Abstract
Ookinete invasion of the mosquito midgut is an essential step for the development of the malaria parasite in the mosquito. Invasion involves recognition between a presumed mosquito midgut receptor and an ookinete ligand. Here, we show that enolase lines the ookinete surface. An antienolase antibody inhibits oocyst development of both Plasmodium berghei and Plasmodium falciparum, suggesting that enolase may act as an invasion ligand. Importantly, we demonstrate that surface enolase captures plasminogen from the mammalian blood meal via its lysine motif (DKSLVK) and that this interaction is essential for midgut invasion, because plasminogen depletion leads to a strong inhibition of oocyst formation. Although addition of recombinant WT plasminogen to depleted serum rescues oocyst formation, recombinant inactive plasminogen does not, thus emphasizing the importance of plasmin proteolytic activity for ookinete invasion. The results support the hypothesis that enolase on the surface of Plasmodium ookinetes plays a dual role in midgut invasion: by acting as a ligand that interacts with the midgut epithelium and, further, by capturing plasminogen, whose conversion to active plasmin promotes the invasion process.
Keywords: transmission-blocking vaccines, insect vectors of disease
Few weapons are available to fight the unbearable burden of malaria (1, 2). For transmission to occur, Plasmodium, the causative agent of malaria, must complete a complex and incompletely understood developmental program in its vector mosquito (3). Plasmodium differentiation in the mosquito begins in the midgut lumen with fertilization and differentiation of the resulting zygote into a motile ookinete. After crossing the midgut, the ookinete differentiates into an oocyst that, when mature, releases thousands of motile sporozoites that, in turn, invade the salivary glands. Ookinete invasion of the midgut is a crucial step, the failure of which results in aborted development and unsuccessful transmission. Little is known about the molecular events that lead to midgut invasion. Circumstantial evidence suggests that invasion of the mosquito midgut by ookinetes requires specific interactions between the parasite and the epithelial surface (4, 5). In an attempt to elucidate these interactions at the molecular level, we have previously screened a phage display library for peptides that bind to the Anopheles midgut epithelium. This screen led to the identification of Salivary gland and Midgut peptide 1 (SM1), a dodecapeptide that binds tightly to the midgut luminal surface and, importantly, efficiently inhibits Plasmodium berghei ookinete invasion (4). Based on these results, we hypothesized that SM1 mimics the domain of an ookinete surface protein ligand involved in the recognition of a midgut receptor.
Here, we show that SM1 is a mimotope of the ookinete surface protein enolase. Moreover, enolase interacts with the abundant mammalian plasma protein plasminogen, and this interaction appears to be essential for progression of the Plasmodium life cycle in the mosquito. The results suggest that in evolution, a close relationship developed between the three relevant organisms, with the parasite having coopted plasminogen from its mammalian host to invade its mosquito vector.
Results
Anti-SM1 Antibody Recognizes Ookinete Surface Component(s).
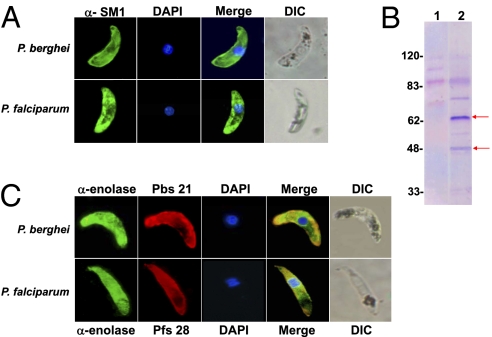
Our previous phage display library screening led to the identification of the SM1 dodecapeptide that not only binds to the luminal surface of the mosquito midgut but, importantly, strongly inhibits ookinete invasion (4). These results led to the hypothesis that SM1 mimics (mimotope) an ookinete surface ligand that interacts with a putative midgut receptor and that this interaction is required for invasion. According to this premise, the peptide would bind to, and sterically shield, the putative mosquito receptor, precluding its interaction with the ookinete ligand. This hypothesis advocates for the similarity between SM1 and an unknown ookinete invasion ligand. To test this prediction, we produced an anti-SM1 antibody and used it as a probe in immunofluorescence assays to determine whether the antibody recognizes an ookinete surface component. As shown in Fig. 1A, the antibody actually binds to the surface of both P. berghei and Plasmodium falciparum ookinetes. Control experiments indicated that antibodies cannot recognize cytoplasmic proteins of nonpermeabilized ookinetes (Figs. S1 and S2). To identify the protein(s) specifically recognized by the antibody, we analyzed ookinete proteins by Western blotting using our anti-SM1 antibody for detection. As shown in Fig. 1B, anti-SM1 antibodies recognized two major P. berghei proteins of ∼65 kDa and ∼48 kDa. Other protein bands were either also present when incubated with control preimmune serum or were not detected reproducibly in repeat experiments. Additional fractionation of the ookinete extracts by 2D gel electrophoresis (Fig. S3), followed by mass spectrometric analysis of the excised proteins, revealed that the ∼65-kDa protein is an RNA helicase and the ∼48-kDa protein is enolase (EC 4.2.1.11). Because RNA helicase is a cytoplasmic protein, we focused our efforts on establishing the possible functional significance of enolase on the ookinete surface.
