Abstract
Objective
Several fastidious bacteria have been associated with bacterial vaginosis (BV), but their role in lactobacilli recolonization failure is unknown. We studied the effect of seven BV-associated bacterial species and two Lactobacillus species on vaginal colonization with L. crispatus CTV-05 (LACTIN-V).
Methods
Twenty four women with BV were given a 5-day course of metronidazole vaginal gel and then randomized 3:1 to receive either LACTIN-V or placebo applied vaginally once daily for 5 initial consecutive days, followed by a weekly application over 2 weeks. Vaginal swabs for L. crispatus CTV-05 culture and 9-bacterium specific 16S rRNA gene quantitative PCR assays were analyzed on several study visits for the 18 women receiving LACTIN-V.
Results
Vaginal colonization with CTV-05 was achieved in 61% of the participants receiving LACTIN-V at either the day 10 or the 28 visit and 44% at day 28. Participants not colonized with CTV-05 had generally higher median concentrations of BV-associated bacteria compared to those who colonized. Between enrollment and day 28, the median concentration of Gardnerella vaginalis minimally reduced from 104.5 to 104.3 16S rRNA gene copies per swab in women who colonized with CTV-05 but increased from 105.7 to 107.3 in those who failed to colonize (p=0.19). Similarly, the median concentration of Atopobium spp. reduced from 102.7 16S rRNA gene copies per swab to below limit of detection in women who colonized with CTV-05 but increased from 102.7 to 106.6 in those who failed to colonize (p=0.04). The presence of endogenous L. crispatus at enrollment was found to be significantly associated with a reduced odds of colonization with CTV-05 on day 28 (p=0.003) and vaginal intercourse during the study significantly impaired successful CTV-05 colonization (p=0.018).
Conclusion
Vaginal concentration of certain BV-associated bacteria, vaginal intercourse during treatment and presence of endogenous L. crispatus at enrollment predict colonization with probiotic lactobacilli.
Keywords: Bacterial vaginosis, BV-associated bacteria, Lactobacillus probiotics, Lactobacillus crispatus CTV-05
INTRODUCTION
Bacterial vaginosis (BV) is a common polymicrobial disorder characterized by an overgrowth of anaerobic or facultative bacteria and a reduction or absence of lactobacilli. BV accounts for 40% to 50% of all cases of vaginitis1;2 and is associated with numerous disorders of the female urogenital tract including adverse pregnancy outcomes,3-5 sexually transmitted infections6-9 and human immunodeficiency virus (HIV) acquisition.10
The microorganisms involved in the BV pathogenesis are diverse, but most frequently include Gram-variable coccobacilli (Gardnerella vaginalis), small Gram-negative bacilli (Prevotella spp. and Porphyromonas spp.), curved Gram-variable bacteria (Mobiluncus spp.), other anaerobic organisms (Peptostreptococcus spp. and Fusobacterium spp.) and Genital Mycoplasmas (Mycoplasma hominis and Ureaplasma urealyticum).11 Recently, several fastidious bacteria have been identified using sequence-based detection methods and reported to be associated with BV. These include Atopobium vaginae, Megasphaera species, Leptotrichia/Sneathia species and bacterial vaginosis associated bacteria (BVAB1, BVAB2 and BVAB3).12-14
BV treatment with antibiotics such as clindamycin and metronidazole results in low cure rates (50%–80%)12 and unacceptably high rates of BV recurrence15, making the use of Lactobacillus probiotics a promising treatment and prevention strategy. Although strains of Lactobacillus fermentum and Lactobacillus rhamnosus have been investigated as probiotics to prevent urogenital infections,16-19 focus on Lactobacillus species commonly recovered from the vagina has been recommended.20;21 L. crispatus CTV-05, a human vaginal strain of L. crispatus, has thus been formulated as a probiotic (LACTIN-V) by Osel Inc. (Santa Clara, CA).
Previous vaginal formulations of L. crispatus CTV-05 at lower concentrations (106 - 5 × 108 colony-forming units (cfu)/capsule) coated by a gelatin capsule appeared to be safe in clinical trials.22;23 A higher concentration of L. crispatus CTV-05 (2 × 109 cfu/dose) administered as a powder using a prefilled tampon-like applicator in healthy women as well as women with BV was also found to be safe and acceptable.24;25 In a phase 2A trial, 61% of women were successfully colonized with L. crispatus CTV-05 at either day 10 or day 28 and 44% colonized at day 28.25 Factors causing a sub-optimal vaginal colonization with exogenous lactobacilli are unknown, but could include high levels of BV-associated bacteria. This study explored the ability of L. crispatus CTV-05 to colonize the vagina in premenopausal women treated for BV and sought to determine the impact of BV-associated bacteria and other lactobacilli on CTV-05 colonization.
METHODS
Study population and participant recruitment
This study was nested in a phase 2A blinded randomized placebo-controlled trial assessing colonization efficiency, safety and acceptability of LACTIN-V at 2 × 109 cfu/dose (600 mg) or placebo, given on 5 consecutive days followed by two additional weekly doses administered vaginally via pre-filled applicator in women with BV immediately after the completion of a 5-day course of metronidazole vaginal gel (MetroGel®).25 The study which was conducted at San Francisco General Hospital (SFGH) recruited 24 pre-menopausal women with BV from clinics in the San Francisco Bay Area and randomized them 3:1 to receive either LACTIV-V or placebo. L. crispatus CTV-05 culture and 9-bacterium specific 16S rRNA gene quantitative PCR assays were then done on vaginal swabs collected on several study visits from the 18 women receiving LACTIN-V. Since this study aimed to determine if the vaginal concentration of certain BV-associated bacteria could affect colonization with a probiotic containing exogenous L. crispatus CTV-05, qPCR assays were not performed for the 6 control participants who did not receive LACTIN-V and were consequently unlikely to present with CTV-05 colonization.
