounts of F-actin already being present. TIRF microscopy revealed that, in the absence of DRR1, most of the filaments were short and thin, whereas DRR1 induced the formation of extended, thick filaments (Fig. 3D). This effect already started to appear after 5 min (Fig. 3E). Thus, the apparent increase in F-actin in cells may be due to the bundling capability of DRR1.
DRR1 Localizes to Neurites and Affects Neurite Outgrowth in Vitro.
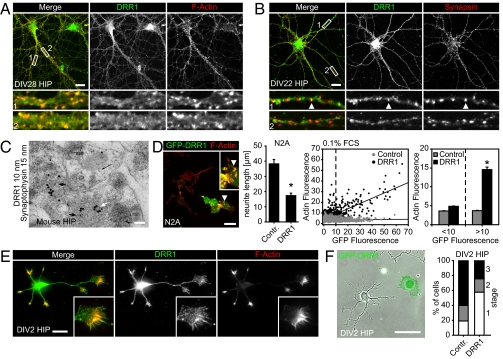
To reveal the subcellular localization of DRR1, we stained primary neuronal cultures from rat hippocampus, the forebrain region with the highest expression of DRR1 (Fig. 1). The cultures were maintained for 3–4 wk to allow for development of the neuronal structures that most commonly form in primary cells. DRR1 staining produced a punctate pattern along neurites, colocalizing with actin (Fig. 4A, correlation coefficient = 0.813; details in Fig. S4B). A similar distribution was revealed in cerebellar primary neurons (Fig. S4A). Furthermore, we also observed a partial overlap with synapsin, which suggests that DRR1 localizes to some, but presumably not all, synapses (Fig. 4B). Accordingly, correlation coefficients vary between 0.1 and 0.8, depending on which subregion of the neurites is analyzed (Fig. S4B).
Fig. 4.
DRR1 localizes on neurites in actin-rich regions and interferes with neurite outgrowth. (A) Rat hippocampal neurons cultivated for 28 days in vitro (DIV28) were stained with DRR1 antibodies together with phalloidin to visualize actin. (B) DIV22 rat hippocampal neurons were stained with DRR1 in combination with synapsin antibodies to visualize synaptic clusters. Laser scanning microscopy overlay showed DRR1 colocalizing with synapsin in some clusters. (C) Ultrathin section of mouse hippocampal CA3 stratum lucidum after double immunogold labeling with DRR1 (10-nm particles, black arrows) and synaptophysin (15-nm particles, white arrow). (D) Expression of DRR1-GFP reduces neurite outgrowth in N2A cells (upon differentiation) and primary hippocampal neurons (F). (D) GFP-DRR1 (green) also induces F-actin accumulations visualized by fluorescent phalloidin (red). (Inset) Enlargement of the accumulations indicated by the arrowhead. (Right) Quantification of neurite length of GFP-DRR1 (n = 132) and GFP (n = 102) overexpressing cells (+SEM) revealed a significant decrease in neurite length (P = 0.000038). Quantification of actin fluorescence revealed a significant increase in DRR1-transfected cells (<10). (E) DRR1 localizes to neurite tips in hippocampal neurons cultivated for 36 h (DIV2). (F) Rat HIP neurons were transfected with plasmids expressing GFP-DRR1 or GFP as control, and the developmental stages were assessed after DIV2. (Scale: A, B, D, and E, 20 μm; C, 100 nm; F, 50 μm.)
To visualize DRR1 localization at higher resolution in the hippocampal CA3 via electron microscopy, we performed double immunogold labeling together with synaptophysin. DRR1 was shown to be predominantly presynaptic (Fig. 4C). Analysis of 48 images (total area assessed was 610 μm2) revealed that 62% of the examined giant boutons (n = 112) were positive and colabeled with the presynaptic marker synaptophysin, whereas only 23% of the postsynaptic thorns were DRR1 positive.