Fig. 1.
Binding of the anti-SM1 and anti-Plasmodium enolase antibodies to ookinetes. (A) Anti-SM1 antibody was incubated with fixed but nonpermeabilized P. berghei (Upper) or P. falciparum (Lower) ookinetes, and binding was detected with Alexa Fluor-488–labeled (green) secondary antibody. Nuclei were detected by DAPI staining (blue). The last panel in each row shows a differential interference contrast (DIC) image of the same ookinete. (B) Western blot of purified P. berghei ookinetes (5 × 106 per lane). Lane 1 shows incubation with preimmune serum. Lane 2 shows incubation with anti-SM1 antibody from the same rabbit. The arrows point to proteins specifically and reproducibly recognized by the anti-SM1 antibody. (C) Binding of an anti-P. falciparum enolase antibody to ookinetes. (Upper) Nonpermeabilized P. berghei ookinete incubated with a mixture of antienolase antibody (green), antibody against the surface Pbs21 protein (red), and the DAPI nuclear stain (blue). (Lower) Nonpermeabilized P. falciparum ookinete incubated with a mixture of antienolase antibody (green), an antibody against the surface protein Pfs28 (red), and the DAPI nuclear stain (blue). Between 72 and 110 ookinetes were analyzed for each of the staining protocols, and 100% of the ookinetes displayed the pattern illustrated. A small proportion (<10%) of the ookinetes did not stain with any of the reagents and may represent dead parasites.
Enolase Occurs on the Ookinete Surface.
To investigate whether enolase is indeed present on the ookinete surface, we produced recombinant P. falciparum enolase that was used to generate a rabbit polyclonal antibody. Immunofluorescence assays provided evidence that enolase is on the surface of both P. berghei and P. falciparum ookinetes (Fig. 1C). Control experiments showed that general antibody access to internal structures required permeabilization (Figs. S1 and S2), thus supporting the conclusion that the anti-SM1 and antienolase antibodies bind to a surface ookinete component. Developmental studies indicated that surface enolase cannot be detected in gametocytes, gametes, and zygotes and that it is initially detected at the retort (immature ookinete, ∼12 h) and later stages of ookinete differentiation, both for P. berghei and P. falciparum (Fig. S4). Immunoelectron microscopy confirmed the presence of enolase on the ookinete surface, especially on the apical pellicle complex that is involved in invasion (Fig. 2 A and B). Enolase is a cytosolic protein involved in glycolysis, but a significant proportion (10%) of the protein was detected on the ookinete surface (Fig. 2C).
Fig. 2.
Ultrastructural detection of enolase in Plasmodium ookinetes. (A and B) Two examples of enolase localization (gold particles) using a polyclonal anti-P. falciparum enolase antibody and cultured P. berghei ookinetes. Surface localization is indicated by arrows. (C) Stereological analysis of immunogold-labeled structures. The density (gold particles per mm2) of labeled structures was determined from 20 cryosections. The percentage of individual intracellular compartment density was determined from the sum of gold density normalized for the variation in expression of enolase. Although the majority of enolase localized to the cytoplasm, a significant proportion was located on the surface and in the nucleus, consistent with the enolase distribution in blood forms (20). Gold particles on single- or double-membrane–bound compartments excluding the nucleus (organelles) correspond to negligible background. DV, digestive vacuole; IMC, inner membrane complex; mi, secretory microneme organelle; PM, plasma membrane. (Scale bar: 200 nm.)
Antienolase Antibody Inhibits Oocyst Formation.
The polyclonal anti-P. falciparum enolase antibody was used to investigate whether surface enolase plays a role in midgut invasion. Oocyst formation was used as a proxy for ookinete invasion of the mosquito midgut. We found that the anti-Plasmodium enolase antibody efficiently inhibited P. berghei (Fig. 3A) and P. falciparum (Fig. 3B) oocyst formation, consistent with the concept that surface enolase plays an important role in mosquito midgut invasion. Moreover, these experiments provided further in vivo evidence for the presence of enolase on the ookinete surface.
Fig. 3.
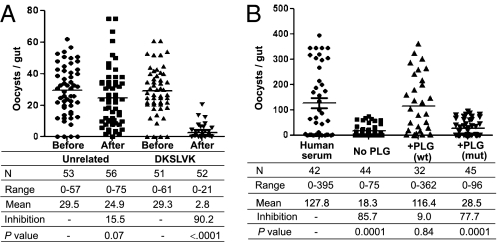
Inhibition of P. berghei and P. falciparum oocyst formation by antienolase antibody. (A) Inhibition of P. berghei oocyst formation by antienolase antibody. For each experiment, a group of control An. gambiae mosquitoes was fed on a P. berghei-infected mouse (before). The mouse was then injected i.v. with 1 mg (total protein) of preimmune or antienolase serum, and after 10 min, a group of experimental mosquitoes was fed on the same mouse (after). Data for three independent experiments were pooled. N represents the number of mosquitoes analyzed, range represents the minimum and maximum number of oocysts per gut, mean indicates the mean number of oocysts per gut, and inhibition indicates the percent decrease of oocyst numbers. The P value was calculated by the Mann–Whitney test. (B) Inhibition of P. falciparum oocyst formation by antienolase antibody. P. falciparum gametocytes were mixed with preimmune or immune sera (1 mg/mL total serum protein) and fed to An. gambiae mosquitoes. Data for three separate experiments were pooled.
