Percutaneous treatment of chylothorax was developed as a minimally invasive alternative to surgical options. The treatment consists of diagnostic pedal lymphangiography followed by transabdominal catheterization of the cisterna chyli (CC)/thoracic duct (TD) and embolization of the TD proximal to the chyle leak. Initially developed by Cope et al,1 thoracic duct embolization (TDE) has become a viable primary treatment for chylous leaks. Potential advantages include the minimally invasive nature of the procedure, which results in reduction of mortality and morbidity, as well as the ability to identify chyle leaks and variations in TD duct anatomy.2
PREPROCEDURE
Imaging prior to TDE is generally unnecessary. However, in cases of nontraumatic chylous leaks, we perform preprocedure magnetic resonance (MR) ductography to visualize the cause of the leak, which, in most cases, is the occlusion of the upper part of the TD.3 In patients without chest tubes, a baseline chest X-ray is ordered. A large chylothorax is treated with thoracentesis if respiratory status is compromised. To reduce the risk of peritonitis in the setting of possible transintestinal TD access, antibiotics are administered: intravenous (IV) cefazolin 1 g, or levofloxacin 500 mg IV in those with an allergy to penicillins and cephalosporins.
Bilateral Pedal Lymphangiography
In the past, lymphangiography was the method of choice for imaging of the lymphatic system. Because noninvasive cross-sectional imaging methods of the lymph nodes have supplanted lymphangiography, the skills and expertise for performing lymphangiography have gradually declined. However, lymphangiography remains the gold standard for imaging of the disorders of lymphatic flow and there are multiple texts and articles describing technique of pedal lymphangiography.4,5
Positioning of the feet is important, as even very slight movement can compromise cannulation of the tiny lymphatic vessels and/or dislodge the needle. The feet are placed in a comfortable parallel position and the big toes are taped together. To opacify pedal lymphatic vessels and facilitate lymphatic vessel dissection, ~0.2 mL of isosulfan blue 1% (Lymphazurin™, US Surgical, Norwalk, CT) mixed in a 2:1 ratio with lidocaine 1% is injected subcutaneously into each of the web spaces of the toes. In our experience, the web space between the fourth and fifth toes tends to be the most pain-sensitive for patients. Five to 10 minutes later, a 2-cm superficial horizontal incision is made on the upper dorsal foot. The identification of the lymphatic vessels can be augmented by gently milking the dorsum of the foot from the toes toward the incision, allowing the isosulfan blue to opacify the vessels. Gentle dissection of the lymphatic duct from the subcutaneous tissues and fat is performed using tenotomy scissors and curved forceps (Fig. 1). It is extremely useful to use magnification glasses while accessing the duct.
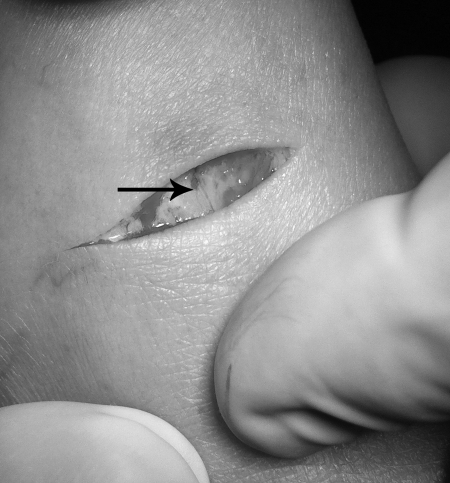
Figure 1.
Lymphatic duct dissection. Photography shows dissection of the lymphatic vessel (blue vessel, black arrow) following injection of the Lymphazurin™.
After dissection of the lymphatic duct, a 4–0 silk suture is placed under the duct at the distal/cranial end to serve as a tourniquet for facilitation of duct distention. To better visualize the vessel, a small waterproof wedge (we fashion one from the backing of sterile strips) is placed behind the vessel inside the wound. To secure the needle in the vessel, a loose knot (4–0 silk suture) is placed around the proximal/caudal end of the vessel (Fig. 2A). A 30-gauge needle (Cook Inc., Bloomington, IN) is placed initially through the skin for stabilization, and then through the loose knot. The foot is massaged to distend the vessel and the needle is placed into the duct. The loose knot is tightened around the cannulated vessel to secure the needle in place (Fig. 2B).