Neurite outgrowth requires reorganization of actin filaments and thus might be affected by DRR1, given its potency to modulate actin dynamics. To reveal the effects of enhanced levels of DRR1 on the development of neuronal processes, we used ectopic expression of DRR1 from transfected plasmids and extended the study to the murine neuroblastoma cell line Neuro2a (N2a), an established model sensitive to alterations in actin dynamics (8). GFP-DRR1 did not change N2A cell morphology in regular medium containing 10% FCS (Fig. S4C). However, upon induction of differentiation in N2A cells, GFP-DRR1 caused a steep decrease in the development of neurites (Fig. 4D and Fig. S4C). Both the number of cells developing neurites and the neurite length (Fig. 4D) were reduced significantly. This effect is not due to general toxicity (Fig. S4 F and G) and is specific for DRR1 because expressing GFP alone did not show any significant influence on neurite formation (Fig. S4C). N2A cells expressing GFP-DRR1 also showed an increase in punctuate F-actin accumulation (Fig. 4D). Both at 0.1% FCS (Fig. 4D) and at 10% FCS (Fig. S4E), DRR1 expression led to an increase of F-actin, which was even more pronounced than in NIH 3T3 cells (cf. Fig. 2D). This suggests that enhanced DRR1 levels change actin organization, leading to reduced neurite outgrowth.
We also analyzed primary hippocampal neurons 36 h after in vitro cultivation, when structures are still being built up. Endogenous DRR1 localized strongly to the ends of the outgrowing protrusions (Fig. 4E). When these primary neurons were transfected before cultivation, the development of outgrowing protrusions was severely impaired (Fig. 4F). This does not indicate a neurite-destructive activity of DRR1 because DRR1 transfection of primary neurons with already developed neurites, as well as using an inducible expression system turning on DRR1 expression in developed N2A cells, did not reveal an effect on neurites (Fig. S4D). Thus, DRR1 does not degrade neurites, but profoundly affects actin-dependent structural reorganization in cells.
DRR1 Influences Synaptic Function and Complex Behavior.
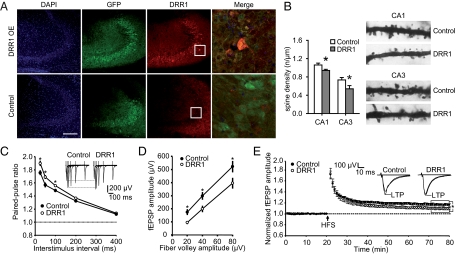
To delineate the contribution of increased DRR1 levels to the overall stress-induced events, we stably increased DRR1 expression in the hippocampus using an adeno-associated viral vector (Fig. 5A). The magnitude of DRR1 overexpression was comparable to that found after food deprivation or dexamethasone treatment on the mRNA as well as on the protein level (Fig. 1 and Fig. S5A). Golgi-Cox staining revealed a significant reduction of spine density on apical dendrites of CA3 and CA1 pyramidal neurons of mice with increased DRR1 expression (Fig. 5B). This finding, together with the predominantly presynaptic localization of DRR1 (Fig. 4C) and its enhancing effect on actin polymerization and bundling (Fig. 3), led us to hypothesize that elevated DRR1 expression might also reduce synaptic neurotransmitter release. The reported increased probability of neurotransmitter release at excitatory hippocampal synapses upon depolymerization of presynaptic actin (9) also supported the hypothesis of an opposite effect of DRR1. We tested the hypothesis by field excitatory postsynaptic potential (fEPSP) recordings performed in acute brain slices from mice with enhanced DRR1 levels. Consistent with a reduced probability of synaptic neurotransmitter release, increasing DRR1 enhanced paired-pulse facilitation at CA3-CA1 synapses at interstimulus intervals of 25, 50, and 100 ms (Fig. 5C) and caused a shift of input–output curves toward smaller fEPSP amplitudes (Fig. 5D). Furthermore, enhancing DRR1 levels reduced the magnitude of long-term potentiation (LTP) (Fig. 5E).
Fig. 5.