Diagnosis of bacterial vaginosis
Women were recruited if they tested positive for BV by both Amsel’s criteria,26 (a score ≥3 considered positive) AND Nugent’s criteria27 (done at Magee Women’s Hospital in Pittsburgh, PA) (a score of 7-10 considered positive).
Clinical and laboratory procedures
At the screening visit (day -30 to -6) eligible women diagnosed with BV were instructed to complete a 5-day MetroGel® treatment during the first half of their next menstrual cycle and to return for the enrollment visit (day 1) within 24-72 hours after the termination of the antibiotic treatment. Treatment with LACTIN-V or placebo was commenced at enrollment once a day for 5 consecutive days and thereafter one additional dose in week 2 (day 12) and week 3 (day 19). Vaginal samples were collected before commencement of the antibiotic treatment at screening, before treatment with the study product at enrollment and on two follow-up visits (day 10 and day 28). Other study procedures, inclusion/exclusion criteria, details of participant follow-up and all laboratory tests used for detection of sexually transmitted infections, pregnancy and urinary tract infection are described elsewhere.25
Lactobacillus crispatus CTV-05 Identification using rep-PCR Assays
Samples of vaginal fluid collected with a sterile swab were placed in a Port-a-Cul anaerobic transport system and analyzed semi-quantitatively for presence of Lactobacillus by culture (at Cedars-Sinai Medical Center, Los Angeles, CA). To differentiate exogenous L. crispatus CTV-05 from other L. crispatus strains, genomic DNA was extracted from Lactobacillus-positive cultures and subjected to repetitive-sequence polymerase chain reaction (rep- PCR) DNA fingerprinting at Consolidated Laboratory Services, Van Nuys, CA.28
Sample collection, DNA extraction and qPCR Assays
To obtain specimens for the performance of qPCR assays, a polyurethane foam swab (Catch-All; Epicenter) was brushed against the lateral vaginal wall to collect vaginal fluid, re-sheathed, and frozen until the DNA extraction step. These swabs were stored dry (no media) at - 80°C.
DNA extraction from vaginal swabs and bacterium specific qPCR assays were performed on samples taken at screening (before metronidazole treatment), at enrollment (before treatment with LACTIN-V) and day 28, following a protocol previously described by Fredricks et al. (2009)29 and Srinivasan et al. (2010)30. qPCR assays targeted several vaginal bacteria that are significantly associated with BV or vaginal health,14 including a Megasphaera-like bacterium, Atopobium spp., the closely related Leptotrichia and Sneathia species (single assay), G. vaginalis, L. crispatus, L. iners and three Clostridium-like bacteria which have previously been designated BVAB1, BVAB2, and BVAB3. Bacterial levels were expressed as 16S rRNA gene copies per swab.
Data management and analysis
Data was managed using the Datafax Clinical Database Management System (version 3.7) and exported to STATA™ version 10 (College Station, Texas, USA) for analysis. An intent-to- treat (ITT) analysis was performed for colonization efficiency for the 18 participants in the treatment arm of the study who were exposed LACTIN-V and could potentially colonize with L. crispatus CTV-05. Medians and interquartile ranges of seven BV-associated bacterial species and two Lactobacillus species were calculated based on CTV-05 colonization status at the day 28 visit. The concentrations of bacterial rRNA genes per swab at screening/enrollment were compared with day 28 levels using the Wilcoxon signed-rank test within subjects who colonized with CTV-05 and within subjects who did not colonize. Changes in the concentration of bacterial rRNA genes per swab between screening/enrollment and day 28 within subjects who later colonized with CTV-05 were compared to those within subjects who did not colonize using the Wilcoxon rank-sum (Mann-Whitney) test. To assess if the different species of bacteria and sociodemographic and sexual history predicted subsequent colonization with L. crispatus CTV- 05 at day 28, univariate exact logistic regression was also performed.
Ethical approval
This study was done between April 2008 and January 2009 at the Clinical Translational Science Institute (CTSI) Clinical Research Center at the University of California, San Francisco (UCSF), USA, and approved by the UCSF Committee on Human Research at UCSF (#H43476-32139). The study’s sub-protocol was also approved by the Ethical Review Committee of the Kenya Medical Research Institute, Nairobi, Kenya. Safety oversight was provided by a Safety Monitor. This trial is registered at www.clinicaltrials.gov (NCT00635622).
RESULTS
Other results of this Phase 2A study assessing colonization efficiency, safety and acceptability of LACTIN-V are described elsewhere.25
Participants’ socio-demographic and sexual behavior characteristics
The median age of the participants was 30.5 years with a range of 18-44 years (Table 1). Of the 18 women in this sub-study, the majority 13 (72%) had a steady sexual partner, and three (17%) were married. Six participants (33%) were current smokers.
Table 1.