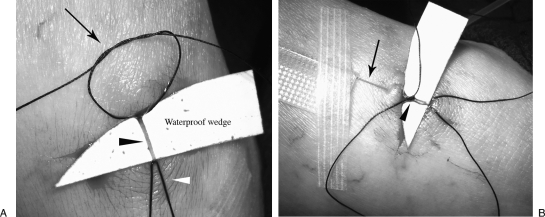
Figure 2.
Lymphatic duct catheterization. (A) Preparation for access of the lymphatic duct: tourniquet suture (white arrowhead), retention knot (arrow), lymphatic vessel (black arrowhead). (B) Access of the lymphatic duct with 30-gauge lymphangiography needle (black arrow). The needle initially placed through the skin for stabilization. Retention knot is then tied around the needle (black arrowhead).
Proper cannulation is tested by injection of saline or lidocaine 1% through the needle. The needle is then attached to the dedicated lymphangiogram pump (Cordis, Johnson and Johnson, Miami Lakes, FL). As an alternative to the dedicated lymphangiogram pump (rarely available in radiology departments currently), a balloon inflation device can be used.5 Usually, a small bubble is present at the interface between the oily contrast and saline inside the needle tubing. The progress of this bubble in the needle tubing indicates that there is forward flow of the contrast.
We inject a maximum of 5 mL of ethiodized oil (Ethiodol®, Savage Laboratories, Melville, NY) through the needle. Using a large amount of contrast can flood the intestinal lymphatic ducts and CC, thus obscuring the target. A large amount Ethiodol® can also delay glue polymerization and prevent complete embolization of the TD. To improve and hasten visualization of the TD and CC, we follow the contrast injection with 20 mL of saline in each leg (termed “saline push”). In our experience, adding a saline push significantly decreases procedure time and improves the success rate of embolization.
Thoracic Duct Cannulation
Initially the lymphographic dye progresses slowly through the network of pelvic and retroperitoneal lymphatic vessels to approximately the L1–L2 level. At this level, the lymphatic ducts quickly lose their definition and clarity, an indicator of lymph inflow from intestinal and hepatic lymphatics (Fig. 3). To prevent chyle leakage from an access point in the CC, we recommend accessing the TD below this inflow point, through one of the lumbar lymphatic ducts (preferably the right). Even though it is more challenging to cannulate a lumbar lymphatic duct due to its small size, this difficulty is offset by its more constant and defined appearance during lymphangiography.
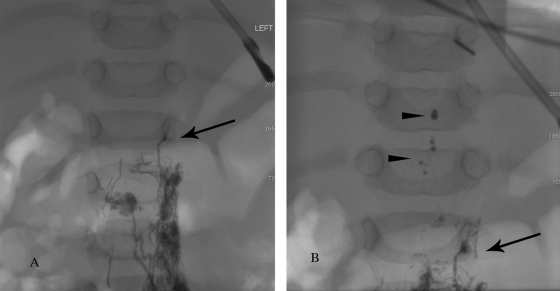
Figure 3.
Fluoroscopic image demonstrating the inflow of the intestinal lymph (chyle) and liver lymph in 3-month-old child. (A) Initially contrast slowly advances through the pelvic lymphatic ducts up to the level of the lower abdomen (black arrow). (B) At the level of the lower abdomen, the inflow of nonopacified lymph flushes the contrast (contrast drops, black arrowheads) into the thoracic duct.