Effects of virus-mediated DRR1 expression on synaptic transmission and plasticity at hippocampal CA3-CA1 synapses. (A) Visualization of DRR1 expression in the hippocampal CA3 region 5 wk following injection of DRR1-expressing virus (Upper panels) or control virus (Lower panels). (Scale, 200 μM.) (B) Increased hippocampal DRR1 significantly reduced spine density on the apical dendrites of neurons in CA1 and CA3 (CA1: T10 = 2.500, P = 0.031; CA3: T10 = 2.308, P = 0.044). (C) Increased hippocampal DRR1-enhanced paired-pulse facilitation at CA3-CA1 synapses at interstimulus intervals of 25 ms (T26 = −2.125; P = 0.025), 50 ms (T26 = −2.215; P = 0.023), and 100 ms (T26 = −2.118; P = 0.038; n = 14 slices for each group). (D) DRR1 caused a shift of input–output curves toward smaller fEPSP amplitudes (20 μV: T26 = 2.863, P = 0.007; 40 μV: T26 = 2.377, P = 0.022; 80 μV: T26 = 2.131, P = 0.048; n = 14 slices for each group). (E) The magnitude of LTP induced by high-frequency stimulation (HFS, 100 Hz for 1 s) in slices from control animals was higher than in slices from mice with increased DRR1 expression (T26 = 2.128; P = 0.043; n = 14 slices for each group). fEPSP amplitudes were normalized to the mean fEPSP amplitude of the last 10 min of baseline recording.
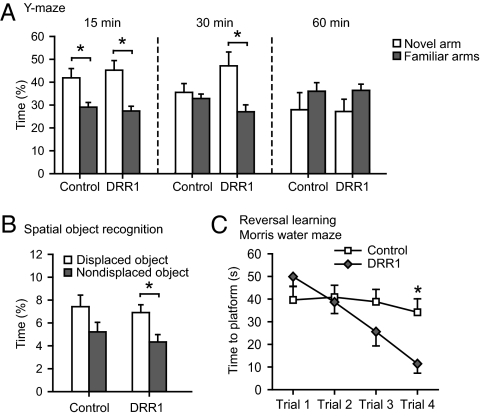
The DRR1-induced changes in spine density and synaptic function in hippocampal slices (Fig. 5) prompted us to test the hypothesis that DRR1 may also play a role in the execution of stress effects on hippocampus-dependent cognitive function. Enhanced DRR1 expression had no obvious negative effects on the animals. Furthermore, we did not detect any alteration in locomotor activity, either under home-cage conditions or in an open field arena (Fig. S5 B and C). In the Y-maze test for hippocampus-dependent spatial memory, enhanced DRR1 expression prolonged the time animals remembered the acquisition setting (Fig. 6A). This finding is supported by another hippocampus-dependent test, the object relocation test. Here, animals with enhanced DRR1 expression distinguished between the replaced and the nonreplaced object following a 30-min intertrial interval, whereas the controls did not (Fig. 6B). In the Morris water maze, the animals behaved normally, and there were no significant differences in the acquisition of the task (Fig. S5D). However, animals with enhanced expression of DRR1 showed a significantly improved cognitive flexibility in the reversal learning task (Fig. 6C). Thus, DRR1 not only affects synaptic function, but intriguingly, also improves cognitive performance, despite its suppressive effect on LTP.
Fig. 6.
Virus-induced hippocampal DRR1 expression improves cognition. (A) Virus-mediated expression of DRR1 in the hippocampus improved spatial memory in the Y-maze test at a retention interval of 30 min (Center) (15 min: control—T30 = 2.804, P = 0.009; DRR1—T30 = 3.81, P = 0.001); 30 min: DRR1—T26 = 2.957, P = 0.007). (B) Animals with enhanced DRR1 expression show improved cognition in the object relocation test (T28 = 2.747; P = 0.010). (C) DRR1-overexpressing animals displayed a significantly improved cognitive flexibility in the reversal learning task of the Morris water maze [ANOVA: time (F1,28 = 33.653; P = 0.0002); treatment–time interaction (F1,28 = 18.957; P = 0.0001)].