Baseline socio-demographic factors and sexual history
| Variable | Frequency (%) |
|---|---|
| Age (years) | |
| 18 – 29 | 8 (44.4) |
| 30 – 39 | 8 (44.4) |
| > 40 | 2 (11.1) |
| Ethnicity | |
| Black or African American | 7 (38.9) |
| White | 7 (38.9) |
| Other | 4 (22.2) |
| Highest level of education | |
| High school education or below | 13 (72.2) |
| Some college education | 3 (16.7) |
| Graduate degree | 2 (11.1) |
| Marital Status | |
| Married | 3 (16.7) |
| Divorced or Separated | 1 (5.7) |
| Single (never married) | 4 (22.2) |
| Steady partner, cohabiting | 5 (27.8) |
| Steady partner, not cohabiting | 5 (27.8) |
| Employment Status | |
| Student | 3 (16.7) |
| Employed (full-time or part-time) | 6 (33.3) |
| Unemployed | 9 (50.0) |
| Smoking | |
| Yes | 6 (33.3) |
| No | 12 (66.7) |
| Sexual debut | |
| ≤15 years | 9 (50.0) |
| 16 – 17years | 5 (27.8) |
| ≥18 years | 4 (22.2) |
| Lifetimenumber ofsexual partners | |
| 1 | 1 (5.6) |
| 2 – 10 | 10 (55.6) |
| >10 | 7 (38.9) |
| Male sexual partners in the last 6 months | |
| 0 | 2 (11.1) |
| 1 | 12 (66.7) |
| ≥2 | 4 (22.2) |
| Female sexual partners in the last 6 months | |
| 0 | 14 (77.8) |
| 1 | 2 (11.1) |
| ≥2 | 2 (11.1) |
| Sexual intercourse in the last 30 days | |
| Had sex | 13 (72.2) |
| Always protected sex | 4 (69.2) |
| Not always protected sex | 9 (46.2) |
| Ever pregnant | |
| Yes | 14 (77.8) |
| No | 4 (22.2) |
| Ever douched | |
| Yes | 14 (66.7) |
| No | 4 (33.3) |
| Age at first BV episode | |
| ≤18 years | 3 (20) |
| >18 years | 12 (80) |
| Lifetime (previous) BV episodes | |
| 1 – 5 | 11 (64.7) |
| 6 – 10 | 3 (17.6) |
| >10 | 3 (17.6) |
| Previous BV episodes in the last 12 months | |
| 0 | 1 (6.3) |
| 1 | 9 (56.3) |
| ≥2 | 6 (37.5) |
| Sexual intercourse during trial | |
| No | 7 (38.9) |
| Yes, ALWAYS protected (male condom) | 5 (27.8) |
| Yes, NOT ALWAYS protected (no male condom) | 6 (33.3) |
| Menses during trial | |
| Yes | 13 (72.2) |
| No | 5 (17.8) |
The women reported a median of 10 (range 1 – 99) lifetime sexual partners. Of the 16 participants reporting having had male sexual partners in the past six months, four (25%) reported having had two or more partners. Four participants (22%) reported female sexual partners in the 6 months preceding this study. Of the 13 women who reported having had sex in the 30 days preceding this study, four (31%) participants reported having had protected sex (used condoms), six (46%) unprotected sex and three (23%) women had both protected and unprotected sex.
Six women (33%) had experienced more than 5 BV episodes in their lifetime and 15 women had had at least one BV episode in the preceding 12 months before enrollment. Fourteen participants (78%) reported ever having douched or used vaginal preparations. During the trial, 11 participants (61%) had sexual intercourse, and six of those (55%) reported inconsistent condom use.
The effects of BV-associated bacteria concentration on L. crispatus CTV-05 vaginal colonization
Overall, L. crispatus CTV-05, as measured by rep-PCR, was recovered at either the day 10 (follow-up) visit and/or the day 28 (final) visit in 11 participants (61%); eight participants (44%) had colonization at the day 28 visit. Seven participants (39%) did not colonize with CTV-05 at any of the two follow-up visits.
The median vaginal concentrations of all seven BV-associated bacteria declined between screening, when metronidazole treatment was started, and enrollment. In participants who subsequently colonized with L. crispatus CTV-05, this trend was maintained throughout to the day 28 visit when levels of six species were below limits of detection (either 375 or 750 16S rRNA gene copies per swab) with up to 7-log reductions in median values. However, participants who did not colonize with CTV-05 also experienced an initial decline of the vaginal levels of all BV-associated bacteria between screening and enrollment, but the concentrations of G. vaginalis, Leptotrichia/Sneathia species, A. vaginae and BVAB2 resurged between enrollment and day 28. L. iners levels changed little during follow-up in both those who subsequently colonized with CTV-05 and those who did not.
Table 2 shows median values and interquartile ranges for bacterial rRNA gene concentrations at screening (before both metronidazole and L. crispatus CTV-05 treatment) and at day 28 in the two outcome groups (colonized and not colonized with L. crispatus CTV-05 at day 28). At screening, the median BV-associated bacteria rRNA concentrations were generally elevated for all participants. As expected, the median concentrations of L. crispatus species rRNA increased significantly from below limit of detection at screening to 107.8 at day 28 in participants who colonized with L. crispatus CTV-05 (p=0.01). Although the median L. crispatus 16S rRNA gene concentration also increased from 102.8 to 104.2 copies per swab between screening and day 28 in participants who did not colonize with CTV-05 at day 28 (p=0.07), the increase in concentration of L. crispatus was more pronounced in participants who subsequently colonized with CTV-05 compared to those who did not (p=0.01). Additionally, Atopobium spp. showed a significantly greater reduction in concentrations between screening and day 28 (p=0.03) among participants who colonized with CTV-05 (from 107.1 to 102.5 16S rRNA gene copies per swab) - compared to those who did not (from 107.5 to 106.6 16S rRNA gene copies per swab).
Table 2.
Concentration of bacteria in vaginal fluid expressed as log10 16S rRNA gene copies per swab at the screening visit and 28 days after probiotic treatment in women grouped according to their post-treatment (Day 28) L. crispatus CTV -05 colonization status
| Bacterium | Colonized with L. crispatus CTV-05 (n=7)
|
Not colonized with L. crispatus CTV-05 (n=11)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Day 28 | Screening | Day 28 | P value¥ for Colonization vs. non-colonization | |||||||
| median | IQR* | median | IQR* | P value† | median | IQR* | median | IQR* | P value‡ | ||
| Gardnerella vaginalis | 7.88 | 1.11 | 4.33 | 3.23 | 0.01 | 8.10 | 0.82 | 7.31 | 1.41 | 0.05 | 0.29 |
| Leptotrichia/Sneathia spp. | 6.17 | 4.60 | BLDπ | 1.81 | 0.15 | 7.01 | 3.53 | 4.48 | 4.93 | 0.26 | 0.79 |
| Megasphaera spp. | 6.73 | 3.48 | BLDπ | 1.94 | 0.08 | 5.50 | 5.48 | BLDπ | 3.18 | 0.10 | 0.66 |
| Atopobium spp. | 7.09 | 1.34 | BLDπ | 2.53 | 0.01 | 7.45 | 1.60 | 6.62 | 3.83 | 0.15 | 0.03 |
| BVAB1 | BLDφ | 2.74 | BLDφ | 0.15 | 0.16 | BLDφ | 0.30 | BLDφ | 0.30 | 0.94 | 0.22 |
| BVAB2 | 5.62 | 4.04 | BLDφ | 1.25 | 0.03 | 6.64 | 3.29 | 4.17 | 4.39 | 0.54 | 0.18 |
| BVAB3 | BLDφ | 2.40 | BLDφ | 0.73 | 0.29 | BLDφ | 3.94 | BLDφ | 3.41 | 0.27 | 0.85 |
| Lactobacillus iners | 6.82 | 3.99 | 6.78 | 2.22 | 0.94 | 6.84 | 0.41 | 6.50 | 1.78 | 0.96 | 0.98 |
| Lactobacillus crispatus | BLDφ | 0.46 | 7.98 | 2.63 | 0.01 | 2.81 | 1.29 | 4.24 | 3.87 | 0.07 | 0.01 |
IQR Interquartile range
Compares changes in the bacterial rRNA gene levels between screening and day 28 within subjects who colonized with L. crispatus CTV-05 and was obtained using the Wilcoxon signed-rank test.