Cannulation is performed using a 21- or 22-gauge 15- to 20-cm Chiba needle (Cook). We introduce a slight 5–10 degree bend at the distal 2 cm of the needle for directionality. Efforts to avoid the aorta are made by using a right transabdominal approach, and fluoroscopic guidance is used to avoid the large intestine. However, avoiding these structures is not critical, as there have been no reports of morbidity or mortality resulting from traversal of vessels or visceral organs.2
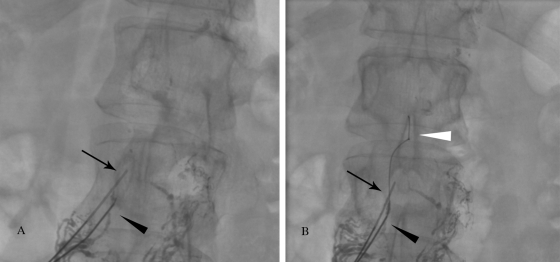
Quick, deliberate jabs with the Chiba needle are made, such that the needle is not deflected by intervening structures. Generally, a double wall technique is used, and a stiff 0.018-inch guidewire (V18 Control, Boston Scientific, Natick, MA) is used to probe for the duct while retracting the needle (Fig. 4A). When the TD is accessed, the wire should pass easily into its cranial aspect (Fig. 4B). Next, a 65-cm microcatheter (Rapid Transit®®, Cordis) is advanced into the TD over the wire. Once the catheter is in the TD, the wire is removed and iodinated contrast is injected using a 1 mL syringe to detect the site of leakage or occlusion (Fig. 4C).
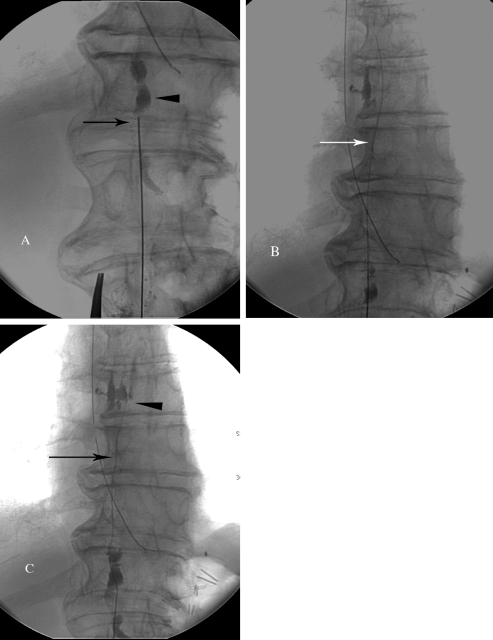
Figure 4.
Technical steps of thoracic duct embolization. (A) Fluoroscopic image of the access of the cisterna chyli (arrowhead) with a 21-gauge needle. (B) Fluoroscopic imaging of the V-18 wire advanced into the thoracic duct (arrow). (C) Injection the contrast into the thoracic duct through the catheter (arrow) to identify the chylous leak (arrowhead).
Thoracic Duct Embolization
After identification of the cause of chyle leakage (extravasation or obstruction), embolization of the TD is performed proximally. First, coils are placed to provide a matrix for glue polymerization. Coils can be slightly oversized, as the TD is quite distendable. However, very large and long coils run the risk of forcing the catheter out of the TD, and care must be exercised during deployment. A slight back-and-forth motion during deployment encourages proper coil formation.
Next, a maximum of 0.2 mL of D5W is used to flush the catheter to prevent intracatheter glue polymerization. Injection of larger amounts of D5W can fill the TD and significantly slow the polymerization of glue, resulting in recanalization of the glue cast. N-Butyl cyanoacrylate (n-BCA) (Truefill®, Cordis, J&J, Warren, NJ) diluted 1:1 in Ethiodol is used for embolization. The speed of n-BCA polymerization depends on the rate of exposure of the glue to ionic compounds. Due to relatively stagnant flow in lymphatic vessels, ionic exposure is significantly less than in blood vessels. Therefore, a 1:1 glue/Ethiodol® dilution provides ample time for injection and removal of the catheter. The TD is filled with the glue mixture just proximal to the leak or occlusion (Fig. 5). If the TD is accessed via a lumbar lymphatic duct, we recommend injecting a small amount of glue at the duct entry site. Immediately after glue injection, the microcatheter is removed.
