Abstract
Microwave ablation is a developing treatment option for unresectable lung cancer. Early experience suggests that it may have advantages over radiofrequency (RF) ablation with larger ablation zones, shorter heating times, less susceptibility to heat sink, effectiveness in charred lung, synergism with multiple applicators, no need for grounding pads, and similar survival benefit. Newer microwave ablation devices are being developed and as their use becomes more prevalent, a greater understanding of device limitations and complications are important. Herein we describe a microwave lung ablation complicated by bronchocutaneous fistula (BCF) and its treatment. BCF treatment options include close monitoring, surgical closure, percutaneous sealant injection, and endoscopic plug or sealant in those who are not surgical candidates.
Keywords: Microwave ablation, bronchopleural fistula, lung cancer treatment
Primary lung cancer incidence is the leading cause of worldwide cancer-related mortality, with 1.3 million deaths per year.1 Although operative resection may be indicated in up to stage IIIa tumors, not all patients may qualify depending on comorbid conditions precluding surgery. Furthermore, patients who have recurrent disease may not be suitable for repeat resection. Percutaneous lung ablation was first reported in 2000,2 and continues to have increasing interest. Moreover, percutaneous lung ablation appears to confer advantages of reduced procedure-related mortality, shorter recovery periods, preservation of lung function, and acceptable 2-year survival rates.3,4,5,6 As newer ablation devices become available and their use more prevalent, increased understanding of device limitations and complications becomes critical. In this report, we describe a case of microwave lung ablation complicated by bronchocutaneous fistula (BCF) and review the current literature on the management of such a complication.
CASE REPORT
Institutional review board approval is not required for retrospective case study at the authors' hospital.
A 63-year-old woman with history of a 7.4 cm moderately differentiated adenocarcinoma in the upper lobe of the left lung underwent resection for stage IIb disease (T2N0). She had a complicated postoperative course with development of pulmonary embolism and after starting anticoagulation developed gastrointestinal bleeding requiring placement of an inferior vena cava (IVC) filter. The patient developed heparin-induced thrombocytopenia (HIT), and was maintained on Fragmin injections. She was treated with systemic chemotherapy for the next 3 years for small recurrent left upper lobe disease with various drug side effects necessitating 5 different lines of chemotherapy. Despite chemotherapy, the patient had documented growth of the left upper lobe nodule by computed tomography (CT) scan, and was referred for percutaneous ablative therapy. Her comorbid conditions included obesity, obstructive sleep apnea, asthma, pulmonary hypertension, and HIT. CT showed 1.3 × 0.5 cm left upper lobe nodule and an adjacent 0.7 cm nodule. These two nodules were thought to be amenable to percutaneous ablation, and the patient was scheduled for CT-guided ablation.
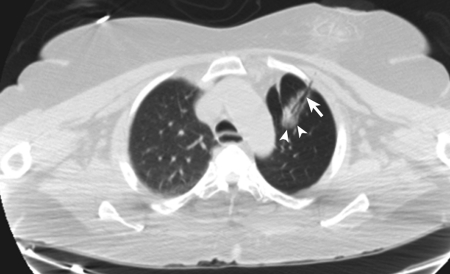
After informed consent was obtained, the patient was positioned on the CT gantry in a supine position. Intravenous moderate sedation was administered with continuous pulse oximetry and intermittent blood pressure monitoring, using a total of 2 mg midazolam and 150 μg fentanyl during the procedure. Limited noncontrast CT of the chest was performed with a radiopaque grid positioned over the left chest. The shortest path to the main nodule was identified and the skin site was marked. The site was prepped and draped in standard sterile fashion. After 1% subcutaneous lidocaine was administered, a monopolar 16-gauge AveCure® microwave antenna (MedWaves Inc., San Diego, CA) was advanced into the nodule using CT guidance. Microwave ablation was performed for 5 minutes using the 915 MHz generator set to 26 W with target temperature 100°C. The antenna was then removed and manual pressure was applied to the site briefly prior to applying a sterile dressing. Immediate postprocedure CT (Fig. 1) revealed expected hazy ground-glass opacification at the ablation site, but also demonstrated a persistent ablation track to the skin.
Figure 1.
Immediate postablation computed tomography scan following removal of 16-gauge AveCure® microwave antenna shows expected hazy ground-glass opacification at the ablation site (arrowheads) with persistent ablation tract to skin (arrow).
