Abstract
Language is typically a function of the left hemisphere but the right hemisphere is also essential in some healthy individuals and patients. This inter‐subject variability necessitates the localization of language function, at the individual level, prior to neurosurgical intervention. Such assessments are typically made by comparing left and right hemisphere language function to determine “language lateralization” using clinical tests or fMRI. Here, we show that language function needs to be assessed at the region and hemisphere specific level, because laterality measures can be misleading. Using fMRI data from 82 healthy participants, we investigated the degree to which activation for a semantic word matching task was lateralized in 50 different brain regions and across the entire cortex. This revealed two novel findings. First, the degree to which language is lateralized across brain regions and between subjects was primarily driven by differences in right hemisphere activation rather than differences in left hemisphere activation. Second, we found that healthy subjects who have relatively high left lateralization in the angular gyrus also have relatively low left lateralization in the ventral precentral gyrus. These findings illustrate spatial heterogeneity in language lateralization that is lost when global laterality measures are considered. It is likely that the complex spatial variability we observed in healthy controls is more exaggerated in patients with brain damage. We therefore highlight the importance of investigating within hemisphere regional variations in fMRI activation, prior to neuro‐surgical intervention, to determine how each hemisphere and each region contributes to language processing. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: functional MRI, language, laterality index, laterality maps, inter‐hemispheric dissociation, left and right hemisphere activity, semantic processing
INTRODUCTION
In this article, we investigate how the relative involvement of the left and right hemisphere in language processing varies with the individual and the anatomical region. Our questions have implications for both theoretical and clinical perspectives of language function. From a theoretical perspective, the key observations are that (a) language is more impaired after lesions to the left than right hemisphere; (b) language processing activates the left more than the right hemisphere in functional neuroimaging experiments; but (c) the degree to which language is left lateralized varies greatly, along a continuum, with some individuals showing bilateral or right hemisphere lateralization [Knecht et al., 2000; Springer et al., 1999]. From the clinical perspective, inter‐subject variability in language lateralization has important implications for predicting how neurosurgical intervention will impact upon language function (e.g., for review see [Bookheimer, 2007; Ganslandt et al., 2009; Stippich et al., 2007; Tharin and Golby, 2007]). For example, if a patient typically uses the right hemisphere for language, then neurosurgery in the right hemisphere may unexpectedly impair language. As language lateralization varies with the anatomical region (for a review, see [Seghier, 2008], the importance of the left or right hemisphere for language function will depend on which region is affected and tested (e.g., [Berl et al., 2005; Deblaere et al., 2004]).
Traditionally, the method used to assess language function prior to neurosurgery is the Wada (intracarotid amobarbital) test [Wada, 1949]. This procedure suppresses the function of each hemisphere individually which allows the language capabilities of each hemisphere to be assessed. There are several factors that complicate the interpretation of the Wada test (e.g., [Baxendale, 2009; Meador and Loring, 1999]) and this invasive procedure is not risk‐free for patients [Loddenkemper et al., 2008]. Its use in the clinical setting is therefore on the decline [Baxendale et al., 2008] and there has been a great deal of interest in replacing the Wada test with functional MRI which is both noninvasive and regionally specific [Abou‐Khalil, 2007; Baxendale, 2009; Bookheimer, 2007; Medina et al., 2007; Rutten and Ramsey, 2010].
Many studies have conducted within subject comparisons of language lateralization assessed with Wada versus fMRI [Binder et al., 1996; Desmond et al., 1995]. Although the results are consistent in ∼90% of cases, the residual inconsistencies have led to the conclusion that clinicians cannot rely on fMRI results, particularly when language lateralization is atypical [Arora et al., 2009; Benke et al., 2006; Giussani et al., 2010; Jayakar et al., 2002; Lee et al., 2008; Paolicchi, 2008; Wellmer et al., 2008; Westerveld et al., 1999; Woermann et al., 2003]. For example, in a recent survey across 26 European epilepsy centres (a review of 1,421 Wada procedures), Haag et al. [ 2008] concluded that clinicians currently do not feel that they can rely solely on fMRI results, with the majority of them agreeing on the necessity of performing a Wada test if fMRI revealed atypical laterality [Haag et al., 2008].
Here we argue that both the Wada and fMRI laterality indices, assessed across hemisphere, are misleading in the clinical setting because they do not indicate which regions within a hemisphere are involved in language function. Nor do they account for spatial heterogeneity in language lateralization. Previous reports have already suggested that lateralization measured with fMRI depends on the location and size of the volume of interest (for a critical review see [Josse and Tzourio‐Mazoyer, 2004; Seghier, 2008]). There is also evidence that the direction of laterality can reverse within the same individual, even when task and stimuli are held constant. For instance, in a patient with focal epilepsy, lexical activation was right lateralized in frontal cortex and left lateralized in temporal cortex [Baciu et al., 2003]. Conversely, in a presurgical fMRI study of a patient with schizencephaly performing a silent reading task, language was left‐lateralized in frontal cortex and right‐lateralized in temporal cortex [Ries et al., 2004]. Recently, this dissociation in laterality between frontal and temporal regions has also been demonstrated in one healthy subject during a verbal fluency task [Jansen et al., 2006a] and in another healthy subject during a semantic matching task [Bethmann et al., 2007]. Critically, however, the studies showing regional differences in language lateralization are based on individuals or small samples of patients in a few regions of interest selected on the basis of a priori knowledge. These intriguing observations motivate a more extensive investigation of regional differences in laterality at the population level using unsupervised and unconstrained laterality measures with high spatial definition. The first aim of our study was therefore to assess regional differences (dissociations) in language lateralization, in a large population of healthy participants, by computing lateralization scores at each voxel across the entire brain [Liegeois et al., 2002].
The need to assess language function in each region individually rather than across the whole hemisphere highlights a useful contribution for fMRI in presurgical investigations because fMRI can provide a rich source of regionally specific information. However, a second aim of our study was to demonstrate that fMRI laterality indices are misleading because they do not take into account the relative contribution of each hemisphere. For example, two patients may both activate and need the left hemisphere to the same degree but, depending on the degree of right hemisphere activation, one of these patients may have left lateralized language function and the other may have right lateralized language function. Thus, right lateralization does not indicate whether the left hemisphere is necessary or not for language. It only indicates that activation is relatively higher in the right than left hemisphere. To address this second aim of our study, we compared individuals with stronger versus weaker left lateralization and asked whether those with stronger left lateralization had more left hemisphere activation, less right hemisphere activation or both.
In summary, we computed individual laterality measures of activation for semantic matching of words at both the global level (across the whole hemisphere) and regional level in 82 left‐ and right‐handed healthy volunteers. Our aims were to (i) characterize the relative contribution of left and right hemisphere activity to lateralization values, and (ii) identify regional dissociations in laterality in an unsupervised manner and with high spatial definition.
MATERIALS AND METHODS
Subjects
Eighty‐two healthy subjects (43 females, 39 males) gave written informed consent to participate in this study. Subjects were native English speakers, had normal or corrected‐to‐normal vision, and had no history of neurological or psychiatric disorders. Their handedness was assessed with the Edinburgh questionnaire [Oldfield, 1971]: 44 were right‐handed and 38 were either left‐handed or ambidextrous. Their age ranged from 13 to 71 years: 21 subjects under 20 years old, 43 subjects between 20 years and 40 years old, and the remaining 18 subjects were more than 60 years old (mean = 30.3 ± 15 years).