Discussion
Deciphering the molecular players is paramount to understanding how stress ultimately leads to changes at the behavioral level. We identified DRR1 as a stress- and glucocorticoid-responsive protein that impacts on F-actin formation, providing a direct molecular link between stress and actin-dependent processes. DRR1, moreover, is able to modify synaptic efficacy and to improve cognition. DRR1 was proposed previously as a tumor suppressor and is thus another example of a multifunctional protein. Given the importance of actin filament reorganization in mitosis and cancer (10), the elucidation of DRR1's molecular function as an actin modulatory factor may also provide further insights into its role in tumorigenesis (11).
As a major cytoskeletal protein expressed in pre- and postsynaptic structures, actin is a key molecule for shaping neuronal morphology and function. DRR1 has the potential to directly affect information processing of single neurons by altering the interplay between presynaptic elements and dendritic spines. Modulation of actin dynamics may thus function as a highly effective way to control synaptic efficacy and complex behavior (5, 12).
Mechanistically, we consider it unlikely that DRR1 acts indirectly on actin, as we observed the bundling effect with purified recombinant DRR1 and purified actin preparations. DRR1 may instead act similarly to the known actin cross-linking proteins β-actinin, filamin, or spectrin (5, 13), with either two binding sites for actin or the ability to dimerize. Our in silico search revealed also a predicted coiled coil region, pointing to the possibility of the protein to dimerize or even oligomerize (Fig. S2A).
DRR1 RNA levels are highest in brain (14). Its brain region-specific expression and stress regulation suggests a rather distinct function, which may be additionally under epigenetic control (6). Of particular relevance to stress physiology is the prominent expression and induction of DRR1 in the hippocampus, as this brain region is particularly rich in corticosteroid receptors and critically influences the stress hormone cascade and cognition (1). The use of a viral expression system allowed us to specifically mimic the stress-induced increase of DRR1 in this brain region, thereby distinguishing its effects from those of the plethora of other stress-induced factors (15).
Enhancing DRR1 reduced the number of spines. A similar reduction in the number of spines is induced after stress exposure in rodents (16) and in depression in humans (17). Because enhanced levels of DRR1 in neuronal cells hampered neurite outgrowth, but had no effect on already developed neurites, it is likely that DRR1 affects the formation and stability of spines, a process recently discovered as essential for learning and memory (18–20). Thus, DRR1 can influence actin dynamics in axon growth and spine dynamics, processes that occur at different developmental stages but that are based on remarkably similar molecular mechanisms (21). In general, the timing and magnitude of DRR1 increase might be crucial, and—similar to stress—long-term exposure may also have maladaptive consequences.
By changing actin dynamics, presynaptic DRR1 has the ability to use an efficient way to impact on the efficacy of neurotransmitter release (9) and adds to the emerging concept of regulation of actin dynamics as a pivotal element in controlling dendritic structure and synaptic transmission (22). For example, latrunculin A—a substance that, by promoting actin depolymerization, exerts an effect on actin dynamics opposite to that of DRR1—also caused the opposite electrophysiological effects, i.e., a decreased paired-pulse ratio and an increased input–output relationship (9). In hippocampal synaptic boutons, vesicle mobility has been shown to be increased upon depolymerization of F-actin (9). We therefore hypothesize that DRR1 reduces the likelihood of synaptic vesicles being recruited into the readily releasable pool at the presynaptic terminal.