Compares changes in the bacterial rRNA gene levels between screening and day 28 within subjects who did not colonize with L. crispatus CTV-05 and was obtained using the Wilcoxon signed-rank test.
Compares changes in the bacterial rRNA gene levels within subjects who colonized with L. crispatus CTV-05 to changes with in subjects who did not colonize and was obtained using the Wilcoxon rank-sum (Mann-Whitney) test.
BLD - Below Limit of Detection (assay detection thresholds) for each bacterium species as shown in Table 1
Assay detection threshold = 375 16S rRNA gene copies per swab
Assay detection threshold between 375 and 750 16S rRNA gene copies per swab
Table 3 shows median values and interquartile ranges for bacterial rRNA gene concentrations at enrollment (following the 5-day course of MetroGel® but prior to CTV-05 treatment) and at day 28 in the two outcome groups. The median concentration of Atopobium spp. in women who colonized with CTV-05 decreased from 102.7 16S rRNA gene copies per swab at enrollment to below limits of detection at day 28, but increased from 102.7 to 106.6 gene copies per swab at enrollment and day 28, respectively, in those who did not colonize with CTV-05 (p=0.04). As expected the median concentration of L. crispatus species rDNA (including the CTV-05 study strain) increased from below limits of detection at enrollment to 108 (p=0.02) at day 28 in participants who subsequently colonized with CTV-05. The increase was less pronounced in participants who did not colonize with CTV-05 at day 28, going from below limit of detection to 104 16S rRNA gene copies per swab (p=0.6). When comparing the change in concentration of L. crispatus rDNA between enrollment and day 28, the increase was significantly greater in participants who colonized with CTV-05 compared to those who did not (p=0.003).
Table 3.
Concentration of bacteria in vaginal fluid expressed as log10 16S rRNA gene copies per swab at the enrollment visit and 28 days after probiotic treatment in women grouped according to their post-treatment (Day 28) L. crispatus CTV -05 colonization status
| Bacterium | Colonized with L. crispatus CTV-05 (n=7)
|
Not colonized with L. crispatus CTV-05 (n=11)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment | Day 28 | Enrollment | Day 28 | P value¥ for Colonization vs. non-colonization | |||||||
| median | IQR* | median | IQR* | P value† | median | IQR* | median | IQR* | P value‡ | ||
| Gardnerella vaginalis | 4.54 | 2.77 | 4.33 | 3.23 | 0.55 | 5.67 | 3.55 | 7.31 | 1.41 | 0.13 | 0.19 |
| Leptotrichia/Sneathia spp. | 3.21 | 1.02 | BLDπ | 1.81 | 0.34 | BLDπ | 0.94 | 4.48 | 4.93 | 0.12 | 0.06 |
| Megasphaera spp. | BLDπ | 2.34 | BLDπ | 1.94 | 0.99 | BLDπ | 2.88 | BLDπ | 3.18 | 0.35 | 0.60 |
| Atopobium spp. | 2.74 | 1.57 | BLDπ | 2.53 | 0.34 | 2.73 | 3.20 | 6.62 | 3.83 | 0.02 | 0.04 |
| BVAB1 | BLDφ | 0.15 | BLDφ | 0.15 | … | BLDφ | 0.30 | BLDφ | 0.30 | 0.32 | 0.37 |
| BVAB2 | BLDφ | 0.00 | BLDφ | 1.25 | 0.91 | BLDφ | 0.60 | 4.17 | 4.39 | 0.22 | 0.40 |
| BVAB3 | BLDφ | 0.15 | BLDφ | 0.73 | 0.09 | BLDφ | 0.30 | BLDφ | 3.41 | 0.05 | 0.48 |
| Lactobacillus iners | 7.75 | 4.53 | 6.78 | 2.22 | 0.93 | 6.71 | 1.23 | 6.50 | 1.78 | 0.96 | 0.99 |
| Lactobacillus crispatus | BLDφ | 0.39 | 7.98 | 2.63 | 0.02 | BLDφ | 4.55 | 4.24 | 3.87 | 0.61 | 0.003 |
IQR – Interquartile range
Compares changes in the bacterial rRNA gene levels between enrollment and day 28 within subjects who colonized with L. crispatus CTV-05 and was obtained using the Wilcoxon signed-rank test.
Compares changes in the bacterial rRNA gene levels between enrollment and day 28 within subjects who did not colonize with L. crispatus CTV-05 and was obtained using the Wilcoxon signed-rank test.
Compares changes in the bacterial rRNA gene levels within subjects who colonized with L. crispatus CTV-05 to changes within subjects who did not colonize and was obtained using the Wilcoxon rank-sum (Mann-Whitney) test.