The study was approved by the National Hospital for Neurology and Institute of Neurology Joint Ethic's Committee
Task and Experimental Design
Our semantic word matching task involved categorization of three visually presented words, each with one target above and two choices below. The subject is required to indicate which of the two choices below is most semantically related to the target above. The baseline task involved the same spatial display of three items but words were replaced with strings of unfamiliar greek letters and participants were instructed to indicate which of the two choices looked identical to the target above. For both tasks, the participants indicated their response on a key pad with either the index and middle finger on their right hand indicating the lower left or lower right stimulus respectively (50 subjects) or the middle and index finger on their left hand indicating the lower left or lower right stimulus respectively (32 subjects). Comparison of the semantic to the perceptual decisions reliably identifies semantic activations and their laterality (e.g., [Josse et al., 2008; Vandenberghe et al., 1996]).
In addition to the semantic decisions on words and perceptual decisions on greek letters, the experiment also included semantic matching on photographs of familiar objects and perceptual matching on photographs of unfamiliar nonobjects. All stimuli during the two semantic matching conditions were derived from a set of 192 objects with three to six letter familiar names (e.g., cat, bus, hat, ship, bell, frog, hand, teeth, camel, snake, spider, dagger, and button) that were first divided into two different sets of 96 items. Half of subjects were presented with the first set of 96 items as written words and the second set of 96 items as pictures, and the other half of subjects were presented with the reverse order of sets. Post hoc checks ensured that inter‐subject variability in lateralization could not be explained by the stimulus set.
Conditions and sets were fully counterbalanced within and across subjects. The two additional conditions of pictures of familiar and unfamiliar objects are not the focus of the current paper but they were used in post hoc tests to examine functional specialization in regions within the semantic word matching network. The four conditions were counterbalanced within each of two experimental runs/sessions. Across sessions, there were 32 stimuli presenting triads of written words, 32 stimuli presenting triads of pictures of objects, 16 stimuli presenting triads of greek letters and 16 stimuli presenting triads of unfamiliar nonobjects. Each stimulus (trial) stayed on the screen for 4.32 s followed by 180 ms fixation before the next stimulus. To maximize efficiency, four stimuli of the same type were presented successfully (i.e., blocks of 18 s). In addition, we included 12 blocks of fixation, each lasting 14.4 s, every two stimulus blocks. To facilitate task switching, each block was preceded by a written instruction for 3.6 s (e.g., “match words”).
Stimulus presentation was via a video projector, a front‐projection screen and a system of mirrors fastened to a head coil. To ensure that the task was understood correctly, all subjects were provided with detailed instructions and underwent a short training session before entering the scanner with a different set of stimuli. Additional details about the paradigm and stimuli can be found in our previous work (c.f., [Josse et al., 2008]).
MRI Acquisition
Experiments were performed on a 1.5T Siemens system (Siemens Medical Systems, Erlangen, Germany). Functional imaging consisted of an EPI GRE sequence (repetition time/echo time/flip angle = 3,600 ms/50 ms/90°, field of view = 192 mm, matrix = 64 × 64, 40 axial slices, 2 mm thick with 1 mm gap). Functional scanning was always preceded by 14.4 s of dummy scans to insure tissue steady‐state magnetization. Anatomical T1‐weighted images were acquired using a three‐dimensional modified driven equilibrium Fourier transform sequence (repetition time/echo time/inversion time = 12.24 ms/3.56 ms/530 ms, matrix = 256 × 224, 176 sagittal slices with a final resolution of 1 mm3).
fMRI Data Preprocessing
Data processing and statistical analyses were performed with the Statistical Parametric Mapping SPM5 software package (Wellcome Trust Centre for Neuroimaging, London UK, http://www.fil.ion.ucl.ac.uk/spm/). All functional volumes were spatially realigned, unwarped, and normalized to the MNI space using the unified normalization‐segmentation procedure [Ashburner and Friston, 2005] with resulting voxels size of 2 × 2 × 2 mm3.
Symmetrical Images
The priors used during the normalization‐segmentation step were a symmetrical version of the default priors implemented in SPM5. These symmetrical priors were created by simply copying, flipping along the x‐axis, and averaging the original and the mirror (flipped) versions of the priors [Salmond et al., 2000]. The resulting normalization‐segmentation parameters were then applied to the subject's functional images thereby rendering them symmetrical. This was relevant to the laterality maps (LM) where we directly compared left and right hemisphere activation; see below. The normalized (symmetrical) functional images were then spatially smoothed with a 6 mm full width half maximum isotropic Gaussian kernel.
First level Analyses
For each individual subject, we carried out a fixed‐effect analysis on all preprocessed functional volumes of that subject, using the general linear model at each voxel. Each stimulus onset was modeled as an event in condition‐specific “stick‐functions” with a duration of 4.32 sec per trial and a stimulus onset interval of 4.5 sec. This event related analysis, in the context of a block design, is more sensitive [Mechelli et al., 2003] and also allows us to exclude trials with incorrect responses. The resulting stimulus functions were convolved with a canonical hemodynamic response function which provided regressors for the linear model. Time‐series from each voxel were high‐pass filtered (1/128 Hz cut‐off) to remove low‐frequency noise and signal drift. The appropriate summary or contrast images were then generated in all subjects for the contrast of interest “semantic matching on words versus perceptual matching on unfamiliar greek letters.”
Global Laterality Index
To express the relative difference in the involvement of the two hemispheres during semantic matching at the global level [Desmond et al., 1995], we computed the laterality index (LI) for each subject. LI values were computed here on the statistical SPM{t} maps using Nagata et al.'s approach [Nagata et al., 2001] that is independent of the statistical threshold and assessed over the whole hemisphere after excluding the cerebellum and mesial part of the brain (i.e., around the inter‐hemispheric fissure). In brief, this procedure calculates the number of left and right hemisphere voxels activated for language relative to baseline, at a range of different statistical thresholds. Nonlinear regression of the shape of the curve, describing the relationship between the number of voxels and the statistical threshold, provides a constant term that is used to compute a normalized difference between left and right hemisphere activity [Nagata et al., 2001]. A positive LI (towards +1) indicates left hemisphere dominance, whereas a negative LI (towards −1) indicates right hemisphere dominance.
Voxel Based Laterality Maps
To express regional differences in the involvement of the two hemispheres, we generated maps of the laterality difference at each voxel for each subject [Baciu et al., 2005; Cousin et al., 2007; Josse et al., 2008; Liegeois et al., 2002; Salmond et al., 2000] as follows: (i) contrast images from the first‐level analysis (i.e., the symmetrical images) were copied and each copy was flipped along the inter‐hemispheric fissure (i.e., x‐axis mirror images), and (ii) the resulting flipped image was then subtracted from its original (unflipped) version to create a map of language laterality. Voxel based LM therefore code the relative difference between the contrast “semantic word matching versus perceptual matching on unfamiliar greek letters” at every voxel and at its homologue in the other hemisphere. Thus, LM images code the interaction [Liegeois et al., 2002] between task (semantic versus perceptual matching) and hemisphere (left versus right) at each voxel. Importantly, LM are symmetrical with respect to the inter‐hemispheric fissure, and can be described by the relationship LM(−x,y,z) = −LM(x,y,z).