Enolase Mediates the Binding of Plasminogen to the Ookinete Surface.
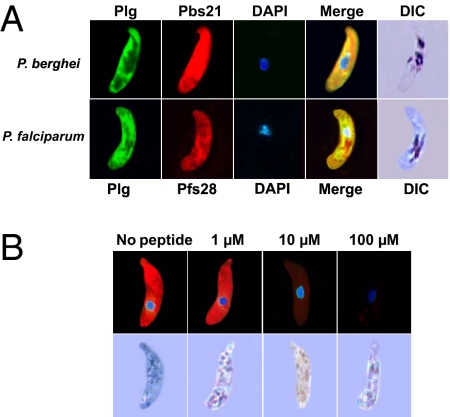
Enolase from various microorganisms has a unique 6-aa motif that is recognized by the lysine-binding Kringle domains of plasminogen from various mammalian species (6, 7). Our bioinformatic analyses revealed that this lysine motif, DKSLVK, is conserved among enolases of several microbial pathogens and is identical in all Plasmodium species sequenced to date (8–13) (Table S1). Based on this information, we predicted that the lysine motif of enolase on the ookinete surface mediates the binding to the mammalian plasminogen present in the ingested blood meal. As shown in Fig. 4A, immunofluorescence assays revealed that plasminogen binds to both P. berghei and P. falciparum ookinetes. This interaction is specific, as indicated by the competitive inhibition of plasminogen binding by the lysine motif peptide DKSLVK (Fig. 4B and Fig. S5). Control experiments indicated that inhibition of plasminogen binding by the DKSLVK peptide is specific because it did not affect binding of anti-Pbs21 and antienolase antibodies to the ookinete surface (Fig. S6 A and B). Furthermore, a peptide derivative that had the two lysines replaced by alanines did not inhibit plasminogen binding (Fig. S6C).
Fig. 4.
Immunolocalization of plasminogen on the ookinete surface and competition with the lysine-motif peptide (100%). (A) (Upper) Nonpermeabilized cultured P. berghei ookinetes were incubated with a mixture of antiplasminogen (Plg; green) and anti-Pbs21 (red) antibodies. Nuclear staining with DAPI is shown in blue. DIC, differential interference contrast microscopy. (Lower) Similar experiment with cultured P. falciparum ookinetes, except that anti-Pfs28 (red) antibodies were used instead of anti-Pbs21. One hundred percent of the ookinetes that stained with the anti-Ps21 or anti-Pfs28 antibodies also stained with plasminogen. (B) (Upper) Plasminogen binding to the P. berghei ookinete surface is competed by the lysine-motif peptide DKSLVK. Peptide concentrations were as indicated in each panel. (Lower) DIC images of the ookinetes shown.
Plasminogens Necessary for Ookinete Invasion of the Mosquito Midgut.
Several experimental approaches were used to investigate the importance of plasminogen binding to the ookinete surface. When mosquitoes fed on P. berghei-infected mice that had been injected with the DKSLVK peptide, parasite development into oocysts was strongly inhibited compared with that in mosquitoes fed on mice receiving a similar dose of an unrelated peptide (Figs. 4B and 5A). Similar results were obtained by use of the lysine analog 6-amino hexanoic acid (6HA) (Fig. S7). These results suggest that the plasminogen-enolase interaction plays an important role in mosquito infection. This role was further tested by feeding mosquitoes on a P. falciparum culture suspended in plasminogen-free medium (plasminogen-depleted human serum, Fig. S8). As shown in Fig. 5B, removal of plasminogen had a strong inhibitory effect on parasite development. This inhibitory effect is indeed caused by the lack of plasminogen, per se, because the ability of the depleted medium to support parasite development could be restored by the addition of recombinant plasminogen (Fig. 5B). Plasminogen is the zymogen form of the broad-spectrum serine protease plasmin. To investigate the importance of the catalytic activity of plasmin for oocyst differentiation, we added a recombinant plasminogen carrying a point mutation (S741A) in the catalytic triad. In contrast to the WT protein, the inactive plasminogen was unable to rescue the impaired oocyst formation phenotype of the plasminogen-depleted serum (Fig. 5B), providing direct evidence for the requirement of unimpaired catalytic activity of the surface-bound plasminogen to complete ookinete invasion. We therefore conclude that mammalian plasminogen plays a crucial role in the ability of the parasite to complete its developmental cycle in the mosquito.
Fig. 5.
