Abstract
Hepatic abscess is a rare complication of yttrium-90 radioembolization of hepatic tumors that most commonly occurs in patients with a history of biliary intervention. Patients usually present several weeks after therapy with pain, nausea, vomiting, and fever. Cross-sectional imaging is necessary in cases of suspected abscess to ensure prompt diagnosis and to help plan treatment, which involves antibiotics and percutaneous drainage.
Keywords: Radioembolization, infection, abscess, embolization
CASE REPORT
The patient was diagnosed with unresectable gastrin-secreting islet cell tumor metastases in 2001 after presenting with vomiting and undergoing a computed tomography (CT) scan that demonstrated hepatic masses. These were biopsied and proven to be neuroendocrine tumor metastases. Treatment consisted of a proton-pump inhibitor and octreotide, and the patient remained stable and symptomatically controlled until July 2006 when a surveillance CT demonstrated an enlarging dominant metastasis in segment 8. Given the disease progression and recurrence of symptoms, including flushing, the patient was sent to the interventional oncology service for consideration of liver-directed therapy. The patient elected to undergo treatment with yttrium-90 (y-90) glass microspheres (TheraSphere®, MDS Nordion, Kanata, ON, Canada).
A planning visceral arteriogram was performed in late July 2006 and the dominant metastatic lesion was found to arise from the distal branches of the anterior right hepatic artery in segment 8. Additionally, a hypertrophied right gastric artery arising from the mid proper hepatic artery was coil embolized, and technetium-labeled macroaggregated albumin was administered via the proper hepatic artery to calculate the shunt fraction. Radioembolization with y-90 glass microspheres was performed 9 days later via a 3-French microcatheter in the anterior branch of the right hepatic artery with a 3 GBq dose vial. Completion angiography demonstrated minimal embolic effect. Of note, the patient was started on an oral course of ciprofloxacin (generic) 250 mg by her primary care physician for mild urinary tract symptoms 1 day prior to the radioembolization procedure. Even though the symptoms resolved, ciprofloxacin 400 mg intravenously (IV) was given as prophylaxis on the day of the procedure. The radioembolization was tolerated well with no immediate complications, and the patient was discharged home that same day.
On postprocedure day 1, the patient developed increasing fevers and right upper quadrant abdominal pain. The patient lived several hours away from the interventional oncology facilities and therefore presented to another hospital as the symptoms persisted. During that admission, the patient was found to be septic and blood cultures were positive for Escherichia coli (E. coli). A CT scan revealed locules of air within the segment 8 treatment site. The patient was managed conservatively with IV antibiotics both in the hospital and as an outpatient after discharge.
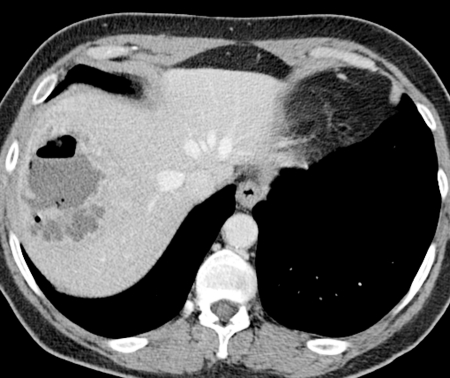
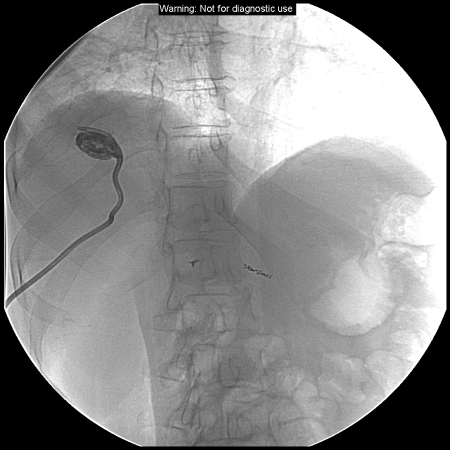
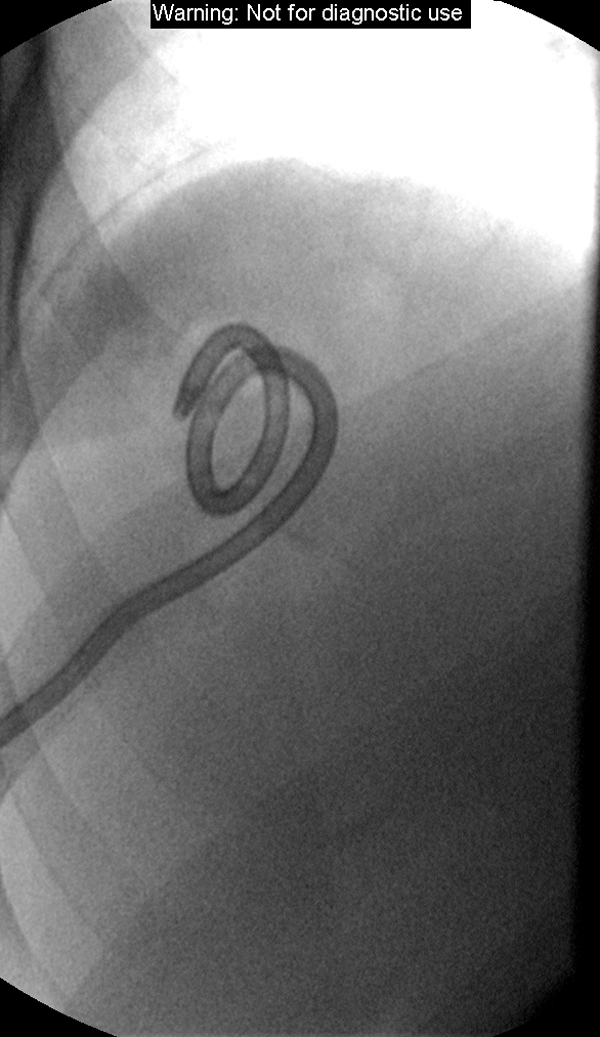
Two weeks after radioembolization and several days after discharge from the outside hospital, the patient presented for follow-up with the interventional oncology service. Conservative management was continued as the fevers and abdominal pain, while still present, had significantly improved. However, these symptoms persisted for 2 more weeks and a CT scan at that time demonstrated a large abscess in the treatment site containing fluid and gas (Fig. 1). Ultrasound-guided percutaneous drainage was then performed, which returned grossly purulent fluid, and a10 French multisided-hole pigtail drainage catheter was placed under fluoroscopy (Fig. 2). Cultures of the purulent fluid were positive for E. coli. The drain was left in place for 3 weeks and removed when the patient was asymptomatic, drainage had ceased, and the abscess cavity had resolved (Fig. 3). The patient recovered completely from the complication, and imaging several months after radioembolization demonstrated decreased size and enhancement of the dominant hepatic metastasis.