It is not unexpected that a multifunctional, actin modulatory protein impacts on cognition (23). However, although there are numerous ways to disrupt memory, factors enhancing cognition have rarely been identified and are of great interest (24). Enhancement of DRR1 in the hippocampus produced increased cognitive capacity and flexibility, as indicated by our data from the Morris water maze, the Y-maze, and the object relocation test. The exact molecular mechanism of DRR1 in enhancing memory still remains to be elucidated in detail, in particular because the combination with the structural and electrophysiological alterations induced by enhanced DRR1 levels is rather unusual. Improved memory is often associated with increased LTP (25). However, there is also evidence that hippocampal LTP in brain-slice preparations does not fully picture the in vivo processes underlying learning and memory (26). For example, mice lacking the AMPA receptor subunit GluR1 have deficits in LTP at CA3-CA1 synapses, but display normal spatial reference memory (27). Thus, although decreased LTP in slices from mice with enhanced levels of DRR1 indicates an impact of DRR1 on hippocampal long-term synaptic plasticity, the data cannot be directly correlated to the behavioral phenotype of the animals.
It is notable that the F-actin–stabilizing capability of DRR1 did not affect memory acquisition, but is apparently important during consolidation or retrieval. Therefore, DRR1 may be involved in mediating the observed time- and context-dependent positive effects of stress on cognition (28, 29).
In sum, among a number of actin-binding proteins (30), DRR1 is specified by its stress-regulation. We suggest it as a candidate protein that translates experience of stressful life events into cellular and structural adaptive mechanisms and the modulation of complex behavioral traits. This proposed molecular route in stress adaptation has to be considered when designing compounds and regimes for drug treatment to prevent activity-dependent, long-term changes in transducing and suppressive systems for stress.
Materials and Methods
Detailed information on experimental procedures is provided in SI Materials and Methods.
Animal Stress Experiments.
Male C57/Bl6N mice were used for all experiments. Maternal separation took place commencing at postnatal day 8 for 24 h. For food deprivation, animals were placed in a new cage without access to food 24 h before testing.
Behavioral Analyses.
In the Y-maze test, the animals were allowed to explore two of the three arms for 10 min. After a 15-, 30-, or 60-min intertrial interval (ITI), a second trial was conducted during which all three arms were accessible for 5 min.
In the spatial object relocation task, the animals explored an open field arena, equipped with two identical aluminum cubes, two times for 10 min with a 15-min ITI. During a 5-min retrieval, 30 min following the last acquisition trial, one of the objects was moved to the opposite corner.
In the water maze, the training phase consisted of four trials each on three consecutive days (ITI 10 min). Probe trial was on the following day without platform for 1 min. Cognitive flexibility was tested in a four-trial reversal learning task with a different platform location.
Neurite Outgrowth.
Neurite formation in N2A cells were induced by serum withdrawal 24 h after transfection, and neurite length was determined 24 h later (ImageJ). For neuronal polarization, hippocampal neurons were transfected at seeding day and analyzed 2 d later.
F-Actin Cosedimentation.
F-actin was formed in vitro by addition of filament-promoting buffer to purified nonmuscle β-actin. For filament breakdown assays, either F-actin was formed and diluted in the presence or absence of DRR1 or F-actin was incubated with DRR1 before dilution. F-actin was separated from G-actin by ultracentrifugation.
Pyrene–Actin Assay.
Actin polymerization was measured by monitoring the change in fluorescence intensity of 4 μM of partially (10%) pyrene-labeled actin. Polymerization reactions were performed at 25 °C and initiated by adding 0.5× filament promoting buffer.
F-Actin Visualization.
Fluorescent F-actin was visualized by total internal reflection microscopy. A total of 1 μM AlexaFluor-568–labeled actin (20% label) was polymerized at 25 °C. Different molar ratios of DRR1 to actin or buffer only were added to the preformed F-actin as indicated.
Viral Overexpression of DRR1.
Adeno-associated bicistronic AAV1/2 vector was used for viral expression of DRR1 together with GFP or with GFP alone as control. Twelve-week-old mice were bilaterally injected in the dorsal hippocampus with 1 μL of either AAV-DRR1 or AAV-EGFP. Testing started 4 wk later.
Electrophysiology.