Voxel Based Second Level Group Analyses
We conducted three different voxel based second‐level analyses on either original (unflipped) contrast images or voxel based LM:
-
1
One sample t‐test over the original contrast images to reveal the most consistently activated voxels for semantic word matching across our 82 subjects.
-
2
One sample t‐test over the voxel based LM to reveal the voxels with the most consistently lateralized activation for semantic word matching across our 82 subjects (as previously illustrated in eight epileptic children [Liegeois et al., 2002]).
-
3
The same analysis as (1) repeated with global LI values included as a covariate of interest. This analysis will show where variability in individual LI values is predicted by signal changes in left and right voxels.
The anatomical location of our effects was inferred from neuroanatomical knowledge after examining the location of the effect in MNI space and reference to the Duvernoy atlas [Duvernoy, 1991] when necessary.
Second Level Clustering and Regional Dissociations
The aim here was to characterize any regional dissociation within the LM, across subjects. Our rationale was to search for regions where an increase in laterality in some voxels was associated with a decrease in laterality in other voxels.
Prior to correlating laterality measures across regions, we needed to reduce the search space. To do this, data from all voxels were summarized into 50 compact clusters using the second‐level fuzzy clustering [Seghier and Price, 2009] on all voxels of the LM showing greater left than right hemisphere activation (at P < 0.05 uncorrected) for the comparison of “semantic word matching versus perceptual letter matching.” About 9,100 voxels within the left hemisphere satisfied these criteria (see Supporting Information Fig. S2). The clustering of these voxels was based on between subject variance such that voxels were clustered together when variance across subjects was strongly correlated. We used the following parameters (c.f., [Seghier and Price, 2009]): 50 for the number of clusters, 1.5 for the degree of fuzziness, and the hyperbolic correlation as a distance metric [Golay et al., 1998]. All voxels that belong to a given cluster are then characterized by the centroid of that cluster. In other words, this data reduction procedure summarized the laterality of all voxels into 50 clusters (i.e., 50 centroids with 82 datapoints in the across subject dimension).
Once the voxel based laterality map had been reduced to 50 compact clusters, we correlated laterality at the centroids of each cluster with that in every other cluster (from 1 to 50 clusters). This resulted in a 50‐by‐50 correlation (similarity) matrix. The aim was to identify any strong negative correlations (at P < 0.001 uncorrected). The results were visualized using multidimensional scaling (MDS, for a similar procedure see [Kherif et al., 2003; Welchew et al., 2002]).
RESULTS
Voxel Based Second Level Analyses
The analysis of the original (unflipped) contrast images from 82 subjects revealed a left dominant pattern of semantic word matching activation, see Figure 1A. In the left hemisphere, this pattern included inferior and middle frontal regions, middle and superior temporal regions, precentral cortex, occipito‐temporal cortex, angular gyrus, and supplementary motor area. In the right hemisphere, activation was only significant in the cerebellum.
Figure 1.
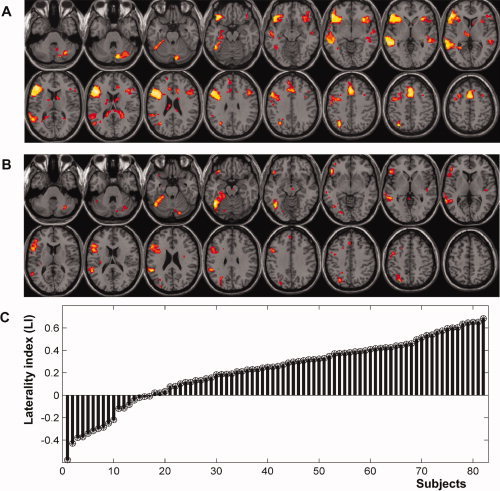
A: Main effect of semantic matching on words relative to perceptual matching on unfamiliar greek letters (random‐effect analysis over 82 subjects, at P < 0.001 uncorrected). Significant effects are shown in red‐to‐yellow color coding and projected on an individual T1‐weighted image in neurological convention. B: Consistent effect of laterality at the voxel level (random‐effect analysis on the LM maps over 82 subjects, at P < 0.001 uncorrected). C: laterality indices (LI) of our 82 subjects. For display purposes, individual LI values are sorted from weak to strong left‐lateralization. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The analysis of the voxel based LM identified the voxels with the most consistently lateralized activation for semantic word matching in our 82 subjects, see Figure 1B. This revealed the typical crossed cerebro‐cerebellar language lateralization, with consistent left lateralization in frontal and occipito‐temporal regions and right lateralization in the cerebellum. The most consistent effect in LM (i.e., the strongest interaction between task and hemisphere) was observed in the left occipito‐temporal cortex at [x = −44, y = −52, z = −16; Z = 7.5].
Calculation of the global LI across the whole hemisphere [Nagata et al., 2001] demonstrated that the majority of our subjects (67 of 82 subjects) showed lateralization to the left hemisphere (global LI >0; median LI = 0.26), as shown in Figure 1C. As expected, only handedness showed a significant effect on global LI values, as right‐handed subjects were more left‐lateralized than left‐handed subjects (t = 3.34, P = 0.001, df = 80). The effect of age on global LI values was not significant across all subjects (see Supporting Information Section 3). The continuous range of LI values, across subjects, was entered as a covariate in the one sample t‐test of the original (unflipped) images to identify voxels that were either positively (red in Fig. 2), or negatively (blue in Fig. 2) correlated with global LI. Our expectation was that differences in global LI between subjects would mainly result from variance in left hemisphere activation. Surprisingly, however, we found strong correlations between global LI and right hemisphere activation (see the extensive blue areas distributed over the 3D‐rendering of the brain in Fig. 2).
Figure 2.
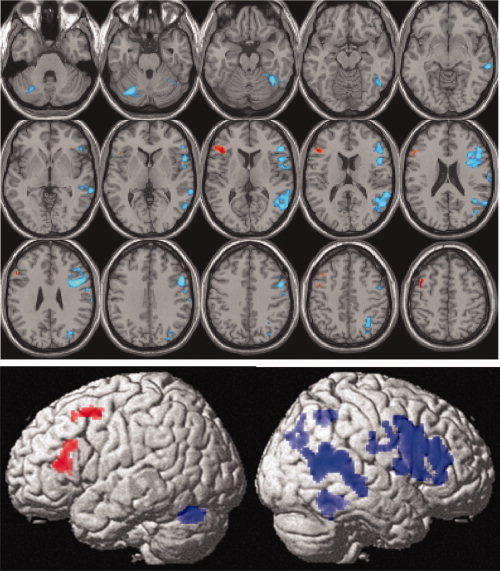
Relationship between global LI values and the parameter estimates of each voxel for the contrast “semantic word matching versus perceptual matching on unfamiliar greek letters.” Positive (red) and negative (blue) relationships are shown in neurological convention on axial slices (top) and 3D‐rendering volume (bottom). Significant effects are shown at P < 0.05 corrected (for height or size). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Second Level Clustering and Regional Dissociations in Laterality
The aim of this analysis was to characterize any regional dissociations in laterality, across subjects performing the same task. Evidence to support this investigation came from observations that voxel based LI was not closely correlated with global LI; see Supporting Information Figure S1. The 50 clusters obtained from the fuzzy clustering are visualized in Figure 3A. A strong negative correlation (r = −0.36, P < 0.001; see scatter plot of Fig. 3B) was observed between laterality in clusters centered on the angular gyrus [−48, −66, 28] and laterality in clusters centered on the ventral precentral gyrus [−58, 2, 16]. Thus, when laterality increased in the angular gyrus (left > right) it decreased in the ventral precentral gyrus. Critically, this double dissociation across subjects was not determined by handedness or any of our other variables (age, gender, stimulus set). Thus there was no evidence that these variables were positively correlated with laterality of one region and negatively corrected with laterality of the other region (or vice‐versa).