Figure 1.
Contrast-enhanced computed tomography image demonstrates a rim-enhancing fluid and gas collection in the treatment site in segment 8. Given the patient's symptoms of pain and fever, this was consistent with an abscess.
Figure 2.
Fluoroscopic image following ultrasound-guided percutaneous placement of a 10 French pigtail drainage catheter in the segment 8 abscess.
Figure 3.
Fluoroscopic image following injection of iodinated-contrast into the drainage catheter several weeks after placement demonstrating no significant residual abscess cavity. The patient's symptoms had resolved and the drainage catheter was removed.
DISCUSSION
Hepatic abscess is a rare but serious complication of y-90 radioembolization. Few cases have been reported in the literature,1,2 and most reports concern complications of other locoregional therapies, such as transcatheter arterial chemoembolization or transarterial embolization. In these patients, the reported incidence is 0.3–5%.3,4,5,6,7,8 Patients with prior biliary intervention resulting in sphincter of Oddi compromise, such as biliary-enteric anastomosis or sphincterotomy, have an increased incidence estimated to be as high as 85%.3 This predisposition may relate to biliary colonization and postembolic biliary ischemia as colonizing enteric bacteria can invade the ischemic biliary walls.9 The incidence has also been reported to be higher in patients with hepatic metastases as opposed to a primary hepatic malignancy.1,4
Patients with hepatic abscess typically present with pain, high-grade fever, nausea, and vomiting ~2–4 weeks after treatment. An earlier presentation, as in this case report, is unusual.2 The time frame of presentation is important as the postembolization syndrome, which commonly occurs after transarterial chemoembolization, has similar symptoms to hepatic abscess.3 However, this syndrome presents in the first days after therapy unlike hepatic abscess, which in most cases presents greater than a week after therapy. The postembolization syndrome is less common in patients treated with y-90 radioembolization as opposed to chemoembolization, and patients often only present with mild symptoms such as fatigue in contrast to fever and abdominal pain.10 Furthermore, it is less common in patients treated with glass y-90 microspheres (Theraspheres®) relative to the resin microspheres (SIR-spheres®; Sirtex Medical, Lane Cove, Australia), which have a higher embolic load.10
When there is clinical concern for hepatic abscess, cross-sectional imaging, often with CT, is necessary to permit prompt diagnosis and treatment. Features suggestive of abscess on CT include fluid, ring enhancement, and air. However, it is vital to interpret these features in the clinical context as ring enhancement and air can normally be seen in the treatment site due to tumor necrosis.11,12,13,14 Laboratory work includes a white blood cell count and aerobic and anaerobic blood cultures, which are positive in ~50% of cases of hepatic abscess.15
Prompt treatment with systemic antibiotics and drainage is necessary due to the risk of sepsis and significant morbidity and mortality. Ultrasound or CT-guided percutaneous drainage has become an important treatment for hepatic abscess as it is well-tolerated, can be performed relatively quickly, and does not require general anesthesia. Surgical drainage may be necessary if the abscess cannot be safely accessed, has overly complicated morphology, ruptures into the peritoneal cavity, or fails to respond to percutaneous drainage. Abscess fluid should be sent for Gram stain, and aerobic and anaerobic cultures.