Ookinete dependence on its surface enolase and on interaction with mammalian plasminogen for invasion of the mosquito midgut. (A) Inhibition of P. berghei oocyst formation by the DKSLVK peptide. For each experiment, a group of control An. gambiae mosquitoes was fed on a P. berghei-infected mouse (before). The mouse was then injected i.v. with 200 μg of the DKSLVK peptide or of an unrelated control peptide (QPQHFR), and after 10 min, a group of experimental mosquitoes was fed on the same mouse (after). Data for three independent experiments were pooled. N indicates the number of mosquitoes analyzed, range indicates the minimum and maximum number of oocysts per gut, mean indicates the mean number of oocysts per gut, and inhibition indicates the percent decrease of oocyst numbers. The P value was calculated by the Mann–Whitney test. (B) P. falciparum dependence on mammalian plasminogen for oocyst formation. An. gambiae mosquitoes were fed on P. falciparum gametocytes suspended in normal human serum in plasminogen-depleted human serum (No PLG) or in plasminogen-depleted serum with added recombinant WT plasminogen [200 μg/mL; +PLG (wt)] or with added recombinant mutant plasminogen carrying a point mutation in the active site (S741A) [200 μg/mL; +PLG (mut)], as indicated. Data for three independent experiments were pooled.
Discussion
SM1 Mimics Enolase.
SM1 is a dodecapeptide that binds tightly to the luminal surface of the mosquito midgut epithelium, and by doing so, it efficiently inhibits ookinete invasion (4, 14). These observations suggest that the peptide binds to a midgut receptor, and by doing so, it competitively inhibits binding to an ookinete ligand, thus interfering with invasion (Fig. 6, Center). In this scenario, peptide conformation resembles that of an ookinete ligand involved in receptor binding. Bioinformatic searches for Plasmodium proteins containing sequences similar to the 8-aa loop were unsuccessful. To address the possible similarity of the SM1 structure to that of an ookinete protein involved in invasion, we generated an anti-SM1 antibody that recognizes enolase, leading to the discovery that this protein is present on the ookinete surface. These observations constitute circumstantial evidence supporting the hypothesis that one function of surface enolase is to interact with a putative mosquito midgut receptor for invasion (Fig. 6). The fact that antienolase antibody inhibits ookinete invasion (Fig. 3 A and B) is consistent with this hypothesis. Our previous findings revealed that SM1 also binds to the outer surface of the salivary glands (4). We found that SM1 mimics the sporozoite ligand thrombospondin-related anonymous protein (TRAP) because this protein is also recognized by the anti-SM1 antibody. As expected, TRAP and SM1 competitively bind to the salivary gland receptor saglin (15). TRAP is not expressed by ookinetes, and saglin is not expressed in midgut epithelial cells, however. It seems that enolase is the ookinete SM1 mimotope (it is recognized by the anti-SM1 antibody) and that it binds to a midgut receptor different from saglin.
Fig. 6.
Hypothetical model for the role of enolase in ookinete midgut invasion. (Left, No SM1 peptide) About midway in their development in the mosquito midgut, ookinetes start expressing enolase on their surface (Fig. S4). (Right) Plasminogen from the surrounding blood meal (Figs. 4 and 5A and Figs. S6 and S7) is captured by the 6-aa C-terminal lysine domain of enolase (lysine motif). Plasminogen is subsequently converted into active plasmin (Fig. 5B), a broad-spectrum serine protease, thus promoting the hydrolysis of glycocalyx components. Local breach of glycocalyx integrity facilitates ookinete access to the midgut epithelium and interaction of a separate enolase domain (SM1-like domain) with a putative midgut receptor, thus promoting invasion. SM1 peptide (+SM1 peptide) mimics the SM1-like domain of enolase [anti-SM1 antibody recognizes enolase (Fig. 1B and Fig. S3) and competes with it for binding to the putative midgut receptor]. When the midgut receptors are occupied by the SM1 peptide, interaction with enolase on the surface of the ookinete is abrogated and invasion is aborted.
Enolase Is Exported to the Ookinete Surface.
Several pathogenic microorganisms from bacteria to Leishmania and Plasmodium merozoites also express enolase on their surface (10, 16–20). We found that enolase is also exported to the surface of P. berghei and P. falciparum ookinetes. None of the enolases identified to date has a signal sequence or a transmembrane domain, and the mechanisms of enolase export and adhesion to the cell surface are unknown (20, 21). In yeast, the N-terminal 169-aa residues of enolase are required for export to the surface, but the exact motif or mechanism has not been identified (22).
Our experiments (Fig. 3 A and B) suggest that enolase may serve as an antigen for transmission-blocking vaccines. There is evidence that enolase also occurs on the surface of merozoites and that antienolase antibodies inhibit the growth of blood-stage parasites in vitro (20). Interestingly, high-titer antienolase antibodies are found in malaria patients in Asia, but the significance of this finding has not yet been assessed (23).
Surface Enolase Captures Plasminogen from the Blood Meal.
