Abstract
Hepatic arterial embolization (HAE) is a treatment used in the management of primary and some metastatic hepatic tumors. Complications of HAE are similar to those seen in other treatments, particularly transcatheter arterial chemoembolization (TACE), but without the possibility for chemotherapy related side effects. Particle reflux into the cystic artery is generally clinically occult but gallbladder ischemia severe enough to require cholecystostomy tube placement can occur. The authors discuss the case of a patient who underwent HAE and subsequently required a cholecystostomy tube due to development of acute cholecystitis.
Keywords: Bland embolization, cholecystitis, hepatic arterial embolization
Hepatic arterial embolization (HAE) and transcatheter arterial chemoembolization (TACE) are treatments that have been used for hepatocellular carcinoma with proven survival benefit.1,2,3,4 Metastatic tumors to the liver, in particular neuroendocrine tumor, have also demonstrated benefit when treated with either HAE or TACE.5,6 The complications of both are similar, including postembolization syndrome (PES), nontarget embolization, hepatic failure, and arterial dissection.7 Reflux during administration of the embolic material can occur with either method and result in nontarget embolization. Nontarget embolization may be clinically silent, or may cause splenic infarction, pancreatitis, gastrointestinal mucosal lesions, and acute cholecystitis.8 We present the case of a woman who developed acute cholecystitis after HAE for hepatocellular carcinoma.
CASE REPORT
A 71-year-old woman presented to the interventional radiology service (IR) with multifocal hepatocellular carcinoma (HCC). The diagnosis was made on a follow-up study after she was incidentally discovered to have a lesion in segment 6 of the liver, when multiple additional masses were discovered in the liver. A fine-needle aspiration was performed and HCC was diagnosed. The patient was hepatitis serology negative and had no evidence of cirrhosis or any other underlying liver disease; however because of the extent of disease, she was not a surgical or transplant candidate (Fig. 1). After 3 embolizations the patient continued to have enhancing tumor in an area incompletely treated (Fig. 2). She returned to IR for retreatment with the knowledge that the cystic artery might be supplying the tumor that had not responded to the previous treatments.
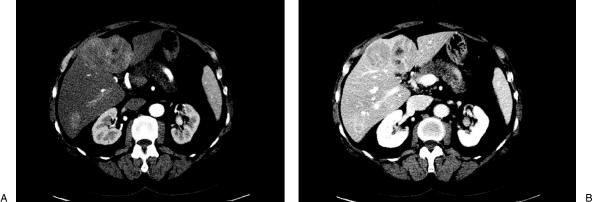
Figure 1.
Triple-phase computed tomography scan at initial presentation showing multifocal hepatocellular carcinoma. (A) Arterial phase showing early arterial enhancement of the tumors. (B) Venous phase showing contrast washout.
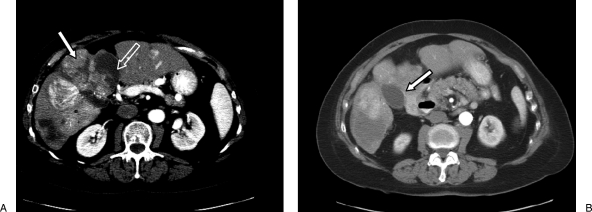
Figure 2.
Triple-phase computed tomography scan prior to the fourth hepatic arterial embolization. (A) Nonenhancing areas represent necrosis from prior embolizations (open arrow) while enhancing areas represent residual tumor (closed arrow). (B) Note the appearance of the gallbladder prior to embolization (closed arrow) and its relationship to the enhancing tumor.
Informed consent was obtained and the patient was brought to the angiographic suite. After puncturing the right common femoral artery and placing a 6 French sheath, a SOS 2 catheter was used to access the celiac artery. A Tracker microcatheter and microguidewire were used to select the right inferior phrenic artery, which was embolized to stasis using 100–300 micron Bead Block and 100 micron PVA. A Simmons 2 catheter was then used to select a replaced common hepatic artery off the superior mesenteric artery (Fig. 3).