BLD -Below Limit of Detection (assay detection thresholds) for each bacterium species as shown in Table 1
Assay detection threshold = 375 16S rRNA gene copies per swab
Assay detection threshold between 375 and 750 16S rRNA gene copies per swab
Figure 1 shows vaginal concentration of the seven BV-associated bacteria species, L. iners and L. crispatus in two representative study subjects. Subject 0106 colonized with CTV-05 and free of BV (Nugent score 0) at day 28 but subject 1606 did not colonize and was again diagnosed with BV (Nugent score 10) at day 28. Figure 2 compares the proportion of participants colonized with any Lactobacillus species and those who subsequently colonized with the L. crispatus CTV-05 at the three sampling points (enrollment, day 10 and day 28 visits), as seen in culture and subsequent rep-PCR.
Figure 1.
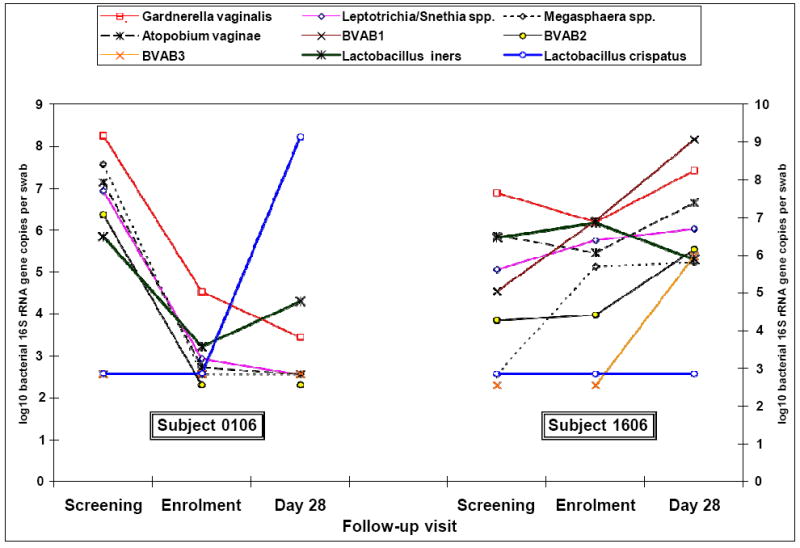
Changes in the concentration of various bacterial species in vaginal fluid expressed as log10 16S rRNA gene copies per swab in two subjects in the 3 follow-up visits. Subject 0106 colonized with CTV-05 but Subject 1606 did not colonize with CTV-05.
Figure 2.
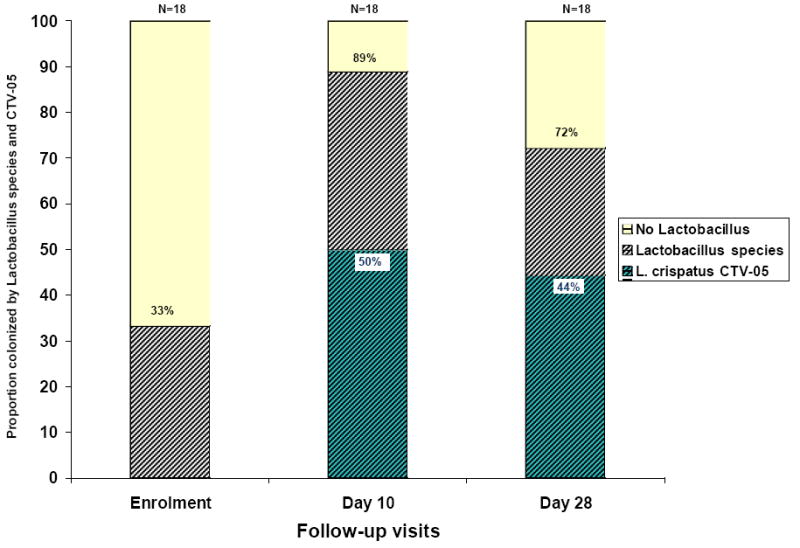
Proportion of participants colonized by Lactobacillus species† and by the probiotic Lactobacillus crispatus CTV-05‡ on follow-up (n=18)
†Colonization determined using culture
‡Colonization determined using repPCR
Univariate analysis: Factors associated with L. crispatus CTV-05 vaginal colonization
Univariate analysis using exact logistic regression suggested an inverse association between vaginal colonization with any of the 7 species of BV-associated bacteria, L. iners and L. crispatus at screening and subsequent colonization with L. crispatus CTV-05 at day 28 (Table 4). A similar association was noted using enrollment as the baseline. However, these associations were not statistically significant.
Table 4.
Un-adjusted risks of colonization with Lactobacillus crispatus CTV-05 at the final follow-up visit (Day 28) for the 18 participants enrolled in the study (exact logistic regression)
|
L. crispatus CTV-05 colonization at Day 28β |
|||||
|---|---|---|---|---|---|
| Risk factors for CTV-05 colonization | Positive n (%) | Negative n (%) | Odds Ratio (OR) | 95%CI | p-value |
| Colonizing species at screening (nα) | n=8 | n=10 | |||
| Gardnerella vaginalis (n=17) | 7 (88) | 10 (100) | 0.80 | 0.0 – 31 | 0.89 |
| Leptotrichia/Sneathia spp.(n=13) | 5 (63) | 8 (80) | 0.44 | 0.03 – 5.3 | 0.76 |
| Megasphaera spp. (n=12) | 5 (63) | 7 (70) | 0.71 | 0.07 – 7.9 | 0.74 |
| Atopobium spp. (n=16) | 7 (88) | 9 (90) | 0.79 | 0.01 – 70 | 1.0 |
| BVAB1 (n=4) | 2 (25) | 2 (20) | 1.31 | 0.07 – 23 | 1.0 |
| BVAB2 (n=13) | 5 (63) | 8 (80) | 0.43 | 0.03 – 5.3 | 0.76 |
| BVAB3 (n=8) | 3 (38) | 5 (50) | 0.62 | 0.06 – 5.6 | 0.59 |
| Lactobacillus iners (n=15) | 6 (75) | 9 (90) | 0.35 | 0.01 – 8.2 | 0.82 |
| Lactobacillus crispatus (n=7) | 3 (38) | 4 (40) | 0.91 | 0.09 – 8.7 | 1.0 |
| Sexual intercourse and menses | |||||
| Sexual intercourse during trial: | |||||
| No | 6 (75) | 1 (10) | 1.00 (referent) | … | … |
| Yes | 2 (25) | 9 (90) | 0.05 | 0.001 – 0.68 | 0.018 |
| Always protected | 0 (0) | 5 (50) | 0.06 | 0.0 – 0.59 | 0.015 |
| Not always protected | 2 (25) | 4 (40) | 0.08 | 0.006 – 1.2 | 0.07 |
| Menses during trial | 6 (75) | 7 (70) | 1.26 | 0.10 – 20 | 1.0 |
Number of participants with concentrations of specific bacterial 16S rRNA gene copies per swab above the detection thresholds shown in table 1.
