Abstract
Mitochondria, dynamic organelles that undergo continuous cycles of fusion and fission, are the powerhouses of eukaryotic cells. Recent research indicates that mitochondria also act as platforms for antiviral immunity in vertebrates. Mitochondrial-mediated antiviral immunity depends on activation of the retinoic acid-inducible gene I (RIG-I)-like receptors signal transduction pathway and the participation of the mitochondrial outer membrane adaptor protein “mitochondrial antiviral signaling (MAVS)”. Here we discuss recent findings that suggest how mitochondria contribute to antiviral innate immunity.
Keywords: Mitochondrion, antiviral innate immunity, MAVS, RLRs pathway, signal transduction, mitochondrial dynamics
Introduction
Mitochondria are double-membrane organelles with a wide variety of functions in eukaryotic cells. Mitochondria contain their own genome, mitochondrial DNA (mtDNA), which is essential for their respiratory function to generate adenosine triphosphate (ATP) [1]. In addition to serving as cell powerhouses via their aerobic respiration, mitochondria are involved in numerous crucial cellular processes, including apoptosis [2], aging [3], calcium homeostasis [4], and multiple cell signaling [5].
One of the most recent discoveries regarding the novel functions of mitochondria is their role in cellular innate antiviral immunity in vertebrates, particularly in mammals [6]. In this review, we discuss a better understanding of mitochondria, as a platform for antiviral immunity.
Antiviral innate immunity and mitochondria
Innate immunity against RNA viral infection involves the activation of multiple signaling steps that culminate in the rapid production of type I interferons, such as IFN-α and -β, and other proinflammatory cytokines that promote the subsequent development of adaptive antiviral immunity [7-9]. Two distinct pathways initiate signal transduction [10] (Figure 1); one is mediated by the endosomal Toll-like receptor 3 (TLR-3), which targets RNA viruses entering the cell by endocytosis; the other system is prompted by retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), which recognize cytoplasmic viral-derived double-stranded (ds)RNA; both systems ultimately activate intracellular signaling cascades that result in the killing of viruses. Mitochondrial antiviral immunity depends on its activation of the RLR signaling pathway, and the participation of the mitochondrial outer membrane protein, mitochondrial antiviral signaling (MAVS) [6] (also known as IPS-1 [11], VISA [12], and Cardif [13]). MAVS cellular deficiency, either generated in knockout mice [14, 15] or by hepatitis C virus protease (NS3/4A) cleavage [13, 16], abolishes the production of type I IFNs and inflammatory cytokines, underscoring the importance of the link between antiviral innate immunity and mitochondria.
Figure 1.
Overview of innate immunity against RNA viruses in mammals. Viral infection of mammalian host cells is detected by the cell’s recognition of virus-derived double-stranded RNA (dsRNA), which initiates two distinct signaling pathways (TLR-3 and RLR). Although these two pathways differ with respect to their initiating stimuli and downstream effectors, they converge at the point of transcriptional activation (NF-κB or IRF-3/7), resulting in the rapid production of type I interferons and proinflammatory cytokines. Mitochondrial antiviral immunity depends on activation of the RLR signaling pathway.