Plasmodium enolase contains a C-terminal 6-aa lysine motif that is identical in all Plasmodium species sequenced to date and is also conserved in other pathogenic microorganisms (7, 13) (Table S1). This motif endows enolase with plasminogen-binding capacity, thus enabling the capture of plasminogen from the surrounding environment. Binding of plasminogen to the lysine motif of enolase is governed by five Kringle domains in the A-chain of plasminogen (24). Experiments showing that the lysine motif peptide competitively inhibits plasminogen binding (Fig. 4B and Fig. S6C) strongly support the concept that this interaction provides the basis for plasminogen binding to the ookinete surface. 6HA is a lysine analog that has been used in vitro to inhibit clot lysis (25). The Kringle domains of plasminogen bind to the terminal lysine residue of fibrin, triggering its degradation. 6HA binds with high affinity to Kringle domains, thus inhibiting plasminogen activation and fibrin degradation (26, 27). Similarly, 6HA had a strong inhibitory effect on oocyst formation, providing additional evidence that plasmin plays an important role in ookinete invasion of the mosquito midgut.
Plasminogen Is Necessary for Midgut Invasion.
Plasminogen is the most abundant serine protease in mammalian blood (1–2 μM). Several pathogens hijack host plasminogen to assist in tissue and organ invasion (6, 7, 28–32). The present experiments indicate that plasminogen, and its conversion into active plasmin, is essential for invasion of the mosquito midgut. Thus, in evolution, Plasmodium seems to have coopted mammalian plasminogen to invade its invertebrate host. The substrate of the activated plasmin in the mosquito midgut is presently unknown. The luminal surface of the mosquito midgut is lined by a network of proteins and carbohydrates (33) that the ookinete must traverse to reach the surface of the midgut epithelium. Plasmin may degrade components of this network to facilitate invasion of the midgut epithelium (Fig. 6).
Concluding Remarks
Our experiments demonstrate that enolase occurs on the surface of Plasmodium ookinetes, where we hypothesize it acts in two independent capacities: (i) to mediate recognition of the mosquito midgut epithelium during invasion and (ii) to mediate the binding of mammalian plasminogen that is subsequently converted to active plasmin. These two functions may be performed by different domains of the enolase protein (Fig. 6). Identification of the putative midgut receptor will allow a direct test of this hypothesis.
Materials and Methods
Mosquitoes.
The Anopheles gambiae Keele and Anopheles stephensi strains were maintained as described (15).
Parasites.
P. berghei strain ANKA 2.34 was maintained by passage in Swiss Webster mice (34, 35). P. falciparum NF54 strain was maintained according to standard methods (36). Gametocyte cultures were started at 0.5% parasitemia, and the medium was changed daily until mature gametocytes were obtained (usually on day 18) (37).
Peptide Synthesis.
All peptides were obtained from Genmed Synthesis, Inc.
Ookinete Culture.
In vitro differentiation of gametocytes into P. berghei or P. falciparum ookinetes was as described elsewhere (38). P. falciparum ookinetes were produced by incubating a gametocyte-enriched culture for 24 h, after which they were purified (34). Ookinete numbers were determined by microscopic examination with a hemocytometer. For peptide competition assays (Fig. 4B), gametocytes were cultured in ookinete transformation medium with FBS. After 6 h, the culture was centrifuged at 659 × g for 5 min, washed with ookinete medium without serum, and resuspended in ookinete medium containing plasminogen-depleted 10% (vol/vol) FBS, and parasites were allowed to differentiate further. Plasminogen was depleted with lysine-Sepharose beads (GE Healthcare, Inc.) per the manufacturer's protocol. Depletion was verified by Western blot analysis (Fig. S5).
Western Blotting.
About 5 × 106 purified ookinetes were resuspended in 20 μL of Laemmli buffer (39) and lysed by boiling. After centrifugation, the supernatant was fractionated by electrophoresis on a 10% (wt/vol) polyacrylamide-SDS gel, followed by transfer to a PVDF membrane (ISEQ 00010; Millipore Corporation). Remaining procedures were as described elsewhere (15).
2D Gel Electrophoresis and Western Blot Analysis.
Purified P. berghei ookinetes were separated by 2D gel electrophoresis as described elsewhere (40, 41). Three identical gels were run in parallel, two for Western blotting (one with immune serum and another with preimmune serum) and the third for recovering protein spots for sequencing by tandem MS (15, 42).
Immunofluorescence Assays.
Immunofluorescence assays were performed as described elsewhere (4). Frozen samples were prepared as follows. Purified ookinetes were smeared on slides maintained over ice, followed by immersion into 4% (wt/vol) paraformaldehyde solution for 1 h at 4 °C and three washes in cold PBS for 15 min each. Slides were stored at −80 °C until use. Frozen slides containing fixed ookinetes were thawed at room temperature for 30 min and dipped in blocking buffer [4% (wt/vol) BSA in PBS] for 1 h at room temperature. Some samples were permeabilized by incubation in 0.05% Triton X-100 for 30 min at room temperature before dipping in blocking buffer. This was followed by incubation with rabbit anti-SM1 antibody (1:1,000), rabbit antienolase antibody (1:1,000), or mouse anti-Pfs25 monoclonal antibody (1: 2,000) in blocking buffer for 1 h at room temperature. Slides were washed three times in PBS for 30 min each time and incubated in blocking buffer as described above. This was followed by incubation in the dark with Alexa Fluor-488–labeled anti-rabbit IgG (1:1,000, A1108; Invitrogen, Inc.) or with Alexa Fluor-586–labeled anti-mouse IgG (1:1,000, A21043; Invitrogen, Inc.) for 45 min at room temperature. Slides were washed as before and mounted in ProLong Gold antifade reagent with DAPI (P36935; Invitrogen, Inc.), and pictures were taken with a Leica DMLB microscope. Procedures for the experiments illustrated in Fig. S2 followed a similar protocol, except that fresh ookinetes (never frozen) were used and the entire experiment was conducted on the same day. The anti–α-tubulin antibody (T9026; Sigma) was used at a 1:1,000 dilution.
Expression of Recombinant P. falciparum Enolase.
Total RNA was extracted from an asynchronous P. falciparum NF54 culture using the TRIzol Reagent (15596-018; Invitrogen, Inc.). cDNA was synthesized using an oligo-dT18 primer, and the full-length enolase coding sequence was amplified with the following gene-specific primers: forward primer bearing the EcoR1 site 5′-CCGGAATTCATGGCTCATGTAATAACTCGTATTAATGCC-3′ and reverse primer 5′-GCCGCCGTCGACATTTAATTGTAATCTAAATTTTTCAGC-3′ with a Sal1 site. The PCR amplification products were purified with the QIA quick PCR purification kit (28104; Qiagen), followed by digestion with EcoR1 (RO101L; New England Biolabs) and Sal1 (RO138S; New England Biolabs). The digestion product was gel-purified, cleaned, and ligated to the PGEX-4T vector (Amersham Pharmacia Biotech, Inc.) at its EcoR1 and Sal1 sites. Transformed cells were grown at 25 °C, and expression was induced with isopropyl-β-galactosidase (15529-019; Invitrogen, Inc.) to a final concentration of 10 mM (OD 0.6 at 600 nm). Cells were harvested after 3 h of induction, and protein was purified as described elsewhere (43).
Antibodies and Purified Proteins.
Rabbit polyclonal anti-SM1 antibody and a polyclonal rabbit antienolase antibody were produced as described elsewhere (15). Recombinant human plasminogen and its active site-mutated variant (Ser741Ala) were expressed in Drosophila S2 cells and purified by lysine-Sepharose chromatography (44). The eluted product was buffer-exchanged into PBS.
Feeding P. falciparum Gametocytes to Mosquitoes and Measurement of Oocyst Numbers.
Gametocyte feeding was done as described elsewhere (38). A gametocyte or ookinete culture was added to each feeding chamber, making sure that the culture covered the entire feeding surface. Mosquitoes were allowed to feed on these feeders, and 8 (P. falciparum) or 15 (P. berghei) d later, the midguts were dissected for microscopic determination of oocyst numbers.
Competition Assays.
Slides containing fixed ookinetes (stored at −80 °C) were thawed at room temperature for 30 min and blocked in 4% (wt/vol) BSA solution in PBS (blocking buffer) for 1 h at room temperature. Each slide was incubated for 1 h at room temperature in blocking buffer containing plasminogen (2 μg/mL; Innovative Research, Inc.) or in blocking buffer containing the same concentration of plasminogen and increasing concentrations (1 μM, 10 μM, or 100 μM) of the lysine motif (DKSLVK) or the mutated (DASLVA) peptides. The plasminogen/peptide mixtures were preincubated for 1 h at room temperature before addition to the slides. Slides were washed with PBS three times for 30 min each time, followed by 30 min in blocking buffer. Slides were washed and blocked again as described above and were incubated for 1 h at room temperature with rabbit anti-mouse plasminogen antibody (1:1,000 dilution; Innovative Research, Inc.). Slides were washed and mounted in ProLong Gold antifade, and the images were recorded with the aid of a fluorescence microscope as described above. For experiments illustrated in Fig. S6 A and B, ookinete-containing slides were incubated with 100 μM lysine motif peptide (DKSLVK) for 1 h at room temperature and slides were washed and blocked as before. Slides were then incubated with anti-Pbs21 antibody (1:1,000) or antienolase antibody (1:1,000) for 1 h and washed, and staining was completed using Alexa Fluor-488–labeled anti-mouse IgG (1:1,000) for anti-Pbs21 antibody and Alexa Fluor-488–labeled anti-rabbit IgG (1:1,000) for antienolase antibody. Pictures were taken as described before.
Peptide Inhibition Assays.
Mouse infection with P. berghei was followed by daily measurement of exflagellation events. When exflagellation reached one to two events per field at a magnification of 40×, mice were anesthetized and a group of ∼50 starved An. gambiae females (control group) was allowed to feed for 10 min at 19 °C. A total of 200 μg of the lysine motif peptide (DKSLVK) dissolved in PBS was then injected into the tail vein of the same mouse. After an interval of 10 min to allow dispersal of peptide through the circulation, another group of ∼50 starved An. gambiae females (experimental group) was allowed to feed. Mosquitoes that did not feed or only partially fed were removed from the group, and the remaining mosquitoes were kept in an insectary at 19 °C with a 10% (wt/vol) sugar solution. On day 15, mosquitoes were dissected and the oocyst number per midgut was determined.
Transmission Blocking Assays.
P. berghei-infected mice (1–2 exflagellations per field) were anesthetized and exposed for 10 min at 19 °C to a group (control) of ∼50 starved An. gambiae females. Antienolase antibody, preimmune serum (1 mg), or peptides dissolved in 250 μL of PBS were injected through the tail vein. After a 10-min pause to allow for the dispersal of the injected material, the same mouse was exposed to another group (experimental) of ∼50 starved An. gambiae females. Remaining procedures and oocyst counts were done as described in the previous section. P. falciparum gametocytes were produced as described above. Gametocytes were mixed with the antienolase antibody (final concentration of 1 mg/mL), or the preimmune serum of the same rabbit and membrane was fed to a group of ∼50 starved An. gambiae females. Alternatively, 6HA (400 μg/mL PBS) was mixed with P. falciparum gametocytes and fed to mosquitoes in the same way, using PBS as a control. Mosquitoes were kept in a humidified chamber at 24 ± 2 °C, and midgut oocysts were counted on day 8 as described above.
Immunoelectron Microscopy.
P. berghei ookinetes were fixed in 4% (wt/vol) paraformaldehyde (Electron Microscopy Sciences) in 0.25 M Hepes (pH 7.4) for 1 h at room temperature, followed by 8% (wt/vol) paraformaldehyde in same buffer overnight at 4 °C. The fixed ookinetes were frozen and sectioned as previously described. Sections were immunolabeled with rabbit antienolase antibodies (1:250 in PBS/1% fish skin gelatin) and then with anti-rabbit IgG, followed by 10 nm of protein A-gold particles. Sections were examined with a Philips CM120 Electron Microscope at 80 kV (45).
Supplementary Material
Acknowledgments
We thank the Johns Hopkins Malaria Research Institute mosquito and P. falciparum core facilities for help with mosquito rearing and parasite cultures. Receipt of the Pfs28 antibody from the Malaria Research and Reference Reagent Resource Center (MR4) is gratefully acknowledged. This work received financial support from National Institutes of Health Grant AI031478. Additional support was provided by the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation. Supply of human blood was supported by National Institutes of Health Grant RR00052.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103657108/-/DCSupplemental.
References
- 1.Breman JG, Egan A, Keusch GT. The intolerable burden of malaria: A new look at the numbers. Am J Trop Med Hyg. 2001;64(Suppl 1–2):iv–vii. doi: 10.4269/ajtmh.2001.64.iv. [DOI] [PubMed] [Google Scholar]
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh AK, Edwards MJ, Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: Hopes for the new century. Parasitol Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh AK, Ribolla PE, Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc Natl Acad Sci USA. 2001;98:13278–13281. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinglasan RR, et al. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci USA. 2007;104:13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lottenberg R, Minning-Wenz D, Boyle MD. Capturing host plasmin(ogen): A common mechanism for invasive pathogens? Trends Microbiol. 1994;2:20–24. doi: 10.1016/0966-842x(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 7.Ehinger S, Schubert WD, Bergmann S, Hammerschmidt S, Heinz DW. Plasmin(ogen)-binding alpha-enolase from Streptococcus pneumoniae: Crystal structure and evaluation of plasmin(ogen)-binding sites. J Mol Biol. 2004;343:997–1005. doi: 10.1016/j.jmb.2004.08.088. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann S, Rohde M, Chhatwal GS, Hammerschmidt S. alpha-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol Microbiol. 2001;40:1273–1287. doi: 10.1046/j.1365-2958.2001.02448.x. [DOI] [PubMed] [Google Scholar]
- 9.Rios-Steiner JL, Schenone M, Mochalkin I, Tulinsky A, Castellino FJ. Structure and binding determinants of the recombinant kringle-2 domain of human plasminogen to an internal peptide from a group A Streptococcal surface protein. J Mol Biol. 2001;308:705–719. doi: 10.1006/jmbi.2001.4646. [DOI] [PubMed] [Google Scholar]
- 10.Jong AY, et al. Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J Med Microbiol. 2003;52:615–622. doi: 10.1099/jmm.0.05060-0. [DOI] [PubMed] [Google Scholar]
- 11.Jolodar A, et al. Molecular cloning of an alpha-enolase from the human filarial parasite Onchocerca volvulus that binds human plasminogen. Biochim Biophys Acta. 2003;1627:111–120. doi: 10.1016/s0167-4781(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 12.Bernal D, et al. Identification of enolase as a plasminogen-binding protein in excretory-secretory products of Fasciola hepatica. FEBS Lett. 2004;563:203–206. doi: 10.1016/S0014-5793(04)00306-0. [DOI] [PubMed] [Google Scholar]
- 13.Read M, Hicks KE, Sims PF, Hyde JE. Molecular characterisation of the enolase gene from the human malaria parasite Plasmodium falciparum. Evidence for ancestry within a photosynthetic lineage. Eur J Biochem. 1994;220:513–520. doi: 10.1111/j.1432-1033.1994.tb18650.x. [DOI] [PubMed] [Google Scholar]