Figure 3.
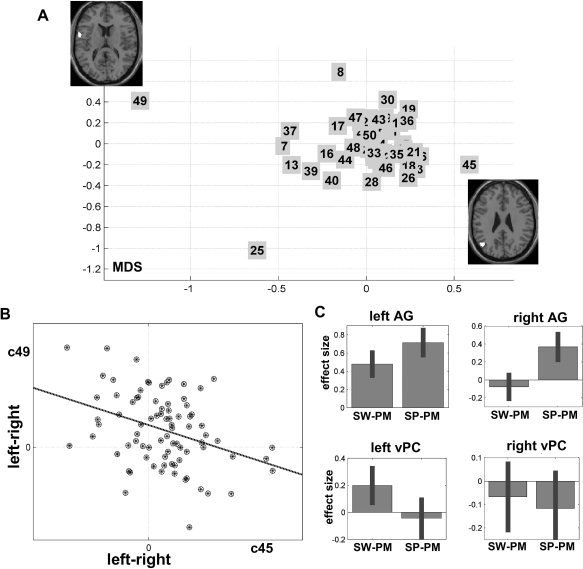
A: Multidimensional scaling projection of the distance between the 50 clusters (numbered from 1 to 50). The regions belonging to the most distant clusters (clusters 45 and 49) are displayed on axial slices. B: Scatter‐plot of the negative correlation between laterality in the angular gyrus (cluster 45) and ventral precentral gyrus (cluster 49). Each subject is shown by a circle‐dot shape. C: Effect size of left and right angular and ventral precentral gyri during semantic matching on words and pictures. This displays the mean parameter estimates over all subjects that code the differences in activation signal during semantic versus perceptual matching. SW, semantic word matching; SP, semantic picture matching; PM, perceptual matching on unfamiliar stimuli.
Post Hoc Tests
To investigate whether the opposing effects of laterality in the left angular gyrus and left ventral premotor cortex were driven by left or right hemisphere activation, we compared activation in the left and right angular gyrus and left and right ventral precentral gyrus, using data from the original (unflipped) images for the contrasts “semantic word matching versus perceptual matching” and “semantic picture matching versus perceptual matching” (Fig. 3C). The left angular gyrus was activated by semantic matching on both words and pictures, whereas left ventral precentral activation was only significant for semantic word matching (see Fig. 3C). These regions were not consistently activated in the right hemisphere during semantic word matching; but the right angular gyrus was activated by semantic picture matching.
DISCUSSION
In this article, we have highlighted two important findings that have implications for the clinical interpretation of language lateralization indices. The first finding is that, across hemisphere, global LI was significantly correlated with extensive activation across the right hemisphere (see Fig. 2). Thus, individual variation in the degree to which language is left or right lateralized at the global level was primarily driven by right hemisphere activation, and was not simply a reflection of the degree to which the left hemisphere supports language. The second finding is that healthy subjects who have relatively high left lateralized activation in the angular gyrus also have relatively low left lateralized activation in the ventral precentral gyrus. This demonstrates regional dissociations in language lateralization in a large population of healthy controls and is consistent with previous studies of single subjects or small samples of patients that have demonstrated the reversal of language lateralization in different regions, even when task and stimuli are held constant [Baciu et al., 2003; Jansen et al., 2006a; Ries et al., 2004].
The Influence of the Right Hemisphere on laterality Indices
Although many surgeons do use laterality cautiously, a recent international survey across 31 countries [Baxendale et al., 2008] has shown that the majority (86%) of centers treating patients with temporal lobe epilepsy would take into account language laterality, if this information was available. Once language laterality is known, neurosurgical resections are usually less extensive in the language dominant side than the nondominant side. Our findings highlight major limitations in the use of such global lateralization indices for the clinical assessment of language function. Specifically, the demonstration that inter‐subject variability in language lateralization is primarily driven by right rather than left hemisphere activation indicates that right lateralization does not imply that the left hemisphere has a redundant role in language function. On the contrary, our findings from a reasonably large population of subjects indicate that two patients with different global laterality values could have identical left hemisphere activation. In this case, the left hemisphere may be as essential for language processing in the patient with weaker left hemisphere language lateralization as a patient with strong left hemisphere language lateralization. Conversely, two patients with identical global LI values may differ in their dependency on left hemisphere activation. This is consistent with prior claims that superior linguistic capability in the left hemisphere does not imply that the right hemisphere is irrelevant for language (for a review see [Lindell, 2006]).
Understanding the relative contributions of the left and right hemisphere in language processing is also important for understanding the different intra‐ and inter‐hemispheric mechanisms of recovery after brain insult [Cousin et al., 2008; Lazar et al., 2000; Rosenberger et al., 2009; Seghier et al., 2001], particularly the interpretation of laterality indices after brain damage (e.g., [Knecht et al., 2002]). For example, given the normal inter‐subject variability that we observed in the right hemisphere, it is not surprising that patients also show wide variability in right hemisphere language activation following damage to the dominant left‐hemisphere.
Regional Dissociation in Laterality
Our second finding, that language lateralization has high spatial heterogeneity, also has clinical implications because it implies that one global laterality value is not sufficient to reflect the underlying spatial variability [Josse and Tzourio‐Mazoyer, 2004], irrespective of how laterality is assessed at the global level. The opposing effects of laterality that we observed in the angular gyrus and the ventral precentral gyrus (see Fig. 3) are particularly interesting because they indicate that, when healthy subjects have increased left lateralization in the angular gyrus they also had decreased left lateralization in the ventral precentral activation, even though the stimuli and task are held constant across subjects. This contrasts to rare observations of mixed lateralizations (e.g., [Kamada et al., 2006; Kurthen et al., 1992; Lee et al., 2008; Risse et al., 1997]) in patients who showed strong lateralization for expressive language function to one hemisphere and the opposite lateralization for receptive language ability.
The negative covariance in regional laterality that we observed across subjects may reflect the use of different task strategies that are supported by different regions (e.g., [Reinke et al., 2008; Seghier et al., 2008b; Seghier and Price, 2009; Sugiura et al., 2007]. For example, our post hoc tests indicated that left angular gyrus activation was strongly associated with amodal semantic processing (activated for semantic matching on both words and pictures) but left ventral precentral activation was more activated for words than pictures (see Fig. 3C) consistent with a role in translating orthography to phonology [Fiez et al., 2006]. Irrespective of the exact cause of this regional dissociation in laterality, damage to one region is likely to increase activation and lateralization in the other region, during the semantic word matching task. Therefore, the negative correlation we observed between lateralization in different regions of the same semantic word matching network clearly has implications for interpreting laterality changes following brain damage (e.g., [Crosson et al., 2009; Hertz‐Pannier et al., 2002; Klingman and Sussman, 1983; Saur et al., 2006; Thomas et al., 1997]).
Regional Activation Versus Laterality
Given the difficulties interpreting LI at the individual subject level (irrespective of how LI is assessed), we suggest that the contribution of many right and left hemisphere regions should be considered independently. This highlights the potential contribution of fMRI for presurgical investigations (e.g., [Petrella et al., 2006; Pouratian et al., 2002]). It also emphasizes that, if global or regional lateralization indices are assessed, the relative contributions of the left and right hemispheres need to be considered so that the contribution of the right hemisphere is not ignored.
A further advantage of using regionally specific activations, within hemisphere, for presurgical planning is that this approach circumvents the challenges associated with the computation of regionally specific LI. Specifically, the problem with assessing LI in multiple regions is that voxel based analyses of fMRI data are complex and therefore widespread heterogeneity needs to be reduced to a few manageable variables that minimize the number of regions tested [Chlebus et al., 2007; Deblaere et al., 2004; Hund‐Georgiadis et al., 2002; Seghier, 2008; Spreer et al., 2002]. In this context, Seghier [ 2008] suggested that, to avoid misinterpretation (and the generalization) of a single global LI per subject, a minimum of three regional LI values should be assessed per subject, including LI from an anterior region (frontal), a posterior region (temporal), as well as across the whole hemisphere. More recently, Guillen et al. [ 2009] parcellated the brain into 48 anatomical regions and calculated an LI for each region [Guillen et al., 2009]. They also suggested combining LI measures (e.g., one frontal and one temporal) in a 2D projection plane that displays the laterality of each subject in a more intuitive manner (see similar discussion in [Seghier et al., 2004]).
Furthermore, assessing laterality in patients presents further complications due to the impact of the damage upon structure and vasculature. Previous reports have shown that even lateralities at the regional level might be misleading when the regions of interest are at the vicinity of the damage (e.g., Ulmer et al., 2004; Wellmer et al., 2009]. The optimal procedure for computing regional LIs would obviously depend on the nature and the location of the damage (for more details see Wellmer et al., 2009]. At the voxel level, Liegeois et al. [ 2004] have recommended that high definition laterality measures should not be used in patients, as the matching between homologues structures of both hemispheres can be hindered by the spatial deformations that are caused by lesions (e.g., mass effect). In this particularly challenging context, there are some general principles that can help to optimize LM in patients if necessary, although we are arguing here that such complicated methodological procedures could be avoided if the relative contribution of each hemisphere is preserved (see below). First, improved normalization is the key for accurate LM, using for instance the unified normalization‐segmentation algorithm [Ashburner and Friston, 2005] that has previously been shown to be robust in brain‐damaged subjects [Crinion et al., 2007]. Second, using procedures that can explicitly model lesions as an additional class would minimize tissue misclassification during segmentation [Seghier et al., 2008c] and thus would improve brain normalization. Third, excluding lesioned voxels (with abnormal signal) and their homologue voxels in the other hemisphere, by explicit masking procedures, would avoid mixing voxels with abnormal/atypical signal. Fourth, at the group level, it is more judicious to limit second‐level analyses on LM to patients with comparable lesions (those showing maximum lesion overlap). Irrespective of the exact procedure, our point here is that these challenges are avoided if presurgical planning focuses on region specific activations within hemisphere at the site of the intended surgery.
Beyond Laterality in the Clinical Setting
All current methods for assessing laterality in fMRI act as reductionist tools that aim to homogenize (simplify) the complexity of fMRI maps. Despite all the well‐known limitations in assessing LI in fMRI [Chlebus et al., 2007; Jansen et al., 2006b; Seghier, 2008], there is still a growing interest in optimizing such laterality measures in clinics (e.g., [Suarez et al., 2009; Wang et al., 2009]). This over‐reliance on LI in the clinical setting is a direct consequence of the history of the development of this field that aimed to convince clinicians about the validity and reliability of fMRI. This validation era, inaugurated by two seminal papers [Binder et al., 1996; Desmond et al., 1995], was mainly concerned with demonstrating the concordance between fMRI LI values and well‐established clinical tools such as the Wada test. However, this historical approach is still motivating further investigation into how the consistency between laterality indices from fMRI and Wada can be further improved (e.g., see [Arora et al., 2009; Lee et al., 2008; Rutten and Ramsey, 2010; Suarez et al., 2009; Wellmer et al., 2008]), despite the fact that a total agreement between Wada and fMRI assessments may never be reached because they are measuring different things (see critical review in [Rutten and Ramsey, 2010]).
In the context of using fMRI for preoperative assessments, we suggest that a shift needs to be made in the way fMRI is presented and implemented in the clinical setting. The rich source of information provided by fMRI needs to be preserved rather than over‐simplified into laterality indices that can be misleading rather than helpful. This perspective is in accordance with the recent attempts to integrate fMRI maps into clinical navigation systems which assist clinicians in making optimal decisions during surgery (e.g., [Ganslandt et al., 2009; Rutten et al., 2003; Sankar and Cosgrove, 2009; Wurn et al., 2008]). As recently stressed, neurosurgeons are more comfortable with interpreting fMRI results when shown in a navigation system. This approach provides high spatial definition (e.g., [Yoo et al., 2004]) which can be especially helpful in defining the extent of the surgical exposure [Wurn et al., 2008].
A shift from fMRI based language LI to fMRI based language mapping for preoperative investigations would be beneficial for the whole field of clinical fMRI. Nevertheless, to pursue this perspective, and to address previous criticisms of the clinical interpretation of fMRI maps [Bell and Racine, 2009; Desmond and Chen, 2002; Giussani et al., 2010; Matthews et al., 2006; Robinson, 2004], further investigations are required to: (i) establish reliable ways of differentiating indispensable regions from less critical regions [Paolicchi, 2008; Rutten and Ramsey, 2010], (ii) minimize the occurrence of false negatives in the presence of abnormal neuronal or hemodynamic responses (e.g., [Jayakar et al., 2002; Westerveld et al., 1999]), and (iii) characterize variability across subjects that may confound the definition of normative functional responses [Seghier et al., 2008a]. This will necessitate standardization of fMRI protocols (tasks and analyses), and their validation in different clinical populations [Allen and Fong, 2008; Binder et al., 2008; Rutten et al., 2002].
CONCLUSION
In summary, our findings highlight the limitations of interpreting laterality indices, irrespective of whether lateralization is assessed with fMRI or the WADA test. From a nonclinical theoretical perspective, the interpretation of global LI needs to consider the relative contribution of the left and right hemispheres as well as between subject dissociations in regional lateralization. Future work is also required to further our understanding of how laterality changes: with the underlying anatomy (e.g., [Josse et al., 2008, 2009]), with genetic variables (e.g., [van Rijn et al., 2008]); and following brain damage [Crosson et al., 2009; Hertz‐Pannier et al., 2002]. Specifically, the implications of our findings need to be tested in patients with variable focal or nonfocal damage. This will allow the impact of damage on laterality to be assessed while considering the relative contribution of the ipsilesional and contralesional regions. Extensive postsurgical language testing would also allow the presurgical conclusions to be evaluated in a systematic way so that the contribution of both fMRI maps and laterality values can be better understood.