Colonization determined using culture and repPCR (BV-associated bacteria species, L. iners and L. crispatus were all determined using qPCR)
Sexual intercourse during the trial was negatively associated with CTV-05 colonization whether or not the sex was protected (OR 0.05; 95% CI 0.001 – 0.68). CTV-05 colonization was not significantly influenced by having menses during the clinical trial (OR 1.26; 95% CI 0.10 – 20) (Table 4).
DISCUSSION
For this study, we reasoned that persistently high concentrations of BV-associated bacteria could prevent colonization with exogenous Lactobacillus. Vaginal colonization with L. crispatus CTV-05 was achieved in 61% of women at either day 10 and/or day 28; while 44% were colonized with CTV-05 at day 28. A comparison with other studies on probiotics for BV treatment is difficult because most used different strains, tested colonization in healthy women, used a vaginal or oral capsules, and/or they did not measure specific colonization but assessed BV recurrence using Nugent’s criteria or clinical cure.
Antonio et al. (2008)23 studied L. crispatus CTV-05 in 90 healthy women without BV using gelatin vaginal capsules and reported a colonization efficiency of 69% at one or more follow-up visits and of 59% at day 28. Mastromarino et al. (2008)31, reported treatment success after 21 days of follow-up (Nugent score <7) in 61% of Lactobacillus-treated patients compared to 19% in the placebo-treated group and reached a general Lactobacillus species colonization rate of 74% at day 21. Martinez et al. (2009)19, using vaginal capsules of L. rhamnosus GR-1 and L. reuteri RC-14 over 4 weeks following a single 2g dose of tinidazole, reported a cure rate of 87.5% in the Lactobacillus group compared to 50% in the placebo group. However, often the reasons for failure to colonize or for BV recurrence are not explored in detail.
Our qPCR data on the vaginal concentrations of fastidious BV-associated bacteria prior to treatment suggest an association between these levels and subsequent colonization with exogenous L. crispatus CTV-05 and also suggest that high concentrations may be a more important influence on CTV-05 colonization than the mere presence of the bacteria. Although the median concentrations of BV-associated bacteria were fairly similar at screening and enrollment (after MetroGel® treatment) for both groups, the change from these baseline values to day 28 (after LACTIN-V treatment) was more pronounced in participants who colonized with CTV-05, especially for Atopobium spp. (p=0.04). Higher median levels of BV-associated bacteria at the screening, enrollment and day 28 follow-up visits were generally associated with a decreased likelihood of colonization with L. crispatus CTV-05 at the final (day 28) follow-up visit.
Swidsinski et al. (2008)32 followed 18 patients with BV after a 7-day treatment with oral metronidazole and reported consistently observing the resurgence of a dense and active bacterial bio-film on the vaginal mucosa, primarily consisting of G. vaginalis and A. vaginae. It is possible that these metronidazole resistant biofilms were present in those of our study participants with sustained high concentration of specific BV-associated bacteria and that these bio-films prevented the exogenous L. crispatus CTV-05 from adhering to the vaginal epithelial cells. Consequently, it may be crucial for future probiotic studies to break down these biofilms before treatment with Lactobacillus probiotics using higher doses of oral and/or intravaginal antibiotics and/or longer treatment courses. Additionally, longer periods of probiotic treatment could optimize vaginal colonization with high numbers of H2O2 and lactic acid producing lactobacilli.
Vaginal Gram stains from healthy women without BV typically show lactobacilli, but geographic and racial variations regarding the predominant Lactobacillus have been recorded. Studies in China33, Japan34, Europe21;35 and USA13;36 have reported the predominance of L. crispatus in normal women including pregnant women. In contrast, Anukam et al. (2006)37 reported that L. iners is the most abundant vaginal Lactobacillus species in premenopausal Nigerian women, many of them with BV. Matu et al. (2009)38 reported a higher diversity of lactobacilli in Kenyan women with normal flora compared to women with BV, with L. jensenii as the predominant species in addition to L. iners. Fredricks et al. (2005)13, using a combination of broad-range PCR assays of 16S rRNA genes and fluorescence in situ hybridization (FISH) performed directly on vaginal fluid, found L. crispatus to be the predominant species in BV-negative women and L. iners to be the predominant lactobacillus species in BV-positive women. Our study also found high levels of L. iners, maintained throughout the study, in participants who colonized with CTV-05 as well as in those who did not. This suggests that the vaginal presence of L. iners neither hinders nor aids CTV-05 colonization and that L. iners may be more resistant to replacement by BV-associated bacteria.
Vaginal presence of endogenous L. crispatus at baseline was found to be associated with a reduced odds of colonization with the L. crispatus CTV-05 strain, similar to findings of Antonio et al. (2009)23 who studied L. crispatus CTV-05 colonization in women without BV. While 15 of 18 participants receiving LACTIN-V (83%) had qPCR detectable L. crispatus species at day 28, only eight (53%) of these participants had the exogenous L. crispatus CTV-05 strain detectable by rep-PCR, suggesting that endogenous strains of L. crispatus could have prevented CTV-05 colonization. Based these findings, future study designs should generally include either rep-PCR probes or qPCR probes to directly detect the administered strain (e.g. L. crispatus CTV-05) and to differentiate it from endogenous lactobacilli.