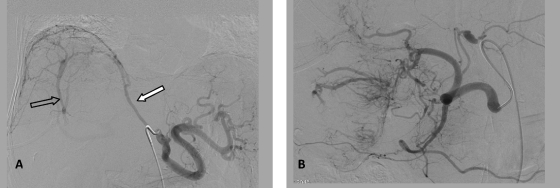
Figure 3.
Right inferior phrenic (A) and replaced common hepatic (B) angiograms. (A) Note the hypertrophy of the right inferior phrenic artery (closed arrow) and a portal vein shunt (open arrow). (B) Numerous branches of the right hepatic artery supplying tumor surrounding the gallbladder are evident.
A branch of the right hepatic artery was selected and an angiogram was performed. Multiple enhancing tumors were seen as well as the cystic artery. Because the cystic artery itself was supplying tumor and it was impossible to treat the tumor completely while excluding this non-target vessel, a decision was made to embolize from this location. The vessel was embolized to stasis using 100–300 micron and 300–500 micron Bead Block with a small amount of 100 micron PVA (Fig. 4). A non-contrast CT obtained immediately following treatment confirmed embolization of the cystic artery/gallbladder (Fig. 5).
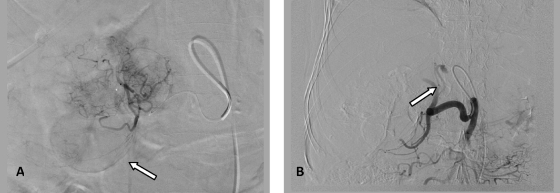
Figure 4.
(A) Angiogram of a branch of the right hepatic artery prior to embolization shows tumor blush and enhancement of the gallbladder (closed arrow). (B) Postembolization common hepatic angiogram shows complete stasis of the right hepatic artery (closed arrow).
Figure 5.
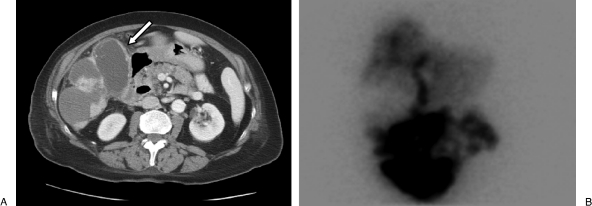
Imaging findings consistent with the patient's clinical picture of acute cholecystitis. (A) Computed tomography scan on postoperative day 4 shows a dilated gallbladder with thickened wall (white arrow). Note excellent radiographic response of previously enhancing pericholecystic tumor. (B) Hepatobiliary scintigraphy shows lack of gallbladder filling.
The patient did well during and immediately after the procedure except for some mild right upper quadrant “achiness.” She developed a fever as high as 39.2 Celsius on postoperative day (POD) 1 with associated rigors. Her white blood cell count was normal at 7.7 K/μL and her total bilirubin and alkaline phosphatase were within expected limits postembolization at 1.1 mg/dL and 434 u/L respectively. Over the next several days, she did well clinically and did not complain of pain; however, she continued to spike fevers up to 38.8° Celsius despite treatment with antibiotics, and her blood culture returned positive for Klebsiella pneumoniae. Her white blood cell count slowly climbed and peaked at 14.5 K/μL on POD 5. A CT scan was performed on POD 4, which demonstrated a dilated gallbladder with wall enhancement and thickening. Because of the complete absence of symptoms, including right upper quadrant pain, hepatobiliary scintigraphy was performed on POD 5. This showed a lack of filling of the gallbladder with radiotracer (Fig. 5).