Mitochondrial antiviral signaling (MAVS)
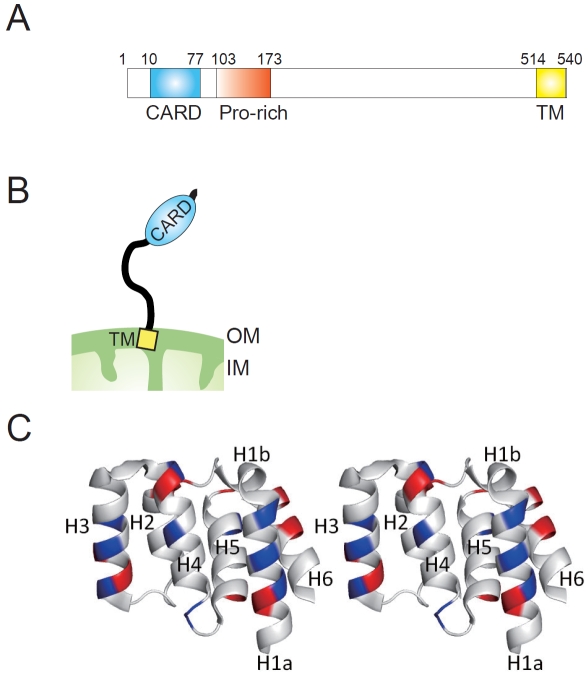
MAVS, comprising 540 amino acids in humans, is a mitochondrial integral outer membrane protein with a predicted molecular mass of 56 kD (Figure 2A and B), and forms a supramolecular (approximately 600 kD) assembly on the outer mitochondrial membrane under physiologic conditions [17]. Structurally, MAVS contains an N-terminal caspase activation and recruitment domain (CARD) comprising six helices, three (H1a, H3, and H4) that form a flat positively-charged surface and two (H2 and H6) that form an acidic negatively-charged surface on the opposite side [18] (Figure 2C). The RLRs (RIG-I and MDA-5), upstream of MAVS, also contain tandem CARDs at their N-terminal [19] that interact with the CARD of MAVS, resulting in the activation of intracellular signaling cascades.
Figure 2.
MAVS structure. (A). A schematic view of human MAVS, showing the location of the CARD domain (CARD), proline-rich region (Pro-rich), and the transmembrane segment (TM). The amino acid positions are indicated above the structure. (B). Schematic representation of MAVS molecule on the mitochondrial outer membrane. OM: mitochondrial outer membrane, IM: mitochondrial inner membrane. (C). A stereo view of the CARD domain crystal structure of human MAVS [18]. Basic potentials are colored blue, and acid regions are red, respectively.
MAVS also contains a C-terminal transmembrane (TM) domain that is responsible for proper mitochondrial localization [6] and its self-association through the stacking of aromatic residues (see next section) [20]. These properties of MAVS, i.e., mitochondrial localization, dimerization, and possession of the CARD domain, seem to be the minimal requirements for its in vivo function. Chen and colleagues demonstrated that overexpression of a MAVS mutant containing only the CARD and TM domains (called mini-MAVS) is sufficient to induce signal transduction [6, 16].
Aside from its CARD and TM domains, MAVS also contains a proline-rich region (PRR) that is involved in downstream signaling via its interaction with the tumor necrosis factor receptor-associated factor (TRAF) family, including TRAF2, TRAF3, and TRAF6 [12, 21]. It remains unclear, however, why a MAVS mutant lacking the PRR retains its signaling capacity.
MAVS activation and inhibition
Upon RNA viral infection, the RNA helicases (RIG-I or MDA5) recognize dsRNA, which leads to their activation [19] and recruitment to the outer membrane of the mitochondria to interact with MAVS following the recruitment of downstream signaling actors, such as TRAF family members [9, 10, 22] (Figure 3). What is the machinery that activates MAVS post RLR-binding and how is it regulated? Two groups revealed that MAVS leads to oligomerization, which is essential for activating the transcription factors, nuclear factor κB (NF-κB) and interferon regulatory factor 3 (IRF-3) [20, 23]. Based on fluorescence (FRET) and bioluminescence resonance energy transfer (BRET) analyses, MAVS forms specific self-interactions via TM domain that is crucial for downstream signaling. Interestingly, Wang and colleague observed that RLR recruitment to MAVS enhances the self-association of MAVS [23], providing a model for the events upstream of the RLR signaling pathway.
Figure 3.
MAVS-mediated antiviral signaling. Viral infection can activate RLRs leading to its translocation to the mitochondria, and the RLRs associate with MAVS via a CARD-CARD interaction. MAVS activation is then triggered by its self-associations, which leads to downstream signaling. Mitochondrial membrane potential (ΔΨm) is capable of supporting MAVS signaling. MAVS-mediated signal transduction can be inhibited by several mitochondrial proteins through direct interaction with MAVS.