Limited information is available about the influence of sexual intercourse on levels of vaginal bacteria or its effect on vaginal colonization with exogenous Lactobacillus. In participants with a history of douching, sex within the past week was associated with increased likelihood of BV.39 Schellenberg et al. (2008)40 found that longer self-reported time since last sexual intercourse was independently associated with increased counts of bacterial cell-units (BCU) per gram of vaginal fluid. High BCU were associated with normal Hay–Ison score41 suggesting the presence and higher quantities of Lactobacillus in these women with longer periods of sexual abstinence. In the present study, we found that vaginal intercourse during the trial significantly decreased the likelihood of successful CTV-05 colonization. A similar observation was previously also reported by Antonio et al. (2009).23 The high pH of seminal fluid or one of its components may affect the adherence of CTV-05 to vaginal epithelial cells and/or its survival in the vaginal vault.
We recognize that our study has several limitations. First, the number of time points for assessment was limited and our sample size was small, having been drawn from the treatment arm of a Phase 2A trial designed to investigate safety and colonization efficiency of L. crispatus CTV-05, and excluding the placebo arm unexposed to L. crispatus CTV-05.25 However, we still found significant association between the vaginal concentration of some BV-associated bacteria DNA and the likelihood of colonization with L. crispatus CTV-05. Second, we performed qPCR assays targeting selected fastidious bacteria recently associated with BV using molecular methods. The qPCR platform could be used to assay other vaginal bacteria that may play a role in the pathogenesis of BV. Future research should seek to measure how vaginal levels of these bacteria correlate with the BV status and how they influence colonization with endogenous and exogenous lactobacilli. Third, our detection threshold for each assay was 375 to 750 copies per swab. The use of a larger fraction of vaginal fluid DNA for each assay would reduce the detection thresholds but would also compromise one’s ability to run multiple assays. Finally, the results of this study may not be generalizable to women not initially treated with metronidazole before probiotic treatment. One of the strengths of this study is the extensive use of PCR controls to monitor for false-positive and false-negative results, thereby increasing the reliability of the bacterial qPCR data reported.
CONCLUSION
These data suggest that L. crispatus CTV-05 colonization status inversely correlates with vaginal concentrations of BV-associated bacteria DNA, especially those known to create a biofilm, and provide supporting evidence that these bacteria are important in the pathogenesis of BV and the success of treatment with both antibiotics and probiotics. Sexual intercourse negatively affects CTV-05 colonization. Efforts to understand BV and BV recurrence will benefit from a greater understanding of the complex and time-dependent bacterial interactions in the vagina. Furthermore, using these PCR methods can help monitor the antibiotic and probiotic therapy. Lengthening the 5-day metronidazole and the CTV-05 dosing period and/or utilizing other means to disrupt the vaginal biofilm, may increase colonization by the probiotic strain.
Acknowledgments
We wish to express our gratitude to all participants, all referral clinics of Planned Parenthood Golden Gate as well as the San Francisco City Clinic, the study clinicians performing the clinical visits, the staff of the CTSI Clinical Research Center at the San Francisco General Hospital, the data management team at DF/Net Research in Seattle, as well as the study sponsor Osel Inc. of Santa Clara, CA, USA. Daisy Ko at the Fred Hutchinson Cancer Research Center provided technical support for all the qPCR assays performed in this study.
This study was supported by a grant from Osel Inc. This study was also supported by grant UL1 RR024131-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. BMN is a fellow of the Infectious Disease Research Training Program (IDRTP) an affiliate program of the University of California, San Francisco, USA and the Kenya Medical Research Institute, Nairobi, Kenya (NIH Fogarty International Center Grant D43 TW007388).
References
- 1.Mitchell H. Vaginal discharge--causes, diagnosis, and treatment. BMJ. 2004;328(7451):1306–1308. doi: 10.1136/bmj.328.7451.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demba E, Morison L, van der Loeff MS, et al. Bacterial vaginosis, vaginal flora patterns and vaginal hygiene practices in patients presenting with vaginal discharge syndrome in The Gambia, West Africa. BMC Infect Dis. 2005;5(1):12. doi: 10.1186/1471-2334-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaer A, Boylu M, Avsar AF. Vaginitis in Turkish women: symptoms, epidemiologic - microbiologic association. Eur J Obstet Gynecol Reprod Biol. 2005;121(2):211–215. doi: 10.1016/j.ejogrb.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Svare JA, Schmidt H, Hansen BB, et al. Bacterial vaginosis in a cohort of Danish pregnant women: prevalence and relationship with preterm delivery, low birthweight and perinatal infections. BJOG. 2006;113(12):1419–1425. doi: 10.1111/j.1471-0528.2006.01087.x. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsson B, Pernevi P, Chidekel L, et al. Bacterial vaginosis in early pregnancy may predispose for preterm birth and postpartum endometritis. Acta Obstet Gynecol Scand. 2002;81(11):1006–1010. doi: 10.1034/j.1600-0412.2002.811103.x. [DOI] [PubMed] [Google Scholar]
- 6.Schwebke JR. Gynecologic consequences of bacterial vaginosis. Obstet Gynecol Clin North Am. 2003;30(4):685–694. doi: 10.1016/s0889-8545(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw CS, Morton AN, Garland SM, et al. Higher-risk behavioral practices associated with bacterial vaginosis compared with vaginal candidiasis. Obstet Gynecol. 2005;106(1):105–114. doi: 10.1097/01.AOG.0000163247.78533.7b. [DOI] [PubMed] [Google Scholar]