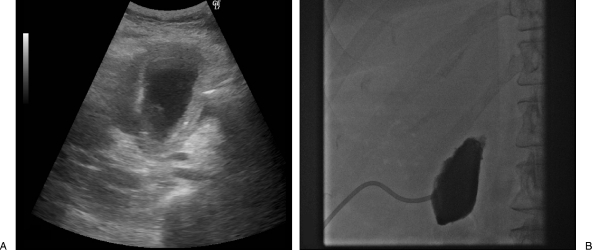
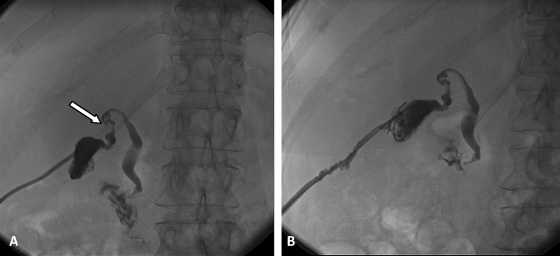
The patient underwent uneventful placement of an 8 French cholecystostomy tube on POD 5 with removal of 50 mL of turbid, brown fluid (Fig. 6). The bile was positive for Klebsiella pneumoniae and the patient defervesced after drain placement. Her leukocytosis gradually resolved, and she was discharged home on POD 9. The patient had a tract study and cholecystogram 3 weeks later at which time the drain was removed (Fig. 7). She did not require a cholecystectomy. Subsequent CT scans showed a thickened gallbladder wall with eventual atrophy of the gallbladder.
Figure 6.
Percutaneous cholecystostomy tube placement. (A) Initial ultrasound shows a dilated, irregular gallbladder consistent with that seen on computed tomography scan. (B) Posttube-placement cholecystogram shows obstruction of the cystic duct.
Figure 7.
Cholecystogram prior to drain removal. (A) Patency of the cystic duct is demonstrated almost 3 weeks after cholecystostomy tube placement (white arrow). (b) Tract study shows a well-formed tract allowing removal of the tube.
DISCUSSION
Cholecystitis is a recognized complication of hepatic artery embolization.8 The cystic artery most commonly arises off of the right hepatic artery. Origins from the left hepatic and proper hepatic arteries are seen less often.9 Treatment of the right hepatic artery can result in embolic material refluxing into the cystic artery. Superselective therapy of right hepatic artery branches theoretically reduces the risk of cystic artery embolization. However, particle reflux can still occur. In an ongoing institutional review board (IRB) approved retrospective study, we have seen contrast within the gallbladder wall on noncontrast CTs obtained immediately after treatment in 22 of 135 patients (16%) following bland embolization, consistent with nontarget cystic artery embolization. None of these patients required a cholecystostomy tube or cholecystectomy.
The reported incidence of acute cholecystitis ranges from 0.3–10% after either HAE or TACE.8,10 Many instances require no surgical or radiologic intervention. Recently, Wagnetz et al demonstrated a 4.9% rate of acute cholecystitis after 355 transarterial chemoembolization treatments performed in 246 patients, all of which were managed conservatively. In the 12 patients that did develop acute cholecystitis, 10 underwent lobar treatment of the right hepatic artery.11 In radioembolization treatment, Atassi et al reported a 0.6% rate of radiation-induced cholecystitis requiring cholecystectomy, with an additional 1.8% rate of abnormal gallbladder wall enhancement on follow-up imaging, and 0.9% rate of asymptomatic gallbladder wall disruption.12 Clearly the incidence of acute cholecystitis after HAE, TACE, or radioembolization is low but is more common following treatment of the right hepatic artery rather than super-selective treatment.
