Abstract
The insulin/insulin-like growth factor-like signaling (IIS) pathway in metazoans has evolutionarily conserved roles in growth control, metabolic homeostasis, stress responses, reproduction, and lifespan. Genetic manipulations that reduce IIS in the nematode worm Caenorhabditis elegans, the fruit fly Drosophila melanogaster, and the mouse have been shown not only to produce substantial increases in lifespan but also to ameliorate several age-related diseases. In C. elegans, the multitude of phenotypes produced by the reduction in IIS are all suppressed in the absence of the worm FOXO transcription factor, DAF-16, suggesting that they are all under common regulation. It is not yet clear in other animal models whether the activity of FOXOs mediate all of the physiological effects of reduced IIS, especially increased lifespan. We have addressed this issue by examining the effects of reduced IIS in the absence of dFOXO in Drosophila, using a newly generated null allele of dfoxo. We found that the removal of dFOXO almost completely blocks IIS-dependent lifespan extension. However, unlike in C. elegans, removal of dFOXO does not suppress the body size, fecundity, or oxidative stress resistance phenotypes of IIS-compromised flies. In contrast, IIS-dependent xenobiotic resistance is fully dependent on dFOXO activity. Our results therefore suggest that there is evolutionary divergence in the downstream mechanisms that mediate the effects of IIS. They also imply that in Drosophila, additional factors act alongside dFOXO to produce IIS-dependent responses in body size, fecundity, and oxidative stress resistance and that these phenotypes are not causal in IIS-mediated extension of lifespan.
Keywords: Drosophila, aging, FOXO, insulin signaling
Introduction
The insulin/insulin-like growth factor (IGF)-like signaling (IIS) pathway of metazoans regulates such diverse processes as growth, developmental timing, body size, metabolism, stress responses, reproduction, and lifespan (Kenyon, 2005; Giannakou & Partridge, 2007). Genetic manipulations that inhibit IIS in the nematode worm Caenorhabditis elegans, the fruit fly Drosophila melanogaster, and the mouse not only increase lifespan but also delay the onset of age-related pathology and disease (Tatar et al., 2003; Kenyon, 2005; Bonkowski et al., 2006; Cohen et al., 2006; Wessells & Bodmer, 2007; Selman et al., 2008; Wessells et al., 2009). Direct downstream targets of IIS in worms, flies, and mammals are the FOXO (Forkhead bOX-containing protein, subfamily O) proteins, a highly conserved family of transcription factors. Phosphorylation of FOXOs by the insulin-activated protein kinases PKB/AKT and SGK leads to their sequestration within the cytoplasm and, as a result, transcriptional inactivation of target gene expression (van der Horst & Burgering, 2007; Partridge & Bruning, 2008). Several direct FOXO target genes have been identified that function during cell cycle control, metabolism, apoptosis, and the regulation of cellular stress responses (Greer & Brunet, 2005, 2008; Partridge & Bruning, 2008; Salih & Brunet, 2008). Hence, the activation of FOXOs and their target genes has been under intense study to identify the transcriptional changes associated with IIS-dependent lifespan extension.
Lifespan extensions induced by decreasing the activity of the insulin/IGF1-like receptor, DAF-2, or downstream components of the IIS pathway in C. elegans are completely dependent upon the activity of the worm FOXO transcription factor, DAF-16 (Kenyon et al., 1993). Thus, mutation of daf-16 or reductions in its expression by RNAi can completely abrogate the lifespan extension observed in mutants for daf-2, the worm insulin/IGF receptor orthologue, or age-1, the worm phosphatidylinositol 3-kinase orthologue (Kenyon et al., 1993). In other model organisms, FOXOs clearly play important roles during lifespan determination: overexpression of dFOXO protein in the adult fat body increases lifespan in Drosophila (Tatar et al., 2003; Giannakou et al., 2004; Hwangbo et al., 2004), while heterozygous knockouts for the insulin receptor substrates, IRS1 or IRS2, are long lived and show increased activity of FOXO1 target genes in murine models (Taguchi et al., 2007; Selman et al., 2008). Furthermore, genetic variation in the Foxo3A gene is associated with longevity in several different human populations (Kuningas et al., 2007; Willcox et al., 2008; Flachsbart et al., 2009). However, it has yet to be shown in these other animal models whether the effects of reduced IIS on lifespan are directly dependent on FOXO activity.
In addition to lifespan extension, decreasing IIS in the worm produces a number of other phenotypic responses that are all dependent upon DAF-16, suggesting that may all be regulated by a common mechanism. For example, daf-2-dependent reproductive delay and oxidative stress resistance are completely suppressed by knockdown of daf-16 expression by RNAi (Larsen, 1993; Honda & Honda, 1999; Dillin et al., 2002). Also, DAF-16 mediates both stress resistance and reduced adult fecundity in age-1 mutants (Larsen, 1993; Tissenbaum & Ruvkun, 1998; Honda & Honda, 1999). dFOXO-dependent effects on IIS-mediated growth control and germline stem cell (GSC) proliferation have been reported in Drosophila (Junger et al., 2003; Puig et al., 2003; Hsu et al., 2008). Nevertheless, in other animals, it remains unclear whether FOXOs mediate all of the phenotypic effects of reduced IIS and thus whether this feature of the signaling pathway has been conserved through evolution.
In this study, we have examined whether several IIS-dependent phenotypes, including increased lifespan, reduced fecundity, increased stress resistance, developmental delay, and growth inhibition, are dependent on dFOXO activity in Drosophila. We combined a newly generated null allele of dfoxo with several models of reduced IIS in Drosophila including ubiquitous expression of a kinase-dead, dominant negative version of the Drosophila insulin receptor both during development and specifically restricted to adulthood, late ablation of the dilp-producing median neurosecretary cells (MNCs) and adult-specific ubiquitous expression of a dominant negative form of PI3 kinase, all of which show phenotypes typical of reduced IIS. We found that mutation of dfoxo almost completely blocked lifespan extension in these IIS-compromised flies. However, removal of dfoxo failed to rescue IIS-mediated developmental delay, small body size, reduced egg laying, and resistance to paraquat. In contrast, increased resistance to the xenobiotic toxin, dichlorodiphenyltrichloroethane (DDT), was completely dependent on dFOXO activity. Our results show that, unlike in C. elegans, where all phenotypic traits produced by reduced IIS are DAF-16 dependent, additional factors besides dFOXO have evolved in Drosophila to mediate the full IIS response.
Results
Generation and characterization of a new dfoxo null allele
Several loss-of-function mutants for the Drosophila dfoxo transcription factor have already been described. These include dfoxo21 and dfoxo25, both of which contain chemically induced nucleotide transversions within the dfoxo coding region, resulting in premature stop codons within the proposed DNA-binding domain of the protein (Junger et al., 2003) and dfoxoW24, which contains a P-element insertion within the first intron of the dfoxo locus (Weber et al., 2005; Fig. 1A). Heteroallelic combinations of these mutants produce viable adults but no detectable protein by western blot analysis and are therefore considered to function as genetic nulls (Junger et al., 2003; Giannakou et al., 2008; Min et al., 2008). We have performed chromatin immunoprecipitation (ChIP) experiments on chromatin extracts prepared from dfoxo21/dfoxo25 transheterozygous flies using a specific dFOXO antibody, the epitope for which would still be present within any translated mutant protein (Fig. 1A). dFOXO DNA binding was assessed using a promoter region of the Drosophila SH2B-encoding gene, Lnk, which we have previously demonstrated to be bound by dFOXO (Slack et al., 2010). Surprisingly, quantitative PCR (qPCR) after dFOXO ChIP from dfoxo21/dfoxo25 samples showed enrichment of the Lnk promoter fragment relative to a control genomic region, similar to wild-type controls (Fig. 1B). Thus, despite the apparent absence of dFOXO protein in dfoxo21/dfoxo25 mutants (Fig. 1C), there still appears to be residual DNA-binding activity in these flies. This allelic combination may therefore function more as a dominant negative rather than as a true null. Interestingly, dominant effects of the dfoxo21 allele have been observed in other studies (Nielsen et al., 2008).
Fig. 1.
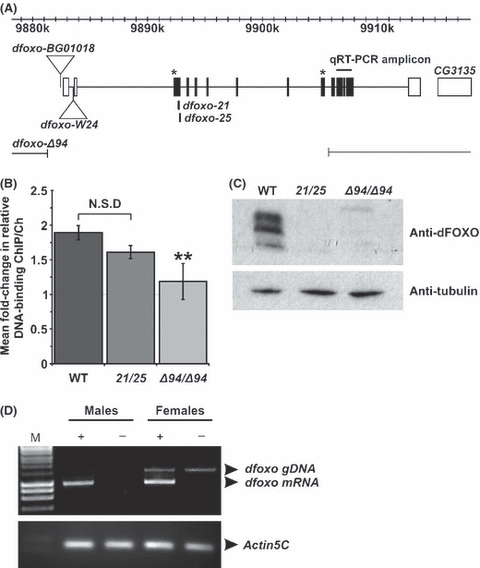
Molecular characterization of the dfoxoΔ94 deletion. (A) Schematic representation of the dfoxo locus. Coding exons are in black and noncoding exons are in white. The position of the P-element insertion (dfoxoBG01018) used to generate the dfoxoΔ94 deletion by imprecise excision and the breakpoints of the dfoxoΔ94 deletion are shown along with the positions of the dfoxo21, dfoxo25, and dfoxow24 mutations. The position of the amplicon amplified by RT-PCR to detect dfoxo mRNA expression is also indicated along with the position of the sequences encoding the peptides from which the dFOXO antibody was generated (marked by *). (B) Quantitative PCR (qPCR) on the Lnk promoter normalized to a control genomic region located 3′ to the Lnk gene to determine the proportion of DNA recovered after chromatin immunoprecipitation (ChIP) using a specific anti-dFOXO antibody from chromatin extracts prepared from wild-type, dfoxo21/dfoxo25 and dfoxoΔ94 homozygous mutant flies. Relative DNA binding was calculated as the proportion of chromatin recovered in the ChIP divided by that in the total chromatin preparation. Data are presented as mean fold change in DNA binding at the Lnk 5′ region compared with the control Lnk 3′ region ± SEM of two (for dfoxo21/dfoxo25) or three biological repeats. (**P <0.05, t-test). (C) Western blot analysis of dFOXO protein expression in extracts prepared from wild-type, dfoxo21/dfoxo25 (21/25) heterozygotes and dfoxoΔ94 (Δ94/Δ94) homozygous mutant flies. Blots were probed with anti-tubulin as a control for protein loading. (D) RT-PCR analysis of dfoxo transcript expression in 7-day-old male and female wild-type (+) and dfoxoΔ94 homozygous mutant (−) flies. M indicates the marker lane. Amplification of the actin5C transcript was used as a control for the RT-PCR protocol.
We therefore generated a new deletion mutant of dfoxo by imprecise excision of a P-element positioned upstream of the first noncoding exon of the dfoxo gene. This deletion (dfoxoΔ94) spans over 20 kb of the dfoxo locus, removing part of the predicted promoter region as well as several coding exons. Homozygotes for the deletion were adult viable, and neither dFOXO protein expression nor DNA-binding activity was detected in these flies (Fig. 1B,C). However, the deletion removes the sequence encoding the epitope site for the dFOXO antibody, and so we could not exclude the possibility that some mutant protein is produced. We therefore examined the expression of dfoxo mRNA by RT-PCR using primers that anneal outside of the deleted region and found that homozygous mutants were completely devoid of dfoxo transcript expression (Fig. 1D). Consequently, this deletion appears to represent a true null allele of dfoxo.
dfoxoΔ94 homozygotes were delayed in egg–adult development time and were also smaller in size than their controls, with significant reductions in both body weight and wing area (Fig. 2A,B). No obvious effects on developmental time or body weight have been previously reported for other dfoxo mutants, with only a small decrease in wing size described for dfoxo21/dfoxo25 transheterozygotes (Junger et al., 2003). Nevertheless, delayed egg-adult development and reduced body size were also observed in transheterozygous dfoxo25/dfoxoΔ94 flies as well as in hemizygous Df(3R)ED5624/dfoxoΔ94 flies using a deficiency that removes the entire dfoxo locus (Supplementary Fig. S1), confirming that the observed effects on developmental time and body size in dfoxoΔ94 homozygotes are specific to the dfoxoΔ94 genetic lesion.
Fig. 2.

Phenotypic analysis of dfoxoΔ94 homozygous mutant flies. (A) Egg-to-adult development time is delayed in both males and females homozygous for the dfoxoΔ94 deletion (dfoxo−) compared with wDahomey controls. Only the eclosion period of the adult flies is shown. Data are shown as percentage of flies eclosing. (For males, n = 114 for wDahomey and n = 106 for dfoxo−. For females, n = 107 for wDahomey and n = 116 for dfoxo−). (B) Homozygous dfoxoΔ94 flies (dfoxo−) have significantly reduced adult body weights and smaller wing sizes than wDahomey controls. Data are represented as means ± SEM (n = 10 for each measurement, **P <0.05, anova). (C) Clonal analysis of the effects of dfoxoΔ94 mutation on cell size and proliferation in the developing wing disk. dfoxoΔ94 mutant cells were generated by mitotic recombination and are marked by the absence of GFP. No obvious differences were observed in clone size nor in the size of in dfoxoΔ94 mutant cells within the clone (GFP-negative cells) compared with adjacent heterozygous cells (GFP-positive cells) outside of the clone. (D) Survival curves of female flies homozygous mutant for dfoxo− mutants (n = 99, median survival = 41 days, maximum survival = 48 days) and wDahomey controls (n = 94, median survival = 61 days, maximum survival = 73 days). dfoxo− mutants are significantly shorter lived than controls (P <0.0001; Log-rank test). Representative of two independent experiments. (E) Average number of eggs laid per dfoxo− mutant 7-day-old female compared with age-matched wDahomey controls. Data are presented as the mean number of eggs laid per female over a 24-h period ± SEM. Eggs were counted from ten separate vials, and each vial contained ten females. dfoxo− females laid significantly fewer eggs than wDahomey controls. (**P <0.05, anova).
The small body size of dfoxoΔ94 homozygotes suggested that dFOXO activity could potentially regulate cell growth or proliferation. To examine this, we generated clones of dfoxoΔ94 mutant cells in an otherwise heterozygous animal by mitotic recombination. Both clone size and dfoxoