Abstract
Angiogenesis is stimulated by vascular endothelial growth factor (VEGF) and antagonized by type 1 interferons, including IFN-α/β. On engaging their respective receptors (VEGFR2 and IFNAR), both stimuli activate protein kinase D2 (PKD2) and type 1 IFNs require PKD2 activation and recruitment to IFNAR1 to promote the phosphorylation-dependent ubiquitination, down-regulation, and degradation of the cognate receptor chain, IFNAR1. Data reveal that PKD2 activity is dispensable for VEGF-stimulated down-regulation of VEGFR2. Remarkably, VEGF treatment promotes the recruitment of PKD2 to IFNAR1 as well as ensuing phosphorylation, ubiquitination, and degradation of IFNAR1. In cells exposed to VEGF, phosphorylation-dependent degradation of IFNAR1 leads to an inhibition of type 1 IFN signaling and is required for efficient VEGF-stimulated angiogenesis. Importance of this mechanism for proangiogenic or antiangiogenic responses in cells exposed to counteracting stimuli and the potential medical significance of this regulation are discussed.
Introduction
Numerous regulators coerce individual cells into collective behavior within a tissue of a multicellular organism. Most of these regulators, including cytokines and growth factors, interact with their cognate receptors on the cell surface to elicit specific signal transduction cascades and ensuing alteration of transcriptional programs within the cell. Levels of specific receptors on the cell surface are therefore critical for the ability of cells to respond to a given ligand. Some of these regulators impose a diametrically opposite set of instructions on a given cell. For example, vascular endothelial growth factor (VEGF) stimulates signal transduction pathways and transcriptional programs through activation of its receptor VEGFR2.1,2 These events are essential for de novo formation of blood vessels (ie, angiogenesis, a process that involves proliferation and migration of endothelial cells).1,3 Conversely, this process is inhibited by cytokines of the type 1 interferon family, including IFN-α/β.4 IFN-α/β exert their effects via binding to the type 1 IFN receptor that consists of IFNAR1 and IFNAR2 chains and subsequent activation of Janus kinases and of signal transducers and activators of transcription (STAT).5–7 Here we report that VEGF promotes phosphorylation-dependent ubiquitination and degradation of IFNAR1 and ensuing attenuation of IFN-α/β signaling; these processes appear to be required for efficient angiogenesis.
Methods
A detailed description is in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The vectors for Flag-IFNAR1 expression and protein kinase D2 (PKD2) knockdown have been previously described.8,9 Human umbilical vein endothelial cells (HUVECs, a gift from M. J. May) and fibrosarcoma U3A cells10 (kindly provided by G. Stark) were maintained as described elsewhere.9 Antibodies against phosphorylated11 or total IFNAR112 were described elsewhere. Immunotechniques and quantification were described elsewhere.13–16
Mice with a Ser526Ala substitution within IFNAR1 were generated using previously characterized ES cells.17 All experiments were conducted with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee. Matrigel Plug assays in WT/WT and heterozygous WT/S526A mice (6-9 weeks old, n = 5 per group) were carried out as described elsewhere.18 Levels of hemoglobin and the presence of infiltrating endothelial cells in plugs were assessed as described.19
Results and discussion
Responsiveness of cells to IFN-α/β is limited by down-regulation of the type 1 IFN receptor driven through the ubiquitination-dependent endocytosis and subsequent degradation of IFNAR1.13 This ubiquitination is facilitated by βTrcp E3 ubiquitin ligase8,20,21 that is recruited to human IFNAR1 on phosphorylation of Ser535 (Ser526 in the murine receptor) mediated by protein kinase D2 (PKD2).9 Intriguingly, VEGF-stimulated ubiquitination and down-regulation of VEGFR2 were also shown to depend on βTrcp.22 Furthermore, VEGF is known to induce PKD2 via a tyrosine phosphorylation-dependent mechanism.23,24
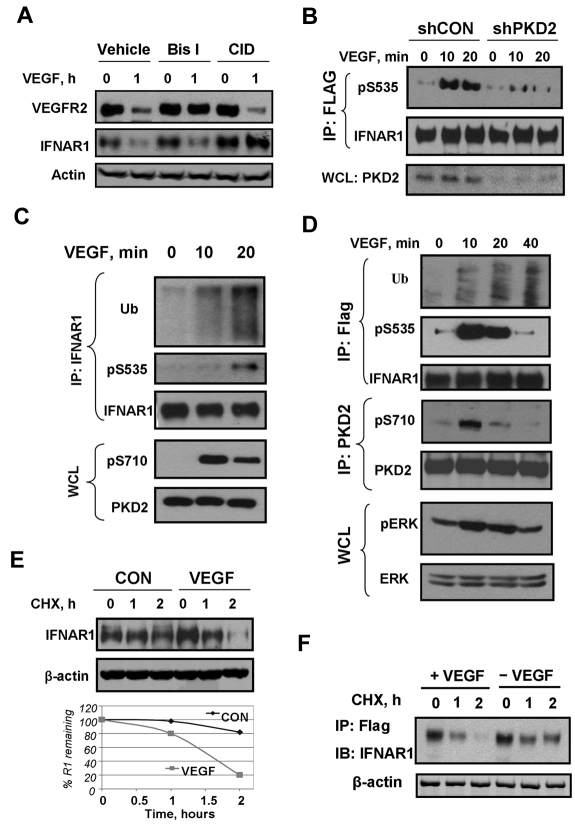
We sought to determine whether PKD contributed to control the levels of the VEGF receptor. Pretreatment of HUVECs with the pan-PKC inhibitor bisindolylmaleimide (Bis-I), but not with the selective PKD inhibitor CID755673, prevented VEGF-stimulated down-regulation of VEGFR2 (Figure 1A). This result implicates PKC (but not PKD) in ligand-induced VEGFR2 down-regulation. Surprisingly, we noticed VEGF treatment also caused a robust decrease in the levels of IFNAR1 in HUVECs (Figure 1A). Furthermore, a 10- to 20-minute treatment of human cells with VEGF led to stimulation of Ser535 phosphorylation on either exogenously expressed (Figure 1B,D) or endogenous (Figure 1C) IFNAR1. Knockdown of PKD2 attenuated IFNAR1 degron phosphorylation (Figure 1B) and pretreatment with the PKD inhibitor CID755673 prevented down-regulation of IFNAR1 in VEGF-treated HUVECs (Figure 1A). Furthermore, VEGF stimulated ubiquitination (Figure 1C-D) and degradation (Figure 1E-F) of endogenous or exogenously expressed IFNAR1. Collectively, these data suggest that VEGF-activated PKD2 mediates phosphorylation, ubiquitination, and degradation of IFNAR1.
Figure 1.
VEGF promotes phosphorylation, ubiquitination, and degradation of IFNAR1. (A) Immunoblot analyses of VEGFR2, IFNAR1, and β-actin in HUVECs pretreated with inhibitors against PKD (CID755673, 100 μM) or PKC (Bis-I, 2 μM) for 2 hours and then treated with VEGF (100 ng/mL) for 1 hour. (B) Immunoblot analysis of phosphorylation and levels of FLAG-tagged IFNAR1 stably expressed in STAT1-deficient U3A cells that received indicated shRNA and were treated with VEGF as indicated. (C) Immunoblot analysis of ubiquitination, phosphorylation, and the levels of endogenous IFNAR1 immunopurified from HUVECs treated with VEGF as indicated. Phosphorylation and levels of PKD2 in whole cell lysates (WCL) were also analyzed. (D) Immunoblot analysis of ubiquitination, phosphorylation, and levels of Flag-IFNAR1 stably expressed in U3A cells treated with VEGF (100 ng/mL) as indicated. Phosphorylation and levels of PKD and Erk in WCL were also analyzed. (E) Cychoheximide (CHX) chase analysis of turnover of endogenous IFNAR1 in HUVECs untreated or treated with VEGF. Equal loading was verified by analysis of β-actin in these samples. The graph depicts percentage of remaining IFNAR1 at the indicated time points. (F) Degradation of Flag-IFNAR1 in U3A cells treated with VEGF (100 ng/mL) and CHX (20 μg/mL) as indicated.