Antibiotic therapy should be started as soon as hepatic abscess is suspected but after blood cultures have been sent. Therapy is directed toward common causative organisms including gram-negative aerobes such as Klebsiella and E. coli, gram-positive aerobes such as Enterococcus and Staphylococcus aureus, and anaerobes such as Bacteroides.15 Common empiric regimens include ampicillin, gentamycin, and metronidazole or ceftriaxone and metronidazole,15 though the treatment should be tailored based on culture sensitivities. Drains are left in place until output becomes negligible and follow-up imaging demonstrates resolution of the abscess cavity. The duration of antibiotic treatment varies based on clinical response though some general guidelines recommend 2 weeks of intravenous therapy followed by 4 to 6 weeks of oral therapy.15 Antibiotic prophylaxis prior to the procedure, as was given in this case, has not been shown to effectively prevent hepatic abscess.3
Untreated liver abscess is uniformly fatal. In patients who have undergone locoregional therapies such as chemoembolization, percutaneous drainage with systemic antibiotics has shown favorable results with most patients improving clinically. However, abscess-related mortality remains significant and ranges from 11% to 50%.4,7,11,16
References
- Atassi B, Bangash A K, Lewandowski R J, et al. Biliary sequelae following radioembolization with yttrium-90 microspheres. J Vasc Interv Radiol. 2008;19(5):691–697. doi: 10.1016/j.jvir.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Mascarenhas N B, Mulcahy M F, Lewandowski R J, Salem R, Ryu R K. Hepatic abscess after yttrium-90 radioembolization for islet-cell tumor hepatic metastasis. Cardiovasc Intervent Radiol. 2010;33(3):650–653. doi: 10.1007/s00270-009-9617-4. [DOI] [PubMed] [Google Scholar]
- Kim W, Clark TWI, Baum R A, Soulen M C. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(8):965–968. doi: 10.1016/s1051-0443(07)61577-2. [DOI] [PubMed] [Google Scholar]
- Song S Y, Chung J W, Han J K, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol. 2001;12(3):313–320. doi: 10.1016/s1051-0443(07)61910-1. [DOI] [PubMed] [Google Scholar]
- Xia J, Ren Z, Ye S, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006;59(3):407–412. doi: 10.1016/j.ejrad.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Tarazov P G, Polysalov V N, Prozorovskij K V, Grishchenkova I V, Rozengauz E V. Ischemic complications of transcatheter arterial chemoembolization in liver malignancies. Acta Radiol. 2000;41(2):156–160. doi: 10.1080/028418500127344966. [DOI] [PubMed] [Google Scholar]
- Kim M H, Choi M S, Choi Y S, et al. Clinical features of liver abscess developed after radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma. Korean J Hepatol. 2006;12(1):55–64. [PubMed] [Google Scholar]
- Chung J W, Park J H, Han J K, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198(1):33–40. doi: 10.1148/radiology.198.1.8539401. [DOI] [PubMed] [Google Scholar]
- Georgiades C S, Hong K, Geschwind J F. Pre- and postoperative clinical care of patients undergoing interventional oncology procedures: a comprehensive approach to preventing and mitigating complications. Tech Vasc Interv Radiol. 2006;9(3):113–124. doi: 10.1053/j.tvir.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Salem R, Thurston K G. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. J Vasc Interv Radiol. 2006;17(9):1425–1439. doi: 10.1097/01.RVI.0000235779.88652.53. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen P J, Yang P M, et al. Clinical and microbiological features of liver abscess after transarterial embolization for hepatocellular carcinoma. Am J Gastroenterol. 1997;92(12):2257–2259. [PubMed] [Google Scholar]
- Atassi B, Bangash A K, Bahrani A, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics. 2008;28(1):81–99. doi: 10.1148/rg.281065721. [DOI] [PubMed] [Google Scholar]
- Akahane M, Koga H, Kato N, et al. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25(Suppl 1):S57–S68. doi: 10.1148/rg.25si055505. [DOI] [PubMed] [Google Scholar]
- Lim H K, Choi D, Lee W J, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221(2):447–454. doi: 10.1148/radiol.2212010446. [DOI] [PubMed] [Google Scholar]
- Johannsen E C, Sifri C D, Madoff L C. Pyogenic liver abscesses. Infect Dis Clin North Am. 2000;14(3):547–563, vii. doi: 10.1016/s0891-5520(05)70120-3. [DOI] [PubMed] [Google Scholar]
- de Baère T, Roche A, Amenabar J M, et al. Liver abscess formation after local treatment of liver tumors. Hepatology. 1996;23(6):1436–1440. doi: 10.1002/hep.510230620. [DOI] [PubMed] [Google Scholar]