Abstract
Numerous modalities for hepatic tumor ablation are currently used including ethanol injection, radiofrequency ablation (RFA), cryoablation, and microwave ablation. The results and complications of these various tumor ablation techniques have been reported extensively, with the most data existing for percutaneous RFA. One of the most serious complications from tumor ablation is the seeding of cancer cells along the ablation tract. The incidence and risk factors for tract seeding in RFA have been reported, but little information regarding this complication with other ablation modalities has been reported. We report a case of tumor seeding into the pleural space following percutaneous cryoablation of hepatocellular carcinoma (HCC).
Keywords: Hepatocellular, carcinoma, cryoablation, tumor seeding
CASE REPORT
An 80-year-old woman initially presented in late 2002 with abdominal discomfort, and was found to have a palpable abdominal mass in the right upper quadrant. She had no history of underlying liver disease and liver function tests at presentation were normal. Further clinical and diagnostic testing revealed a solitary mass in the right lobe of the liver, with subsequent biopsy demonstrating HCC. In December 2002, she underwent surgical resection of liver segments 5 and 6. Pathology again demonstrated HCC, without penetration through the capsule and with negative margins of resection.
Follow-up imaging 4 months postresection was negative, but a subsequent scan 6 months later identified 3 new hepatic lesions. She was then referred to interventional radiology service and had chemoembolization (TACE) performed with a good response. Despite subsequent development of multiple new lesions, multiple liver directed treatments over the span of 4 years controlled her disease, and helped maintain a high quality of life. These treatments included a total of 5 TACE and 1 Therasphere® radioembolization.
In July 2007, surveillance imaging revealed a new lesion in segment 6 (Fig. 1), along with a rising α fetoprotein. Because the patient had previously responded well to TACE, it was decided to attempt this once again. However, the lesion could not be localized angiographically and the TACE was aborted. Percutaneous tumor ablation was then discussed with the patient and her family. Cryoablation was chosen due to the subcapsular location and size of the lesion.
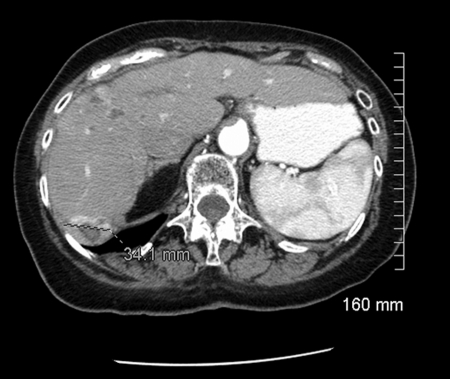
Figure 1.
Enhanced computed tomography study demonstrates enhancing lesion in Segment 6 of the liver.
The cryoablation (Fig. 2) was performed under computed tomography (CT) and ultrasound guidance with standard technique and moderate sedation. A right intercostal approach was chosen based on anatomical considerations, and the pleural space was traversed with the cryoablation probe. Two 10-minute cryoablation cycles were performed, with an interspaced 5-minute thaw. Scans after removal of the cryoablation probe showed no significant perihepatic hemorrhage and adequate coverage of the target lesion. A small, asymptomatic pneumothorax was demonstrated at that time. There were no other immediate complications and the patient tolerated the procedure well.
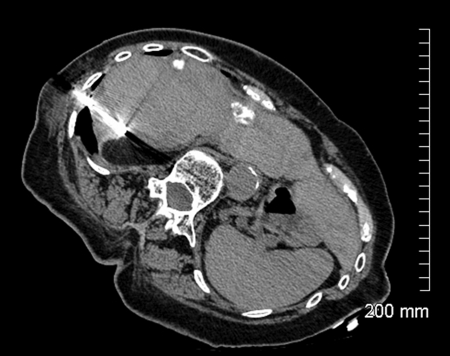
Figure 2.
Unenhanced computed tomography scan shows cryoablation needle within segment 6 mass.
Within a month of the procedure, the patient developed shortness of breath. A chest radiograph (Fig. 3A) and abdominal CT (Fig. 3B) demonstrated increasing, moderate right basilar pleural effusion with associated basilar atelectasis, without associated pleural or parenchymal nodules or masses. A thoracentesis performed on the following day removed 800 mL of serosanguineous effusion.
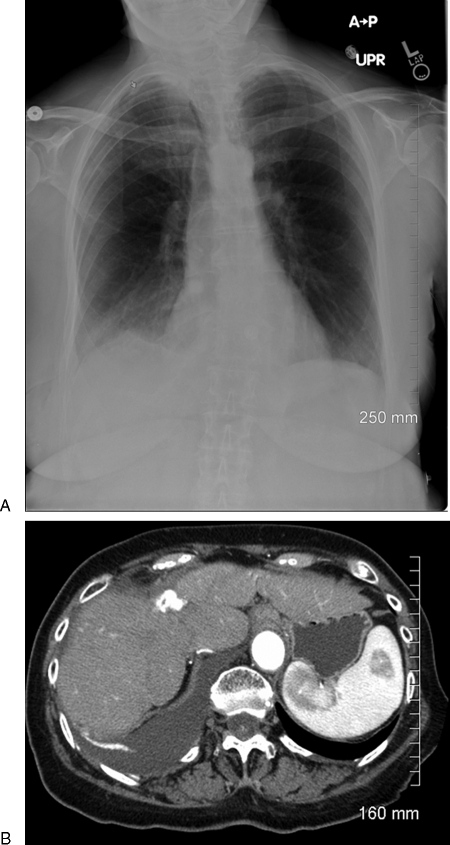
Figure 3.
(A) Chest radiograph showing right pleural effusion. (B) Enhanced computed tomography scan showing right pleural effusion.
An abdominal CT performed in November 2007 (Fig. 4) revealed multiple stable areas of enhancement in the liver consistent with residual tumor, and a persistent moderate pleural effusion with subsegmental atelectasis in the right hemithorax. In addition, the CT demonstrated multiple pleural-based lesions, consistent with pleural metastases. Laboratory values revealed an α-fetoprotein of greater than 15,000, and normal bilirubin level, liver enzymes, and albumin.
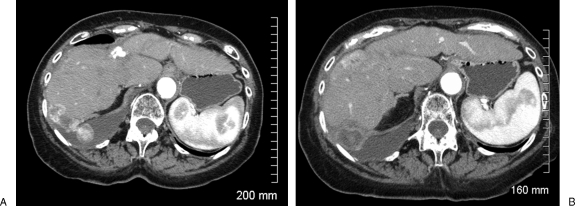
Figure 4.
(A) Computed tomography scan (CT) shows enhancing lesions in the liver and pleural space with right pleural effusion. (B) Enhanced CT shows residual tumor at the cryoablation site.
DISCUSSION
In this case report, we describe tumor seeding of a patient's pleura resulting from cryoablation of HCC. Risk of tumor seeding with percutaneous biopsy and RFA of HCC have been previously reported.1,2,3 To the best of our knowledge, this is the first documented case of tract seeding with cryoablation. Multiple percutaneous cryoablation series have been reported without mention of tumor seeding as a potential complication.4,5,6,7,8,9 These reports outline several complications, including but not limited to liver surface fracture, biliary fistula, bleeding, electrolyte disturbance, and abscess.7,8,9 Retrospective analysis of outcome data in 2 articles,7,8 and 1 published controlled trial5 have investigated the complications without mention of tumor seeding of the tract. A recent article republished in Radiographics reported no tumor seeding in 107 cryoablations.4
Factors specific to cryotherapy that could lead to seeding of the tract include the use of more probes compared with other ablative techniques and an inability to ablate the probe tracts.1 Complications may also result from the preferential usage of cryotherapy in the setting of higher risk (subcapsular) lesions. Despite the lack of observed and documented cases of tract seeding as a result of cryotherapy, these factors make tract seeding plausible.
Malignant pleural effusion and pleural metastases in this case were diagnosed and documented on the follow-up CT scan. Crossing of the pleura with the cryoablation probe was evident from the small pneumothorax demonstrated on the immediate postcryoablation scan.
The patient's prognosis was altered in a meaningful and negative way by the new metastatic lesion within the thoracic pleura, eventually dying as a result of the metastatic disease. Due to the patient's advanced age and comorbidities, palliative care was administered.
References
- Silva M A, Hegab B, Hyde C, Guo B, Buckels J A, Mirza D F. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Epub 2008 Jul 31. Review. Gut. 2008;57:1592–6. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs A K. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33(5):437–447. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Takamori R, Wong L L, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6(1):67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- Dodd G D, III, Soulen M C, Kane R A, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20(1):9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- Korpan N N. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg. 1997;225(2):193–201. doi: 10.1097/00000658-199702000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee M D, Kane R A. Cryosurgery for hepatic tumor ablation. Semin Intervent Radiol. 1997;14(3):285–293. [Google Scholar]
- Morris D L, Ross W B. Australian experience of cryoablation of liver tumors: metastases. Surg Oncol Clin N Am. 1996;5(2):391–397. [PubMed] [Google Scholar]
- Seifert J K, Junginger T, Morris D L. A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb. 1998;43(3):141–154. [PubMed] [Google Scholar]
- Weaver M L, Atkinson D, Zemel R. Hepatic cryosurgery in treating colorectal metastases. Cancer. 1995;76(2):210–214. doi: 10.1002/1097-0142(19950715)76:2<210::aid-cncr2820760208>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]