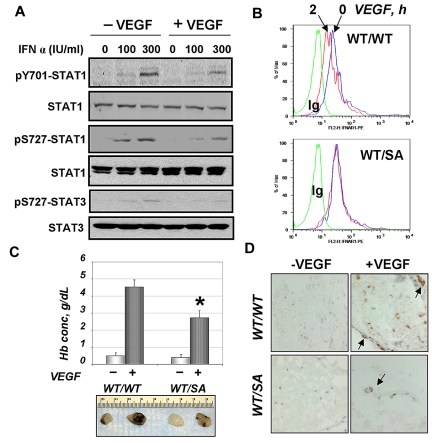
We next determined whether VEGF treatment may regulate the extent of cellular responses to type 1 IFN. Pretreatment of HUVECs with VEGF before addition of IFN-α noticeably inhibited activation of STAT1 and STAT3 (assessed by their phosphorylation, Figure 2A). This effect was not seen if VEGF was added at the same time as IFN-α or after treatment with IFN-α (data not shown). This result suggests that VEGF limits the extent of IFN-α signaling by desensitizing cells to this cytokine. Given that type 1 IFNs are known to inhibit angiogenesis,4 a VEGF-dependent process that involves proliferation and migration of endothelial cells1,3 in a manner dependent on PKD2 activation,25 we hypothesized that VEGF-stimulated phosphorylation of IFNAR1 and its degradation might be of functional importance. As VEGF-activated PKD2 may regulate angiogenesis via phosphorylating diverse substrates, we used a complementary approach that focuses on altering IFNAR1 itself.
Figure 2.
VEGF attenuates IFN-α signaling and requires IFNAR1 degradation for efficient angiogenesis. (A) Immunoblot analysis of levels and phosphorylation of STAT1 (Tyr701 and Ser727) and STAT3 (Ser727) in HUVECs left untreated or treated with VEGF (100 ng/mL for 2 hours) before treatment with the indicated dose of IFN-α (for 15 minutes). (B) Cell surface levels of IFNAR1 in untreated (blue line) or murine VEGF-treated (red line) CD31-positive bone marrow cells from indicated mice. Green line indicates control Ig. (C) In vivo angiogenesis assay in mice of indicated genotype was carried out as outlined in “Methods.” Pictures of retrieved plugs and a graph that depicts measurement of hemoglobin (mean ± SEM). *P < .01 between genotypes for VEGF-induced values. (D) Immunohistochemical analysis of Matrigel plugs retrieved from mice of indicated genotype (untreated or treated with VEGF) was carried out using anti-CD31 antibody and H&E staining. Images were taken using an Olympus BX600 microscope (40×) and a SPOT FIEX camera.
Murine ES cells, in which one wild-type Ifnar1 allele has been replaced with the mutant that lacks Ser526,17 were used to generate knock-in mice that express the mIFNAR1S526A mutant (“SA”). Bone marrow-derived CD31-positive cells from wild-type mice displayed a noticeable decrease in the cell surface levels of IFNAR1 on treatment with murine VEGF (Figure 2B). This effect was much less pronounced in the heterozygous mice (“WT/SA”) indicating that phosphorylation of IFNAR1 is required for down-regulation of IFNAR1 in response to VEGF.
We further used these mice to determine the formation of new vessels stimulated by VEGF in vivo using a Matrigel plug assay. Visual examination of retrieved plugs revealed that SA mice were less responsive to VEGF-stimulated angiogenesis. This observation was independently supported by measurements of the hemoglobin content in the extracts from the Matrigel plugs (Figure 2C) as well as by immunohistochemical analysis of CD31-positive cells within the paraffin-embedded plugs (Figure 2D). These data suggest that phosphorylation-dependent down-regulation of IFNAR1 plays an important role in VEGF-stimulated angiogenesis.
Previous studies have revealed that IFN-α/β down-regulates IFNAR1 via PKD2-mediated IFNAR1 phosphorylation that leads to ubiquitination and degradation of the receptor chain and ensuing restriction of cellular responses to type 1 IFN.8,9,11,13 Data presented here suggest that this mode of regulation could be commandeered by some inducers of unrelated signaling pathways capable of activating PKD2 (eg, VEGF). It is plausible that this mode of regulation may represent a general mechanism by which cells exposed to functionally counteracting stimuli are compelled to select the type of their response. Within this model, a temporal order of exposure would dictate whether sensitivity of a cell to a given stimuli is attenuated (or not) by a heterologous activation of common signaling pathways that directs the elimination of specific receptors.
For example, efficient VEGF-induced formation of vascular networks may depend on the ability of VEGF to counteract the antiangiogenic effects of IFN-α/β. Indeed, VEGF-induced IFNAR1 degradation contributes to angiogenesis efficacy (Figure 2), most likely because of VEGF-induced desensitization of endothelial cell to future encounters with type 1 IFN. Accordingly, generation and characterization of specific small molecules capable of inhibiting PKD2 catalytic activity or of upstream events leading to PKD2 activation by VEGF are expected to provide novel means for potent antiangiogenic therapies.