Figure 5.
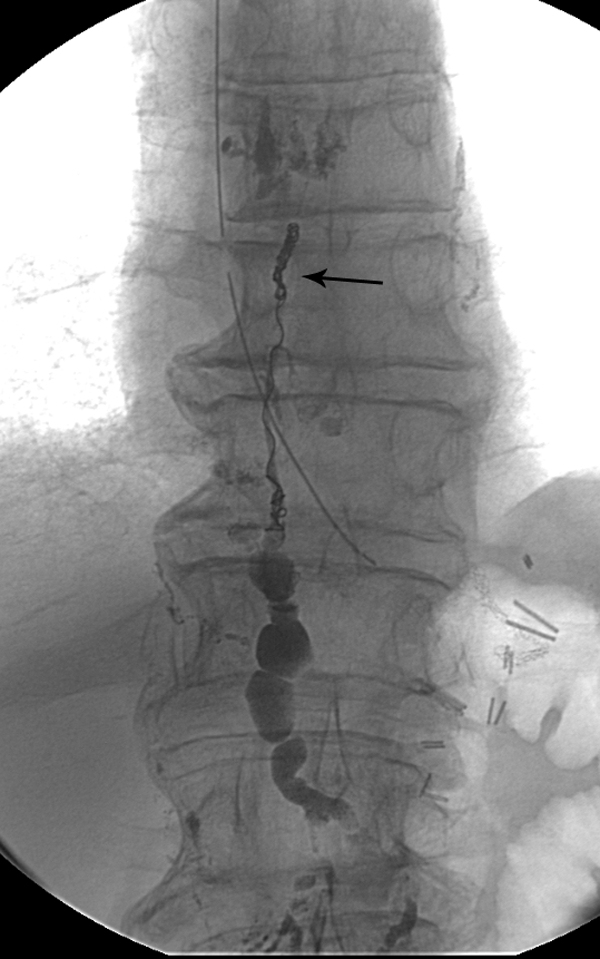
Final fluoroscopy image after embolization of the thoracic duct with microcoils (arrow) and Truefill glue.
We do not recommend use of the Onyx® (Micro Therapeutics, Inc., Irvine, CA) embolic system due to the porosity of the cast that is created. We attribute some of our failures to its use.
POSTPROCEDURE
The foot incisions are closed with at least 3 vertical mattress sutures each, and the percutaneous access site is dressed with a bandage. The patient is observed overnight with a plan to discharge the patient home the next day. Imaging should be obtained immediately and 1-week postprocedure to assess for reaccumulation of chylous effusion.
DISCUSSION
The cure rate of TDE is directly related to the ability to catheterize the CC/TD. One difficulty in accessing the TD arises from the rapid transit of single drops of ethiodized oil through the lumbar lymphatics. When transit is rapid, it allows for only transient opacification of target ducts. To address this problem, a 2-needle technique has been developed in which the first needle is used to externally compress the duct centrally, and the second needle is used to cannulate the duct. External compression results in accumulation of oil in the duct, which allows for better visibility of a stationary target (Fig. 6).
Figure 6.
Fluoroscopic image demonstrating “2-needle” technique. (A) The more superior needle (arrow) compresses the duct, resulting in accumulation of the contrast in the duct below the needle, creating the “target.” The second needle is used to access the duct (black arrowhead). (B) The wire is advanced into the cisterna chyli and thoracic duct (white arrowhead) through the second needle (black arrowhead).
In rare cases, it is possible to visualize a leak from a small branch of the TD. We recommend selective branch catheterization to preserve the integrity of the TD.
On occasion, the duct cannot be accessed after numerous attempts. In these cases, thoracic duct interruption is performed (Fig. 7). This technique consists of multiple needle punctures at the level of the CC, which disrupts flow into the TD. Though this technique results in a cure in 72% of traumatic chyle leaks, it is well documented that lymphangiography alone can result in cessation of chylothorax.6
Figure 7.