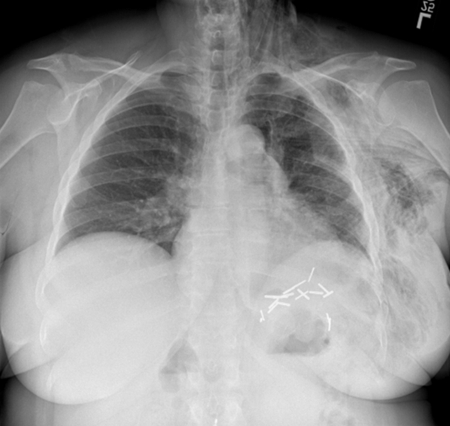
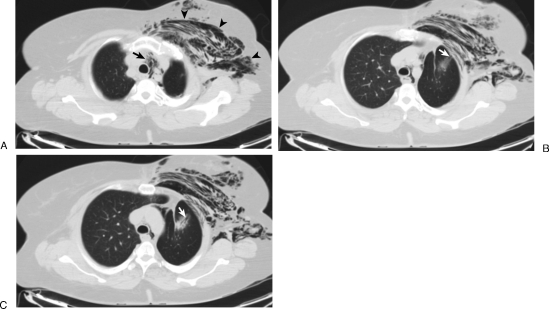
The patient was without complaint at completion of the exam. Upon arrival to the hospital floor, the patient was noticed to have swelling of her face and left chest. Physical exam demonstrated crepitus, and chest radiograph (CXR) was performed at 4 hours and 12 hours (Fig. 2), revealing extensive subcutaneous emphysema without pneumothorax suggesting a diagnosis of BCF. Cardiothoracic surgery was consulted, and given the patient's asymptomatic status, the decision was made to perform close observation. The patient was transferred to the intermediate care ward. CT scan (Fig. 3) and serial CXRs were performed over the next 2 days, and once the subcutaneous emphysema and pneumomediastinum were stable, the patient was discharged home. Outpatient CXR performed at 96 hours postprocedure demonstrated reduced subcutaneous emphysema. At one-month clinical follow-up, the patient was without any swelling or complaints. At 2-month imaging follow-up, the BCF was no longer present and tissue emphysema had completely resolved.
Figure 2.
12-hour delayed chest X-ray after computed tomography-guided microwave ablation reveals extensive subcutaneous emphysema within left chest, breast, and neck, without pneumothorax.
Figure 3.
One-day posttreatment chest computed tomography scan shows subcutaneous (arrowheads) and mediastinal (arrow) emphysema (A). Note continued communication of ablation tract (arrow) to chest wall soft tissues, without pneumothorax (B,C).
DISCUSSION
Complications of percutaneous lung ablation and their relative incidence include pneumothorax (28%), pleural effusion (14%), pain (14%), fever (4.4%), hemoptysis (4.3%), lung infection (1.9%), subcutaneous emphysema (0.2%), BCF (0.4%), and procedure-related death (0.2%).3 Although uncommon, BCF can be difficult to treat; therefore, understanding the cause of and potential means to avoid such a complication is important. In this report, we presented a case of BCF manifested as extensive subcutaneous emphysema and pneumomediastinum immediately following microwave ablation using a MedWaves AveCure® device.
Theoretical advantages of microwave ablation over radiofrequency (RF) ablation in the lung include larger ablation zones, shorter time to heating, less susceptibility to heat sink, improved effectiveness in charred or postsurgical lung, synergism of multiple applicators, and lack of need for grounding pads.7 These differences are attributable to the direct heating from microwave ablation versus resistive heating through electrical currents required of RF ablation in a low conductive medium such as the lung. Currently, there are 9 microwave devices approved or being developed for use.7 The MedWaves device was approved by the United States Food and Drug Administration (FDA) for soft tissue ablation in 2007. It was first approved in open liver ablations and has been utilized in percutaneous liver, lung, and cardiac applications. No published reports of its use or complications in percutaneous lung ablations have been described to date based on our review of the literature.
The MedWaves device is unique, with a monopolar antenna that can increase its ablation zone based on duration of ablation. There is no internal cooling of the antenna. In the case presented, the lack of internal cooling may have caused nontarget shaft heating that could have contributed to the failure of the lung tract to collapse following removal of the device, leaving an open communication to the chest wall soft tissues. The subsequently formed BCF resulted in extensive subcutaneous emphysema and mediastinal emphysema. The patient was clinically asymptomatic, and as such close observation with serial CXRs to evaluate for stability over a course of 2 days was implemented.
In 2005, Radvany et al8 first described delayed extensive subcutaneous emphysema and pneumomediastinum in a patient 7 days after undergoing RF ablation of a recurrent left lung adenocarinoma previously treated with external beam radiation and left upper lobectomy. CT-guided fibrin sealant was injected into the necrotic tumor cavity and resulted in resolution of patient's symptoms of pain at 1 month, as well as radiologic resolution of the emphysema over 1–4 months. The authors hypothesized that either the pneumomediastinum was due to postablation tumoral necrosis that resulted in a bronchial communication to the damaged interstitium allowing tracking to the mediastinum or that postablation tumoral necrosis resulted in a communication to the skin causing a BCF and subcutaneous emphysema tracking to the mediastinum. Of note, both this patient and our patient had previous surgery, the resultant scarring of which may predispose these patients to BCF by precluding appropriate collapse of the lung around the ablation tract.