Coronal slices containing the dorsal hippocampus (350 μm) were prepared from DRR1 overexpressing or control mice (n = 6, two to three slices from each animal). Recordings were in artificial cerebrospinal fluid at 25 °C. Square-pulse electrical stimuli (0.066 Hz, 1–6 V, 50 μs) were delivered within the stratum radiatum of the CA1 region, and evoked fEPSPs were recorded. LTP was induced by high-frequency stimulation (100 stimuli at 100 Hz). The paired-pulse ratio was calculated as fEPSP2 amplitude/fEPSP1 amplitude.
Supplementary Material
Acknowledgments
We thank Carsten Wotjak and Cornelia Kamprath for support with virus application, Nils Gassen and Kathrin Hafner for help with protein purification, Daniela Harbich and Bianca Mayer for help with animal experiments, Anja Kretzschmar for help with caspase staining, and Andreas Schaupp for expert advice on quantitative colocalization analysis. This work was supported by the Klinische Kooperationsgruppe of the Max Planck Institute of Psychiatry and Helmholtz Zentrum Muenchen; by the BMBF-NGFN framework [grants from the German Federal Ministry of Education and Research (NGFN01GS08151 to F.H. and W.W. and DIGTOP-01GS0858 to W.W.)]; and by the Helmholtz Society (Helmholtz Alliance for Mental Health in an Ageing Society).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103318108/-/DCSupplemental.
References
- 1.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 2.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 3.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci. 2006;11:2746–2758. doi: 10.2741/2004. [DOI] [PubMed] [Google Scholar]
- 5.Cingolani LA, Goda Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 6.Awakura Y, Nakamura E, Ito N, Kamoto T, Ogawa O. Methylation-associated silencing of TU3A in human cancers. Int J Oncol. 2008;33:893–899. [PubMed] [Google Scholar]
- 7.Liebl C, et al. Gene expression profiling following maternal deprivation: Involvement of the brain renin-angiotensin system. Front Mol Neurosci. 2009;2:1. doi: 10.3389/neuro.02.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayas CL, Avila J, Wandosell F. Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms. J Neurosci. 2002;22:6863–6875. doi: 10.1523/JNEUROSCI.22-16-06863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 10.Jordan MA, Wilson L. Microtubules and actin filaments: Dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123–130. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 11.Le PU, et al. DRR drives brain cancer invasion by regulating cytoskeletal-focal adhesion dynamics. Oncogene. 2010;29:4636–4647. doi: 10.1038/onc.2010.216. [DOI] [PubMed] [Google Scholar]
- 12.Bamburg JR, Wiggan OP. ADF/cofilin and actin dynamics in disease. Trends Cell Biol. 2002;12:598–605. doi: 10.1016/s0962-8924(02)02404-2. [DOI] [PubMed] [Google Scholar]
- 13.Revenu C, Athman R, Robine S, Louvard D. The co-workers of actin filaments: From cell structures to signals. Nat Rev Mol Cell Biol. 2004;5:635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- 14.Su AI, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soetanto A, et al. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoogenraad CC, Bradke F. Control of neuronal polarity and plasticity: A renaissance for microtubules? Trends Cell Biol. 2009;19:669–676. doi: 10.1016/j.tcb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Rust MB, et al. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29:1790–1791. doi: 10.1038/emboj.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaillend C, Poirier R, Laroche S. Genes, plasticity and mental retardation. Behav Brain Res. 2008;192:88–105. doi: 10.1016/j.bbr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y-S, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau JLW, et al. Enhanced hippocampal long-term potentiation and spatial learning in aged 11beta-hydroxysteroid dehydrogenase type 1 knock-out mice. J Neurosci. 2007;27:10487–10496. doi: 10.1523/JNEUROSCI.2190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hölscher C. Long-term potentiation: A good model for learning and memory? Prog. Neuropsychopharmacol Biol Psychiatry. 1997;21:47–68. doi: 10.1016/s0278-5846(96)00159-5. [DOI] [PubMed] [Google Scholar]
- 27.Reisel D, et al. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- 28.Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revest J-M, et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 30.Uribe R, Jay D. A review of actin binding proteins: New perspectives. Mol Biol Rep. 2009;36:121–125. doi: 10.1007/s11033-007-9159-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.