Supplementary Material
Acknowledgments
The authors thank M. J. May and G. Stark for the reagents.
This work was supported by the National Cancer Institute (PHS grants CA92900 and CA142425; S.Y.F.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.Z., J.Q., C.J.C., and N.A.L. performed the experiments; H.Z. and S.Y.F. prepared the figures; and H.Z., D.P.B., and S.Y.F. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: D.P.B. is an employee of BiogenIdec and owns its shares. The remaining authors declare no competing financial interests.
Correspondence: Serge Y. Fuchs, Department of Animal Biology, University of Pennsylvania, 380 S University Ave, Hill 316, Philadelphia, PA 19104-4539; e-mail: syfuchs@vet.upenn.edu.
References
- 1.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 2.Selleck SB. Signaling from across the way: transactivation of VEGF receptors by HSPGs. Mol Cell. 2006;22(4):431–432. doi: 10.1016/j.molcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ho QT, Kuo CJ. Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol. 2007;39(7):1349–1357. doi: 10.1016/j.biocel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer Res. 1987;47(19):5155–5161. [PubMed] [Google Scholar]
- 5.Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 6.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 7.Huangfu WC, Fuchs SY. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer. 2010;1(7):725–734. doi: 10.1177/1947601910382901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22(20):5480–5490. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng H, Qian J, Varghese B, Baker DP, Fuchs S. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Mol Cell Biol. 2011;31(4):710–720. doi: 10.1128/MCB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKendry R, John J, Flavell D, Muller M, Kerr IM, Stark GR. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc Natl Acad Sci U S A. 1991;88(24):11455–11459. doi: 10.1073/pnas.88.24.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J Biol Chem. 2004;279(45):46614–46620. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- 12.Goldman LA, Zafari M, Cutrone EC, et al. Characterization of antihuman IFNAR-1 monoclonal antibodies: epitope localization and functional analysis. J Interferon Cytokine Res. 1999;19(1):15–26. doi: 10.1089/107999099314379. [DOI] [PubMed] [Google Scholar]
- 13.Kumar KG, Barriere H, Carbone CJ, et al. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179(5):935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Gazdoiu S, Pan ZQ, Fuchs SY. Stability of homologue of Slimb F-box protein is regulated by availability of its substrate. J Biol Chem. 2004;279(12):11074–11080. doi: 10.1074/jbc.M312301200. [DOI] [PubMed] [Google Scholar]
- 15.Marijanovic Z, Ragimbeau J, Kumar KG, Fuchs SY, Pellegrini S. TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem J. 2006;397(1):31–38. doi: 10.1042/BJ20060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soldatenkov VA, Dritschilo A, Ronai Z, Fuchs SY. Inhibition of homologue of Slimb (HOS) function sensitizes human melanoma cells for apoptosis. Cancer Res. 1999;59(20):5085–5088. [PubMed] [Google Scholar]
- 17.Liu J, HuangFu WC, Kumar KG, et al. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe. 2009;5(1):72–83. doi: 10.1016/j.chom.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medhora M, Daniels J, Mundey K, et al. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284(1):H215–H224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- 19.Chiodoni C, Iezzi M, Guiducci C, et al. Triggering CD40 on endothelial cells contributes to tumor growth. J Exp Med. 2006;203(11):2441–2450. doi: 10.1084/jem.20060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs SY. De-regulation of ubiquitin-dependent proteolysis and the pathogenesis of malignant melanoma. Cancer Metastasis Rev. 2005;24(2):329–338. doi: 10.1007/s10555-005-1581-0. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23(11):2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 22.Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol Cell Biol. 2011;31(10):2010–2025. doi: 10.1128/MCB.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin L, Zeng H, Zhao D. Requirement of protein kinase D tyrosine phosphorylation for VEGF-A165-induced angiogenesis through its interaction and regulation of phospholipase Cgamma phosphorylation. J Biol Chem. 2006;281(43):32550–32558. doi: 10.1074/jbc.M604853200. [DOI] [PubMed] [Google Scholar]
- 24.Wong C, Jin ZG. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J Biol Chem. 2005;280(39):33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao Q, Wang L, Zhao ZJ, Tang H. Identification of protein kinase D2 as a pivotal regulator of endothelial cell proliferation, migration, and angiogenesis. J Biol Chem. 2009;284(2):799–806. doi: 10.1074/jbc.M807546200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.