From a clinical perspective, our findings highlight the need for fMRI evaluation of language function because this will provide precision in the spatial localization of language areas. More specifically, we suggest that presurgical language assessments with fMRI should not rely solely on laterality indices but should focus on individual regions, at the site of the intended surgery, and consider the contribution of the right and left hemisphere independently.
Acknowledgements
The authors thank their three radiographers (Amanda Brennan, Janice Glensman, and David Bradbury) as well as Clare Shakeshaft, Laura Stewart, and Tom Schofield for their help with fMRI data collection, Caroline Ellis for her help with data analysis, and Hwee Ling Lee and Sue Ramsden for their valuable help setting up the fMRI database.
REFERENCES
- Abou‐Khalil B ( 2007): Methods for determination of language dominance: The Wada test and proposed noninvasive alternatives. Curr Neurol Neurosci Rep 7: 483–490. [DOI] [PubMed] [Google Scholar]
- Allen MD, Fong AK ( 2008). Clinical application of standardized cognitive assessment using fMRI. II. Verbal fluency. Behav Neurol 20: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora J, Pugh K, Westerveld M, Spencer S, Spencer DD, Todd Constable R ( 2009): Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia 50: 2225–2241. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 2005): Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- Baciu MV, Watson JM, McDermott KB, Wetzel RD, Attarian H, Moran CJ, Ojemann JG ( 2003): Functional MRI reveals an interhemispheric dissociation of frontal and temporal language regions in a patient with focal epilepsy. Epilepsy Behav 4: 776–780. [DOI] [PubMed] [Google Scholar]
- Baciu M, Juphard A, Cousin E, Bas JF ( 2005): Evaluating fMRI methods for assessing hemispheric language dominance in healthy subjects. Eur J Radiol 55: 209–218. [DOI] [PubMed] [Google Scholar]
- Baxendale S ( 2009): The Wada test. Curr Opin Neurol 22: 185–189. [DOI] [PubMed] [Google Scholar]
- Baxendale S, Thompson PJ, Duncan JS ( 2008): The role of the Wada test in the surgical treatment of temporal lobe epilepsy: An international survey. Epilepsia 49: 715–720. [DOI] [PubMed] [Google Scholar]
- Bell E, Racine E ( 2009): Enthusiasm for functional magnetic resonance imaging (FMRI) often overlooks its dependence on task selection and performance. Am J Bioeth 9: 23–25. [DOI] [PubMed] [Google Scholar]
- Benke T, Koylu B, Visani P, Karner E, Brenneis C, Bartha L, Trinka E, Trieb T, Felber S, Bauer G, Chemelli A, Willmes K ( 2006): Language lateralization in temporal lobe epilepsy: A comparison between fMRI and the Wada test. Epilepsia 47: 1308–1319. [DOI] [PubMed] [Google Scholar]
- Berl MM, Balsamo LM, Xu B, Moore EN, Weinstein SL, Conry JA, Pearl PL, Sachs BC, Grandin CB, Frattali C, Ritter FJ, Sato S, Theodore WH, Gaillard WD ( 2005): Seizure focus affects regional language networks assessed by fMRI. Neurology 65: 1604–1611. [DOI] [PubMed] [Google Scholar]
- Bethmann A, Tempelmann C, de Bleser R, Scheich H, Brechmann A ( 2007): Determining language laterality by fMRI and dichotic listening. Brain Res 1133: 145–157. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM ( 1996): Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology 46: 978–984. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS ( 2008): A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia 49: 1980–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S ( 2007): Pre‐surgical language mapping with functional magnetic resonance imaging. Neuropsychol Rev 17: 145–155. [DOI] [PubMed] [Google Scholar]
- Chlebus P, Mikl M, Brazdil M, Pazourkova M, Krupa P, Rektor I ( 2007): fMRI evaluation of hemispheric language dominance using various methods of laterality index calculation. Exp Brain Res 179: 365–374. [DOI] [PubMed] [Google Scholar]
- Cousin E, Peyrin C, Pichat C, Lamalle L, Le Bas JF, Baciu M ( 2007): Functional MRI approach for assessing hemispheric predominance of regions activated by a phonological and a semantic task. Eur J Radiol 63: 274–285. [DOI] [PubMed] [Google Scholar]
- Cousin E, Baciu M, Pichat C, Kahane P, Le Bas JF ( 2008): Functional MRI evidence for language plasticity in adult epileptic patients: Preliminary results. Neuropsychiatr Dis Treat 4: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price CJ, Friston KJ ( 2007): Spatial normalization of lesioned brains: Performance evaluation and impact on fMRI analyses. Neuroimage 37: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Moore AB, McGregor KM, Chang YL, Benjamin M, Gopinath K, Sherod ME, Wierenga CE, Peck KK, Briggs RW, Rothi LJ, White KD ( 2009): Regional changes in word‐production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain Lang 111: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblaere K, Boon PA, Vandemaele P, Tieleman A, Vonck K, Vingerhoets G, Backes W, Defreyne L, Achten E ( 2004): MRI language dominance assessment in epilepsy patients at 1.0 T: Region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology 46: 413–420. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen ASH ( 2002): Ethical issues in the clinical application of fMRI: Factors affecting the validity and interpretation of activations. Brain Cogn 50: 482–497. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Sum JM, Wagner AD, Demb JB, Shear PK, Glover GH, Gabrieli JD, Morrell MJ ( 1995): Functional MRI measurement of language lateralization in Wada‐tested patients. Brain 118 ( Part 6): 1411–1419. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM ( 1991): The Human Brain: Surface, Three Dimensional Sectional Anatomy and MRI. New York: Spring‐Verlag; 354 p. [Google Scholar]
- Fiez JA, Tranel D, Seager‐Frerichs D, Damasio H ( 2006): Specific reading and phonological processing deficits are associated with damage to the left frontal operculum. Cortex 42: 624–643. [DOI] [PubMed] [Google Scholar]
- Ganslandt O, Nimsky C, Buchfelder M, Grummich P ( 2009): fMRI in neurosurgery In: Filippi M, editor. fMRI Techniques and Protocols. Humana Press, New York: pp 737–750. [Google Scholar]
- Giussani C, Roux FE, Ojemann J, Spanzerla EP, Pirillo D, Papagno C ( 2010): Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery 66: 113–120. [DOI] [PubMed] [Google Scholar]
- Golay X, Kollias S, Stoll G, Meier D, Valavanis A, Boesiger P ( 1998): A new correlation‐based fuzzy logic clustering algorithm for fMRI. Magn Reson Med 40: 249–260. [DOI] [PubMed] [Google Scholar]
- Guillen M, Adjouadi M, You X, Barreto A, Rishe N, Gaillard WD ( 2009): Toward fMRI Group Identification based on Brain Lateralization. The IEEE 23rd International Conference on Advanced Information Networking and Applications (WAINA‐09). IEEE Computer Society; Bradford, UK. pp 1025–1030.