Several negative regulators of MAVS-mediated antiviral immunity (Figure 1) localizing to the mitochondria have been identified. NLRX1, a novel nucleotide oligomerization domain (NOD)-like receptors (NLRs), is a ubiquitously expressed mitochondrial protein and inhibits CARD-CARD interactions between RLRs and MAVS, thereby preventing downstream signal transduction [24]. Receptor for globular head domain of complement C1q (gC1qR), is also translocated to mitochondria and binds with MAVS, resulting in the suppression of antiviral immune responses [25]. In addition to these negative regulators, Mitofusin 2 (Mfn2), a mediator of mitochondrial fusion, is also involved, as described in the next section.
Mitochondrial dynamics and antiviral immunity
In many cell types, mitochondria have a tubular morphology (Figure 4A) and undergo continuous cycles of homotypic fusion and fission, opposing processes that control organelle shape, copy number, and mtDNA maintenance [26, 27]. Additionally, mitochondrial dynamics play an important role in apoptosis [28]. Mitochondrial dynamics are controlled by high molecular weight GTPases, and mitofusins (Mfn1 and Mfn2) and OPA1 regulate fusion processes, whereas Drp1 is involved in mitochondrial fission [26, 27] (Figure 4B). In the last several years, several reports have tried to shed light on the connection between mitochondrial dynamics and antiviral innate immunity.
Figure 4.
Mitochondria are dynamic organelles. (A). Mitochondrial morphology in wild-type mouse embryonic fibroblasts (MEFs). Mitochondria are visualized by staining with a monoclonal antibody against mtHsp70 (green). Scale bar, 10 mm. (B). A schematic model of mitochondrial dynamics in mammals. Mitochondria are dynamic organelles that undergo continuous cycles of fusion and fission. Molecules involve in mitochondrial fusion and fission are indicated.
The first evidence of the role of mitochondrial dynamics in antiviral immunity was the discovery that Mfn2, which mediates mitochondrial fusion, inhibits the RLR pathway through MAVS-binding [17]. An increased abundance of Mfn2 is proposed to sequester MAVS in a nonproductive state for propagating a downstream antiviral response, and reducing or obliterating IFN-β expression as well as IRF-3 dimerization. In contrast, loss of endogenous Mfn2 enhances the virus-induced production of IFN-β and thereby decreases viral replication. Interestingly, we have not observed this inhibitory phenotype with the Mfn2 homolog protein, Mfn1, with an approximately 60% sequence identity [17]. Adding to the story of the connection between innate immunity and mitochondrial dynamics, Arnoult and colleagues reported that activation of the RLR pathway via the defective Sendai virus (SeV) strain H4 induces mitochondrial elongation [29]. They also demonstrated that knockdown of either Mfn1 or OPA1, which blocks mitochondrial fusion, decreases virus-induced activation of the transcription factors, nuclear factor κB (NF-κB) and interferon regulatory factor 3 (IRF-3). Conversely, knockdown of Drp1, which depletes mitochondrial fission, increases RLR signaling. Another study reported that viral infection in the host cell triggers MAVS re-distribution on the outer mitochondrial membrane, and the translocation is regulated by Mfn1 because knockdown of the gene abolishes this rearrangement [30]. Furthermore, the authors proposed a model of viral entrapment by mitochondria via a RIG-I-MAVS complex [30].
Based on these studies, Mfn1 or Mfn2 appear to be considerably involved with MAVS and its ability to function in the RLR pathway. Together, these findings suggest that Mfn1 is involved in eliciting the antiviral response, while Mfn2 has an inhibitory effect on MAVS.
Mitochondrial function and antiviral immunity
We recently demonstrated MAVS-dependent signaling in cells with null mutations in both Mfn1 and Mfn2 [31]. Such Mfns-null cells have completely fragmented mitochondria, show no detectable mitochondrial fusion [32, 33], and display impaired RLR signaling. Chan and colleagues reported that the vast majority of Mfns-null cells show widespread heterogeneity of mitochondrial membrane potential (ΔΨm), a physiologic function of mitochondria [33]. Interestingly, treatment of normal cells with CCCP, a protonophore that dissipates ΔΨm, suppresses the antiviral response [31]. In the present study, increasing the abundance of uncoupling protein-2, which induces mitochondrial proton leakage [34], the extent of ΔΨm dissipation correlated with the defect in RLR-induced antiviral responses (Figure 3). Taken together, these data provide a framework for understanding how the physiologic functions of mitochondria are coupled with its functions in antiviral immunity.
Conclusions
Mitochondria are the well-known powerhouses of eukaryotic cells, and recent studies reveal that they also act as a platform for the first line of antiviral defense. Recent studies of mitochondrial mediated-antiviral immune responses suggest a deep interconnection with other organelles, such as the endoplasmic reticulum (ER) and peroxisomes [35, 36]. Further studies are needed to understand the basic physiology of mitochondria and how these organelles collectively achieve antiviral immunity.
Acknowledgements
We are grateful to Koji Okamoto (Osaka University, Japan) for helpful discussions and valuable comments on the paper. We are also grateful to Taro Tamada (Japan Atomic Energy Agency) and Misa Kuboyama (Kyushu University, Japan) for assistance with the figures. This work was supported by the grants-in aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, and Technology of Japan (20770123), the Kato Memorial Bioscience Foundation, The Nakajima Foundation, and The Research Foundation for Pharmaceutical Sciences to T.K.
Abbreviations
- CARD
caspase activation and recruitment domain
- IFNs
interferons
- MAVS
mitochondrial antiviral signaling
- Mfns
mitofusins
- RIG-I
retinoic acid-inducible gene I
- RLR
RIG-I-like receptors
- ΔΨm
mitochondrial membrane potential
References
- [1].Attardi G, Schatz G. Biogenesis of Mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- [2].Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- [4].Rutter GA, Rizzuto R. Regulation of mitochondrial metabolism by ER Ca2+ release: an intimate connection. Trends Biochem Sci. 2000;25:215–221. doi: 10.1016/s0968-0004(00)01585-1. [DOI] [PubMed] [Google Scholar]
- [5].McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- [6].Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kB and IRF3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- [7].Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- [8].Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- [9].Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- [11].Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- [12].Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- [13].Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- [14].Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, Yamamoto M, Uematsu S, Ishii KJ, Takeuchi O, Akira S. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [16].Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci USA. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yasukawa K, Oshiumi H, Takeda M, Ishihara N, Yanagi Y, Seya T, Kawabata S, Koshiba T. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- [18].Potter JA, Randall RE, Taylor GL. Crystal structure of human IPS-1/MAVS/VISA/Cardif caspase activation recruitment domain. BMC Struct Biol. 2008;8:11. doi: 10.1186/1472-6807-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Loo YM, Gale M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baril M, Racine ME, Penin F, Lamarre D. MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. J Virol. 2009;83:1299–1311. doi: 10.1128/JVI.01659-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saha SK, Pietras EM, He JQ, Kang JR, Liu SY, Oganesyan G, Shahangian A, Zarnegar B, Shiba TL, Wang Y, Cheng G. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].West AP, Shadel GS, Ghosh S. Mitochondria in innate immunity responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tang ED, Wang CY. MAVS self-association mediates antiviral innate immune signaling. J Virol. 2009;83:3420–3428. doi: 10.1128/JVI.02623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, Ting JP. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- [25].Xu L, Xiao N, Lui F, Ren H, Gu J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc Natl Acad Sci USA. 2009;106:1530–1535. doi: 10.1073/pnas.0811029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- [27].Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- [28].Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2009;11:133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Onoguchi K, Onomoto K, Takamatsu S, Jogi M, Takemura A, Morimoto S, Julkunen I, Namiki H, Yoneyama M, Fujita T. Virus-infection or 5’ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 2010;6:e1001012. doi: 10.1371/journal.ppat.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- [32].Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- [33].Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- [34].Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- [35].Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, Nibert ML, Superti-Furga G, Kagan JC. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]