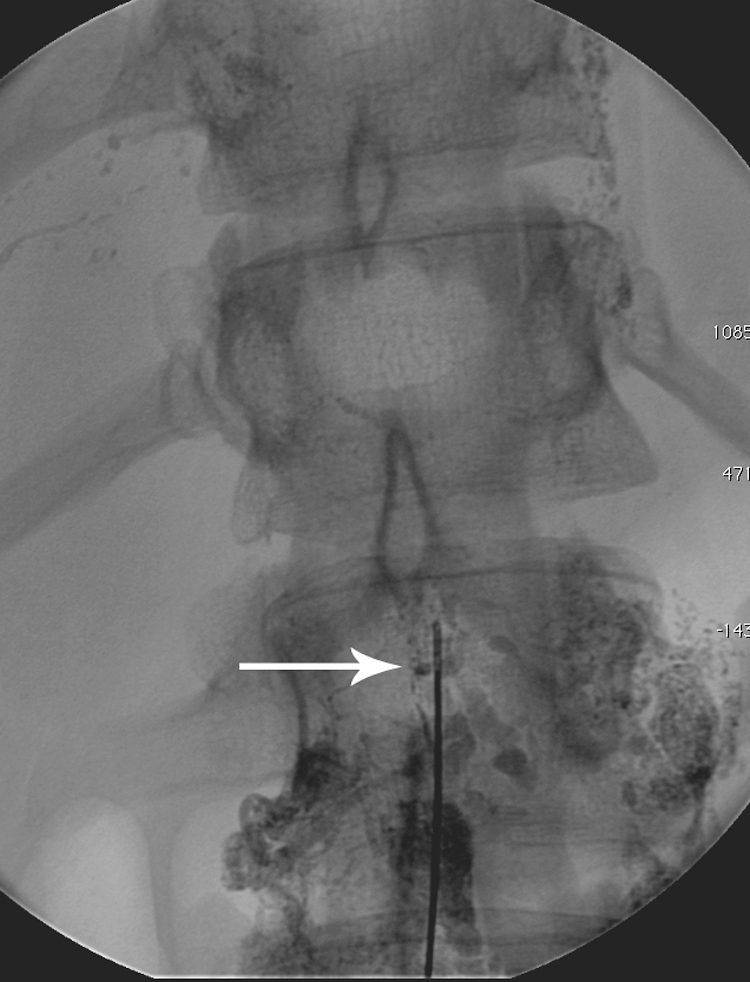
Fluoroscopic image demonstrating disruption of the lymphatic vessels with the needle (arrow).
SUGGESTED READINGS
- Cope C, Salem R, Kaiser L R. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10(9):1248–1254. doi: 10.1016/s1051-0443(99)70227-7. [DOI] [PubMed] [Google Scholar]
- Itkin M, Kucharczuk J C, Kwak A, Trerotola S O, Kaiser L R. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139(3):584–589, discussion 589–590. doi: 10.1016/j.jtcvs.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Okuda I, Udagawa H, Takahashi J, Yamase H, Kohno T, Nakajima Y. Magnetic resonance-thoracic ductography: imaging aid for thoracic surgery and thoracic duct depiction based on embryological considerations. Gen Thorac Cardiovasc Surg. 2009;57(12):640–646. doi: 10.1007/s11748-009-0483-4. [DOI] [PubMed] [Google Scholar]
- Guermazi A, Brice P, Hennequin C, Sarfati E. Lymphography: an old technique retains its usefulness. Radiographics. 2003;23(6):1541–1558, discussion 1559–1560. doi: 10.1148/rg.236035704. [DOI] [PubMed] [Google Scholar]
- Plotnik A N, Foley P T, Koukounaras J, Lyon S M. How I do it: Lymphangiography. J Med Imaging Radiat Oncol. 2010;54(1):43–46. doi: 10.1111/j.1754-9485.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yamagami T, Kato T, et al. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol. 2009;82(976):286–290. doi: 10.1259/bjr/64849421. [DOI] [PubMed] [Google Scholar]