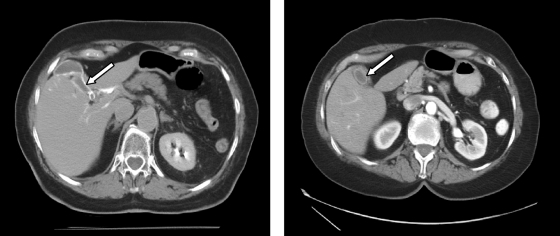
Based on our experience, it is not uncommon to see nontarget cystic artery embolization on immediate posttreatment noncontrast CT scans (Fig. 8). However, the majority of these patients do not develop any clinical symptoms and rarely require cholecystostomy tube placement. We speculate that despite the occurrence of nontarget embolization of the gallbladder in up to 16% of patients following bland embolization at our institution, the rare occurrence of clinical cholecystitis is related to the absence of chemotherapeutic agent in the embolization material. Early surgical literature regarding hepatic arterial infusion pumps showed that chemotherapy alone can cause chemical cholecystitis.13 Although no studies have been performed specifically studying lobar TACE versus selective or superselective TACE, the rate of acute cholecystitis may be higher after lobar TACE should there be nontarget delivery of chemotherapy to the cystic artery. In the study by Wagnetz et al11 discussed above, the incidence of acute cholecystitis with TACE was 4.9% with the majority of patients treated via a lobar approach. A 10% acute cholecystitis rate was reported by Bismuth et al in 291 patients undergoing lobar TACE.10 Other studies reporting rates of acute cholecystitis as low as 0.3% have many patients undergoing selective or superselective TACE,14,15 which should reduce the risk. Despite suspecting that rates of acute cholecystitis are lower following bland embolization, attention should nonetheless be paid to the origin of the cystic artery to avoid embolization whenever possible to minimize the risk of cholecystitis and reduce patient pain.
Figure 8.
Computed tomography (CT) scans from a different patient after transcatheter arterial embolization. (A) CT immediately after embolization showing contrast trapped with particles in the gallbladder wall (white arrow). (B) Arterial-phase CT scan 1 month after treatment showing atrophy of the gallbladder with a thickened, enhancing wall (white arrow). The patient was asymptomatic throughout their course of treatment.
In our experience, when nontarget embolization of the cystic artery occurs after particle embolization of the right hepatic artery, it is usually due to difficulty in identifying the cystic artery. In cases where superselective or left hepatic artery treatment results in nontarget cystic artery embolization, the cause is thought to be due to reflux or anomalous origin of the cystic artery. In some situations, the cystic artery is discovered to supply the tumor as well as the gallbladder and the branch of the cystic artery supplying the gallbladder is protectively coil embolized. When this is not possible, such as in the case described above, a decision may be made to knowingly bland embolize the cystic artery. Nontarget embolization of the cystic artery can be confirmed on a noncontrast CT obtained immediately after the embolization. Diagnosis of postembolization cholecystitis is based on a combination of clinical and imaging findings, but can at times be difficult as it is not uncommon for patients to develop an elevated white blood cell count, fever and right upper quadrant pain in the days following embolization.
An additional important consideration is the percentage of tumor supplied by branches from the cystic artery. In a retrospective analysis of TAE performed at our institution, we found a 56% prevalence of extracystic arterial supply arising from the cystic artery.16 This becomes particularly important when identifying tumor supply for embolization as it appears that embolization of the cystic artery may in fact be necessary in some instances. The knowledge that bland embolization can usually be performed without significant clinical sequelae is important for interventional radiologists to recognize. However, these patients should be informed of the risk prior to the procedure when the location of the tumor suggests this might be the case, and closely followed as they do occasionally require percutaneous cholecystostomy. It is unclear if the risk is similarly low when chemotherapy is used for embolization either in conventional TACE or with drug-eluting beads. Articles describing “TACE of cystic” artery go to great length to make the point that if the tumor-supplying branch of the cystic artery cannot be embolized selectively the procedure should be abandoned.17,18 Indeed, our inclination is to restrict the use of chemotherapeutic agents to vessels supplying liver only.
CONCLUSION
Hepatic arterial embolization is a treatment for hepatocellular carcinoma involving the embolization of tumor-supplying blood vessels using particles only, without the addition of chemotherapy. Nontarget embolization of the cystic artery occurs in up to 16% of cases, typically without clinical sequelae. Infrequently, clinically evident cholecystitis develops. When this occurs, management can usually be conservative; on rare occasions, cholecystostomy may be required.