Air leak following lung ablation may result in uncomplicated pneumothorax, complicated pneumothorax requiring chest tube, subcutaneous emphysema, BCF, and bronchopleural fistula (BPF). Risk factors for pneumothorax after lung biopsy include presence of chronic obstructive pulmonary disease (COPD), increased number of needle passes, older patient age, increased lesion depth, subpleural location, and smaller lesion size.9 For pneumothorax after lung ablation, the only factors increasing risk appear to be central tumors and lack of previous lung surgery.9,10 Uncomplicated pneumothoraces are generally self-limiting, and support with oxygen and close imaging (CXR) follow-up are usually only required. Those pneumothoraces that are larger or symptomatic are typically responsive to chest tube placement. Okuma et al9 reported that subcutaneous emphysema alone following lung RF ablation is usually self-limiting. Abu-Hijleh and Blundin11 added that if air leaks do not resolve within several days, the likelihood of resolution with conservative management, including chest tube drainage, decreases significantly. With reported success rates of 64–95%, instillation of sclerosing agents through a chest tube to produce a pleural symphysis for management of persistent air leaks is acceptable for patients who are not good surgical candidates.12 For secondary pneumothoraces and persistent air leaks, the American College of Chest Physicians expert panel recommends observation for 5 days and urges for surgical intervention if persistent beyond this period.13 The success rate of surgical closure of BPFs has been reported to be between 80–95% and remains the standard of care for those patients who are surgical candidates.12 BPFs have been reported following lung RF ablation, and can be fatal due to their intractable nature. A report of two patients with BPF following RF ablation described unsuccessful treatment with chest tube drainage, bronchoscopic filler, and pleurodesis.14 One patient died at postprocedure day 52 following these measures.14 The other patient had additional unsuccessful treatment attempts including video-assisted thoracoscopic surgery (VATS) closure, fibrin glue injection onto the surface of the BPF, and endobronchial autologous blood into the fistula, and died on postprocedure day 97.14 Others have reported treating BCFs in nonoperative patients with endobronchial plugs, fillers, coils, and sealants, as well as percutaneous injection of plugs (Gelfoam or glue).8,11,12,14 There is no evidence to support one agent as the “best sealant”12.
Understanding the limitations of new devices such as the MedWaves antenna may help to limit risks for complications or assist in their management when anticipated. Future antenna designs may benefit from further elucidation of in-vivo experiences of the device. This report shows the risk for BCF following microwave lung ablation of recurrent adenocarcinoma of the lung and the management of such a complication.
References
- WHO International Agency for Research on Cancer. GLOBOCAN 2008. Lyon, France: WHO; 2010. [Google Scholar]
- Dupuy D E, Zagoria R J, Akerley W, Mayo-Smith W W, Kavanagh P V, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174(1):57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- Chan V O, McDermott S, Malone D E, Dodd J D. Percutaneous radiofrequency ablation of lung tumors: evaluation of the literature using evidence-based techniques. J Thorac Imaging. 2011 Feb;26(1):18–26. doi: 10.1097/RTI.0b013e3181e48d5e. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9(7):621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- Simon C J, Dupuy D E, DiPetrillo T A, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- Wolf F J, Grand D J, Machan J T, Dipetrillo T A, Mayo-Smith W W, Dupuy D E. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- Lubner M G, Brace C L, Hinshaw J L, Lee FT., Jr Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192–203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radvany M G, Allan P F, Frey W C, Banks K P, Malave D. Pulmonary radiofrequency ablation complicated by subcutaneous emphysema and pneumomediastinum treated with fibrin sealant injection. AJR Am J Roentgenol. 2005;185(4):894–898. doi: 10.2214/AJR.04.0235. [DOI] [PubMed] [Google Scholar]
- Okuma T, Matsuoka T, Yamamoto A, et al. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Intervent Radiol. 2008;31(1):122–130. doi: 10.1007/s00270-007-9225-0. [DOI] [PubMed] [Google Scholar]
- Pua B B, Thornton R H, Solomon S B. Ablation of pulmonary malignancy: current status. Review. J Vasc Interv Radiol. 2010;21(8, Suppl):S223–S232. doi: 10.1016/j.jvir.2010.01.049. [DOI] [PubMed] [Google Scholar]
- Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung. 2010;188(3):253–257. doi: 10.1007/s00408-009-9204-0. [DOI] [PubMed] [Google Scholar]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128(6):3955–3965. doi: 10.1378/chest.128.6.3955. [DOI] [PubMed] [Google Scholar]
- Baumann M H, Strange C, Heffner J E, et al. AACP Pneumothorax Consensus Group Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Hiraki T, Mukai T, et al. Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol. 2007;18(1 Pt 1):141–145. doi: 10.1016/j.jvir.2006.10.011. [DOI] [PubMed] [Google Scholar]