- Haag A, Knake S, Hamer HM, Boesebeck F, Freitag H, Schulz R, Baum P, Helmstaedter C, Wellmer J, Urbach H, Hopp P, Mayer T, Hufnagel A, Jokeit H, Lerche H, Uttner I, Meencke HJ, Meierkord H, Pauli E, Runge U, Saar J, Trinka E, Benke T, Vulliemoz S, Wiegand G, Stephani U, Wieser HG, Rating D, Werhahn K, Noachtar S, Schulze‐Bonhage A, Wagner K, Alpherts WC, Boas WE, Rosenow F ( 2008): The Wada test in Austrian, Dutch, German, and Swiss epilepsy centers from 2000 to 2005: A review of 1421 procedures. Epilepsy Behav 13: 83–89. [DOI] [PubMed] [Google Scholar]
- Hertz‐Pannier L, Chiron C, Jambaque I, Renaux‐Kieffer V, Van de Moortele P‐F, Delalande O, Fohlen M, Brunelle F, Le Bihan D ( 2002): Late plasticity for language in a child's non‐dominant hemisphere: A pre‐ and post‐surgery fMRI study. Brain 125 ( Part 2): 361–372. [DOI] [PubMed] [Google Scholar]
- Hund‐Georgiadis M, Lex U, Friederici AD, von Cramon DY ( 2002): Non‐invasive regime for language lateralization in right‐ and left‐handers by means of functional MRI and dichotic listening. Exp Brain Res 145: 166–176. [DOI] [PubMed] [Google Scholar]
- Jansen A, Deppe M, Schwindt W, Mohammadi S, Sehlmeyer C, Knecht S ( 2006a): Interhemispheric dissociation of language regions in a healthy subject. Arch Neurol 63: 1344–1346. [DOI] [PubMed] [Google Scholar]
- Jansen A, Menke R, Sommer J, Forster AF, Bruchmann S, Hempleman J, Weber B, Knecht S ( 2006b): The assessment of hemispheric lateralization in functional MRI‐Robustness and reproducibility. Neuroimage 33: 204–217. [DOI] [PubMed] [Google Scholar]
- Jayakar P, Bernal B, Santiago Medina L, Altman N ( 2002): False lateralization of language cortex on functional MRI after a cluster of focal seizures. Neurology 58: 490–492. [DOI] [PubMed] [Google Scholar]
- Josse G, Tzourio‐Mazoyer N ( 2004): Hemispheric specialization for language. Brain Res Rev 44: 1–12. [DOI] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ ( 2008): Explaining function with anatomy: Language lateralization and corpus callosum size. J Neurosci 28: 14132–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse G, Kherif F, Flandin G, Seghier ML, Price CJ ( 2009): Predicting language lateralization from gray matter. J Neurosci 29: 13516–13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada K, Takeuchi F, Kuriki S, Todo T, Morita A, Sawamura Y ( 2006): Dissociated expressive and receptive language functions on magnetoencephalography, functional magnetic resonance imaging, and amobarbital studies: Case report and review of the literature. J Neurosurg 104: 598–607. [DOI] [PubMed] [Google Scholar]
- Kherif F, Poline JP, Mériaux S, Benali H, Flandin G, Brett M ( 2003): Group analysis in functional neuroimaging: Selecting subjects using similarity measures. Neuroimage 20: 2197–2208. [DOI] [PubMed] [Google Scholar]
- Klingman KC, Sussman HM ( 1983): Hemisphericity in aphasic language recovery. J Speech Hear Res 26: 249–256. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H ( 2000): Language lateralization in healthy right‐handers. Brain 123 ( Part 1): 74–81. [DOI] [PubMed] [Google Scholar]
- Knecht S, Floel A, Drager B, Breitenstein C, Sommer J, Henningsen H, Ringelstein EB, Pascual‐Leone A ( 2002): Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci 5: 695–699. [DOI] [PubMed] [Google Scholar]
- Kurthen M, Helmstaedter C, Linke DB, Solymosi L, Elger CE, Schramm J ( 1992): Interhemispheric dissociation of expressive and receptive language functions in patients with complex‐partial seizures: An amobarbital study. Brain Lang 43: 694–712. [DOI] [PubMed] [Google Scholar]
- Lazar RM, Marshall RS, Pile‐Spellman J, Duong HC, Mohr JP, Young WL, Solomon RL, Perera GM, DeLaPaz RL ( 2000): Interhemispheric transfer of language in patients with left frontal cerebral arteriovenous malformation. Neuropsychologia 38: 1325–1332. [DOI] [PubMed] [Google Scholar]
- Lee D, Swanson SJ, Sabsevitz DS, Hammeke TA, Scott Winstanley F, Possing ET, Binder JR ( 2008): Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav 13: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha‐Khadem F, Baldeweg T ( 2002): A direct test for lateralization of language activation using fMRI: Comparison with invasive assessments in children with epilepsy. Neuroimage 17: 1861–1867. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha‐Khadem F, Baldeweg T ( 2004): Language reorganization in children with early‐onset lesions of the left hemisphere: An fMRI study. Brain 127 ( Part 6): 1229–1236. [DOI] [PubMed] [Google Scholar]
- Lindell AK ( 2006): In your right mind: Right hemisphere contributions to language processing and production. Neuropsychol Rev 16: 131–148. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Morris HH, Moddel G ( 2008): Complications during the Wada test. Epilepsy Behav 13: 551–553. [DOI] [PubMed] [Google Scholar]
- Matthews PM, Honey GD, Bullmore ET ( 2006): Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci 7: 732–744. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW ( 1999): The Wada test: Controversies, concerns, and insights. Neurology 52: 1535–1536. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Henson RN, Price CJ, Friston KJ ( 2003): Comparing event‐related and epoch analysis in blocked design fMRI. Neuroimage 18: 806–810. [DOI] [PubMed] [Google Scholar]
- Medina LS, Bernal B, Ruiz J ( 2007): Role of functional MR in determining language dominance in epilepsy and nonepilepsy populations: A Bayesian analysis. Radiology 242: 94–100. [DOI] [PubMed] [Google Scholar]
- Nagata S, Uchimura K, Hirakawa W, Kuratsu J ( 2001): Method for quantitatively evaluating the lateralization of linguistic function using functional MR imaging. Am J Neuroradiol 22: 985–991. [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Paolicchi JM ( 2008): Is the Wada test still relevant? Yes. Arch Neurol 65: 838–840. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Shah LM, Harris KM, Friedman AH, George TM, Sampson JH, Pekala JS, Voyvodic JT ( 2006): Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology 240: 793–802. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW ( 2002): Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg 97: 21–32. [DOI] [PubMed] [Google Scholar]
- Reinke K, Fernandes M, Schwindt G, O'Craven K, Grady CL ( 2008): Functional specificity of the visual word form area: General activation for words and symbols but specific network activation for words. Brain Lang 104: 180–189. [DOI] [PubMed] [Google Scholar]
- Ries ML, Boop FA, Griebel ML, Zou P, Phillips NS, Johnson SC, Williams JP, Helton KJ, Ogg RJ ( 2004): Functional MRI and Wada determination of language lateralization: A case of crossed dominance. Epilepsia 45: 85–89. [DOI] [PubMed] [Google Scholar]
- Risse GL, Gates JR, FRangman MC ( 1997): A reconsideration of bilateral language representation based on the intracarotid amobarbital procedure. Brain Cogn 33: 118–132. [DOI] [PubMed] [Google Scholar]