- 8.Ness RB, Kip KE, Soper DE, et al. Bacterial vaginosis (BV) and the risk of incident gonococcal or chlamydial genital infection in a predominantly black population. Sex Transm Dis. 2005;32(7):413–417. doi: 10.1097/01.olq.0000154493.87451.8d. [DOI] [PubMed] [Google Scholar]
- 9.Demirezen S, Korkmaz E, Beksac MS. Association between trichomoniasis and bacterial vaginosis: examination of 600 cervicovaginal smears. Cent Eur J Public Health. 2005;13(2):96–98. [PubMed] [Google Scholar]
- 10.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. Aids. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw CS, Mortom AN, Garland SM, et al. Evaluation of a Point-of-Care Test BVBlue and Clinical and Laboratory Criteria for Diagnosis of Bacterial Vaginosis. The Journal of Clinical Microbiology. 2005;43(3):1304–1308. doi: 10.1128/JCM.43.3.1304-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris MJ, Norori J, Zozaya-Hinchliffe M, et al. Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J Clin Microbiol. 2007;45(3):1016–1018. doi: 10.1128/JCM.02085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 14.Fredricks DN, Fiedler TL, Thomas KK, et al. Targeted Polymerase-Chain-Reaction for the Detection of Vaginal Bacteria Associated with Bacterial Vaginosis. J Clin Microbiol. 2007 doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342(8):534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 16.Reid G, Charbonneau D, Erb J, et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized placebo-controlled trial in 64 healthy women. FEMS Immunology and Medical Microbiology. 2003;35:131–134. doi: 10.1016/S0928-8244(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 17.Anukam KC, Osazuwa E, Osemene GI, et al. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006;8(12-13):2772–2776. doi: 10.1016/j.micinf.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Bolton M, van der SA, Cohen CR. Probiotics: potential to prevent HIV and sexually transmitted infections in women. Sex Transm Dis. 2008;35(3):214–225. doi: 10.1097/OLQ.0b013e31815b017a. [DOI] [PubMed] [Google Scholar]
- 19.Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55(2):133–138. doi: 10.1139/w08-102. [DOI] [PubMed] [Google Scholar]
- 20.Wilks M, Wiggins R, Whiley A, et al. Identification and H(2)O(2) production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. J Clin Microbiol. 2004;42(2):713–717. doi: 10.1128/JCM.42.2.713-717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiss H, Kogler B, Petricevic L, et al. Vaginal Lactobacillus microbiota of healthy women in the late first trimester of pregnancy. BJOG. 2007;114(11):1402–1407. doi: 10.1111/j.1471-0528.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 22.Czaja CA, Stapleton AE, Yarova-Yarovaya Y, et al. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol. 2007;2007:35387. doi: 10.1155/2007/35387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonio MA, Meyn LA, Murray PJ, et al. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J Infect Dis. 2009;199(10):1506–1513. doi: 10.1086/598686. [DOI] [PubMed] [Google Scholar]
- 24.Hemmerling A, Harrison W, Schroeder A, et al. Phase 1 dose-ranging safety trial of Lactobacillus crispatus CTV-05 for the prevention of bacterial vaginosis. Sex Transm Dis. 2009;36(9):564–569. doi: 10.1097/OLQ.0b013e3181a74924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmerling A, Harrison W, Schroeder A, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex Transm Dis. 2010;37(12):745–750. doi: 10.1097/OLQ.0b013e3181e50026. [DOI] [PubMed] [Google Scholar]
- 26.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 27.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonio MAD, Hillier SL. DNA Fingerprinting of Lactobacillus cripatus Strain CTV-05 by Repetitive Element Sequence-Based PCR Analysis in a Pilot Study of Vaginal Colonization. J Clin Microbiol. 2003;41(5):1881–1887. doi: 10.1128/JCM.41.5.1881-1887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fredricks DN, Relman DA. Paraffin removal from tissue sections for digestion and PCR analysis. Biotechniques. 1999;26(2):198–200. doi: 10.2144/99262bm04. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastromarino P, Macchia S, Meggiorini L, et al. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect. 2009;15(1):67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 32.Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97–6. doi: 10.1016/j.ajog.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 33.Yan DH, Lu Z, Su JR. Comparison of main Lactobacillus species between healthy women and women with bacterial vaginosis. Chin Med J (Engl) 2009;122(22):2748–2751. [PubMed] [Google Scholar]
- 34.Song YL, Kato N, Matsumiya Y, et al. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J Clin Microbiol. 1999;37(9):3062–3064. doi: 10.1128/jcm.37.9.3062-3064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasquez A, Jakobsson T, Ahrne S, et al. Vaginal Lactobacillus flora of healthy Swedish women. J Clin Microbiol. 2002;40(8):2746–2749. doi: 10.1128/JCM.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180(6):1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 37.Anukam KC, Osazuwa EO, Ahonkhai I, et al. Lactobacillus vaginal microbiota of women attending a reproductive health care service in Benin city, Nigeria. Sex Transm Dis. 2006;33(1):59–62. doi: 10.1097/01.olq.0000175367.15559.c4. [DOI] [PubMed] [Google Scholar]
- 38.Matu MN, Orinda GO, Njagi EN, et al. In vitro inhibitory activity of human vaginal lactobacilli against pathogenic bacteria associated with bacterial vaginosis in Kenyan women. Anaerobe. 2009;20 doi: 10.1016/j.anaerobe.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Schwebke JR, Desmond RA, Oh MK. Predictors of bacterial vaginosis in adolescent women who douche. Sex Transm Dis. 2004;31(7):433–436. doi: 10.1097/01.olq.0000129948.91055.9f. [DOI] [PubMed] [Google Scholar]
- 40.Schellenberg J, Blake BT, Lane M, et al. Flow cytometric quantification of bacteria in vaginal swab samples self-collected by adolescents attending a gynecology clinic. J Microbiol Methods. 2008;73(3):216–226. doi: 10.1016/j.mimet.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Ison CA, Hay PE. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect. 2002;78(6):413–415. doi: 10.1136/sti.78.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]