- 14.Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh AK, et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 2009;5:e1000265. doi: 10.1371/journal.ppat.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candela M, et al. Bifidobacterial enolase, a cell surface receptor for human plasminogen involved in the interaction with the host. Microbiology. 2009;155:3294–3303. doi: 10.1099/mic.0.028795-0. [DOI] [PubMed] [Google Scholar]
- 17.Carneiro CR, Postol E, Nomizo R, Reis LF, Brentani RR. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect. 2004;6:604–608. doi: 10.1016/j.micinf.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal S, Kulshreshtha P, Bambah Mukku D, Bhatnagar R. alpha-Enolase binds to human plasminogen on the surface of Bacillus anthracis. Biochim Biophys Acta. 2008;1784:986–994. doi: 10.1016/j.bbapap.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Quiñones W, et al. Leishmania mexicana: Molecular cloning and characterization of enolase. Exp Parasitol. 2007;116:241–251. doi: 10.1016/j.exppara.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Pal-Bhowmick I, Mehta M, Coppens I, Sharma S, Jarori GK. Protective properties and surface localization of Plasmodium falciparum enolase. Infect Immun. 2007;75:5500–5508. doi: 10.1128/IAI.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pancholi V. Multifunctional alpha-enolase: Its role in disease. Cell Mol Sci. 2001;58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Villar E, et al. Genetic and proteomic evidences support the localization of yeast enolase in the cell surface. Proteomics. 2006;6(Suppl 1):S107–S118. doi: 10.1002/pmic.200500479. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, et al. Application of yeast enolase as antigen for immunodiagnosis of malaria. Southeast Asian J Trop Med Public Health. 2000;31(Suppl1):79–84. [PubMed] [Google Scholar]
- 24.Ponting CP, Marshall JM, Cederholm-Williams SA. Plasminogen: A structural review. Blood Coagul Fibrinolysis. 1992;3:605–614. [PubMed] [Google Scholar]
- 25.Krishnamurti C, Vukelja SJ, Alving BM. Inhibitory effects of lysine analogues on t-PA induced whole blood clot lysis. Thromb Res. 1994;73:419–430. doi: 10.1016/0049-3848(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 26.Rejante MR, Llinás M. Solution structure of the epsilon-aminohexanoic acid complex of human plasminogen kringle 1. Eur J Biochem. 1994;221:939–949. doi: 10.1111/j.1432-1033.1994.tb18809.x. [DOI] [PubMed] [Google Scholar]
- 27.Kupcsik L, Alini M, Stoddart MJ. Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng. 2009;15:2309–2313. doi: 10.1089/ten.tea.2008.0400. [DOI] [PubMed] [Google Scholar]
- 28.Bugge TH, et al. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci USA. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman JL, et al. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 31.Hajjar KA, Krishnan S. Annexin II: A mediator of the plasmin/plasminogen activator system. Trends Cardiovasc Med. 1999;9:128–138. doi: 10.1016/s1050-1738(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh AK, Jacobs-Lorena M. Surface-expressed enolases of Plasmodium and other pathogens. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):85–90. doi: 10.1590/s0074-02762011000900011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zieler H, Garon CF, Fischer ER, Shahabuddin M. A tubular network associated with the brush-border surface of the Aedes aegypti midgut: Implications for pathogen transmission by mosquitoes. J Exp Biol. 2000;203:1599–1611. doi: 10.1242/jeb.203.10.1599. [DOI] [PubMed] [Google Scholar]
- 34.Ranawaka GR, Fleck SL, Blanco AR, Sinden RE. Characterization of the modes of action of anti-Pbs21 malaria transmission-blocking immunity: Ookinete to oocyst differentiation in vivo. Parasitology. 1994;109:403–411. doi: 10.1017/s0031182000080653. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez MC, et al. Plasmodium berghei: Routine production of pure gametocytes, extracellular gametes, zygotes, and ookinetes. Exp Parasitol. 2002;101:73–76. doi: 10.1016/s0014-4894(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 36.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 37.Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh AK, Dinglasan RR, Ikadai H, Jacobs-Lorena M. An improved method for the in vitro differentiation of Plasmodium falciparum gametocytes into ookinetes. Malar J. 2010;9:194–200. doi: 10.1186/1475-2875-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 41.O'Farrell PZ, Goodman HM, O'Farrell PH. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 42.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 43.Jethwaney D, et al. Fetuin-A, a hepatocyte-specific protein that binds Plasmodium berghei thrombospondin-related adhesive protein: A potential role in infectivity. Infect Immun. 2005;73:5883–5891. doi: 10.1128/IAI.73.9.5883-5891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsen SL, Castellino FJ. Expression of human plasminogen in Drosophila Schneider S2 cells. Protein Expr Purif. 1999;16:136–143. doi: 10.1006/prep.1999.1045. [DOI] [PubMed] [Google Scholar]
- 45.Fölsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.