- Robinson R ( 2004): FMRI beyond the clinic: Will it ever be ready for prime time? PLoS Biol 2: e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger LR, Zeck J, Berl MM, Moore EN, Ritzl EK, Shamim S, Weinstein SL, Conry JA, Pearl PL, Sato S, Vezina LG, Theodore WH, Gaillard WD ( 2009): Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology 72: 1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF ( 2010): The role of functional magnetic resonance imaging in brain surgery. Neurosurg Focus 28: E4. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF, van Rijen PC, Noordmans HJ, van Veelen CW ( 2002): Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas. Ann Neurol 51: 350–360. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey N, Noordmans HJ, Willems P, van Rijen P, Berkelbach van der Sprenkel JW, Viergever M, van Veelen C ( 2003): Toward functional neuronavigation: Implementation of functional magnetic resonance imaging data in a surgical guidance system for intraoperative identification of motor and language cortices. Technical note and illustrative case. Neurosurg Focus 15: E6. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha‐Khadem F, Gadian DG, Friston KJ ( 2000): Detecting bilateral abnormalities with voxel‐based morphometry. Hum Brain Mapp 11: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar T, Cosgrove GR ( 2009): Functional MRI in image guided neurosurgery In: Lozano AM, Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. Springer, Berlin: pp 287–298. [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C ( 2006): Dynamics of language reorganization after stroke. Brain 129: 1371–1384. [DOI] [PubMed] [Google Scholar]
- Seghier ML ( 2008): Laterality index in functional MRI: Methodological issues. Magn Res Imaging 26: 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Price CJ ( 2009): Dissociating functional brain networks by decoding the between‐subject variability. Neuroimage 45: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M, Lazeyras F, Momjian S, Annoni J‐M, de Tribolet N, Khateb A ( 2001): Language representation in a patient with a dominant right hemisphere: fMRI evidence for an intrahemispheric reorganisation. NeuroReport 12: 2785–2790. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Zimine I, Mayer E, Michel CM, Khateb A ( 2004): Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp 23: 140–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Khateb A ( 2008a) Group analysis and the subject factor in functional magnetic resonance imaging: Analysis of fifty right‐handed healthy subjects in a semantic language task. Hum Brain Mapp 29: 461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ ( 2008b) Inter‐subject variability in the use of two different neuronal networks for reading aloud familiar words. Neuroimage 42: 1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Ramlackhansingh A, Crinion J, Leff A, Price CJ ( 2008c) Lesion identification using unified segmentation‐normalisation models and fuzzy clustering. Neuroimage 41: 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreer J, Arnold S, Quiske A, Wohlfarth R, Ziyeh S, Altenmuller D, Herpers M, Kassubek J, Klisch J, Steinhoff BJ, Honegger J, Schulze‐Bonhage A, Schumacher M ( 2002): Determination of hemisphere dominance for language: Comparison of frontal and temporal fMRI activation with intracarotid amytal testing. Neuroradiology 44: 467–474. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM ( 1999): Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 122 ( Part 11): 2033–2046. [DOI] [PubMed] [Google Scholar]
- Stippich C, Blatow M, Krakow K ( 2007): Presurgical functional MRI in patients with brain tumors In: Stippich C, editor. Clinical Functional MRI: Presurgical Functional Neuroimaging. Berlin, Heidelberg: Springer; pp 87–134. [Google Scholar]
- Suarez RO, Whalen S, Nelson AP, Tie Y, Meadows ME, Radmanesh A, Golby AJ ( 2009): Threshold‐independent functional MRI determination of language dominance: A validation study against clinical gold standards. Epilepsy Behav 16: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Friston KJ, Willmes K, Shah NJ, Zilles K, Fink GR ( 2007): Analysis of intersubject variability in activation: An application to the incidental episodic retrieval during recognition test. Hum Brain Mapp 28: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharin S, Golby A ( 2007): Functional brain mapping and its applications to neurosurgery. Neurosurgery 60( 4 Suppl 2): 185–201. [DOI] [PubMed] [Google Scholar]
- Thomas C, Altenmuller E, Marckmann G, Kahrs J, Dichgans J ( 1997): Language processing in aphasia: Changes in lateralization patterns during recovrey reflect cerebral plasticity in adults. Eletroencephalogr Clin Neurophysiol 102: 86–97. [DOI] [PubMed] [Google Scholar]
- Ulmer JL, Hacein‐Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, Meyer GA, Krouwer HG, Schmainda KM ( 2004): Lesion‐induced pseudo‐dominance at functional magnetic resonance imaging: Implications for preoperative assessments. Neurosurgery 55: 569–579. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Vink M, Sommer I, Kahn RS ( 2008): Effects of an extra X chromosome on language lateralization: An fMRI study with Klinefelter men (47,XXY). Schizophr Res 101: 17–25. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ ( 1996): Functional anatomy of a common semantic system for words and pictures. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Wada J ( 1949): A new method for the determination of the side of cerebral speech dominance. A preliminary report on the intra‐carotid injection of sodium amytal in man [in Japanese]. Igaku Seibutsugaki 14: 221–222. [Google Scholar]
- Wang Z, Mechanic‐Hamilton D, Pluta J, Glynn S, Detre JA ( 2009): Function lateralization via measuring coherence laterality. Neuroimage 47: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchew DE, Honey GD, Sharma T, Robbins TW, Bullmore ET ( 2002): Multidimensional scaling of integrated neurocognitive function and schizophrenia as a disconnexion disorder. Neuroimage 17: 1227–1239. [DOI] [PubMed] [Google Scholar]
- Wellmer J, Weber B, Weis S, Klaver P, Urbach H, Reul J, Fernandez G, Elger CE ( 2008): Strongly lateralized activation in language fMRI of atypical dominant patients—Implications for presurgical work‐up. Epilepsy Res 80: 67–76. [DOI] [PubMed] [Google Scholar]
- Wellmer J, Weber B, Urbach H, Reul J, Fernandez G, Elger CE ( 2009): Cerebral lesions can impair fMRI‐based language lateralization. Epilepsia 50: 2213–2224. [DOI] [PubMed] [Google Scholar]
- Westerveld M, Stoddard KR, Spencer DD, McCarthy K, Schlosser M, Constable T ( 1999). Case report of false lateralization using fMRI: Comparison of fMRI language localization, Wada testing, and cortical stimulation. Arch Clin Neuropsychol 14: 162–163. [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A ( 2003): Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 61: 699–701. [DOI] [PubMed] [Google Scholar]
- Wurn G, Schnizer M, Fellner C ( 2008): The impact of fMRI on multimodal navigation in surgery of cerebral lesions: four years clinical experience. Int J Comput Assist Radiol Surgery 3: 191–199. [Google Scholar]
- Yoo SS, Talos IF, Golby AJ, Black PM, Panych LP ( 2004): Evaluating requirements for spatial resolution of fMRI for neurosurgical planning. Hum Brain Mapp 21: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