References
- Llovet J M, Real M I, Montaña X, et al. Barcelona Liver Cancer Group Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Lo C M, Ngan H, Tso W K, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- Chang J M, Tzeng W S, Pan H B, Yang C F, Lai K H. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma. A randomized controlled study. Cancer. 1994;74(9):2449–2453. doi: 10.1002/1097-0142(19941101)74:9<2449::aid-cncr2820740910>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30(1):6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- Martin RCG, Robbins K, Tomalty D, et al. Transarterial chemoembolisation (TACE) using irinotecan-loaded beads for the treatment of unresectable metastases to the liver in patients with colorectal cancer: an interim report. World J Surg Oncol. 2009;7:80. doi: 10.1186/1477-7819-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K T, Koh B Y, Brody L A, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Interv Radiol. 1999;10(4):397–403. doi: 10.1016/s1051-0443(99)70055-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto I, Aso N, Nagaoki K, et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18(3):605–619. doi: 10.1148/radiographics.18.3.9599386. [DOI] [PubMed] [Google Scholar]
- López-Benítez R, Richter G M, Kauczor H U, et al. Analysis of nontarget embolization mechanisms during embolization and chemoembolization procedures. Cardiovasc Intervent Radiol. 2009;32(4):615–622. doi: 10.1007/s00270-009-9568-9. [DOI] [PubMed] [Google Scholar]
- Michels N A. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112(3):337–347. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- Bismuth H, Morino M, Sherlock D, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163(4):387–394. doi: 10.1016/0002-9610(92)90039-t. [DOI] [PubMed] [Google Scholar]
- Wagnetz U, Jaskolka J, Yang P, Jhaveri K S. Acute ischemic cholecystitis after transarterial chemoembolization of hepatocellular carcinoma: incidence and clinical outcome. J Comput Assist Tomogr. 2010;34(3):348–353. doi: 10.1097/RCT.0b013e3181caaea3. [DOI] [PubMed] [Google Scholar]
- Atassi B, Bangash A K, Lewandowski R J, et al. Biliary sequelae following radioembolization with Yttrium-90 microspheres. J Vasc Interv Radiol. 2008;19(5):691–697. doi: 10.1016/j.jvir.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Pietrafitta J J, Anderson B G, O'Brien M J, Deckers P J. Cholecystitis secondary to infusion chemotherapy. J Surg Oncol. 1986;31(4):287–293. doi: 10.1002/jso.2930310413. [DOI] [PubMed] [Google Scholar]
- Sakamoto I, Aso N, Nagaoki K, et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18(3):605–619. doi: 10.1148/radiographics.18.3.9599386. [DOI] [PubMed] [Google Scholar]
- Poggi G, Pozzi E, Riccardi A, et al. Complications of image-guided transcatheter hepatic chemoembolization of primary and secondary tumours of the liver. Anticancer Res. 2010;30(12):5159–5164. [PubMed] [Google Scholar]
- Wang X, Shah R P, Maybody M, et al. Cystic artery localization using a three-dimensional angiography vessel tracking system compared with conventional two-dimensional angiography. J Vasc Interv Radiol. 2011;22:549–550. doi: 10.1016/j.jvir.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Miyayama S, Matsui O, Nishida H, et al. Transcatheter arterial chemoembolization for unrespectable hepatocellular carcinoma fed by the cystic artery. J Vasc Interv Radiol. 2003;14:1156–1161. doi: 10.1097/01.rvi.0000086534.86489.10. [DOI] [PubMed] [Google Scholar]
- Miyayama S, Matsui O, Taki K, et al. Extrahepatic blood supply to hepatocellular carcinoma: angiographic demonstration and transcatheter arterial chemoembolization. Cardiovasc Intervent Radiol. 2006;29(1):39–48. doi: 10.1007/s00270-004-0287-y. [DOI] [PubMed] [Google Scholar]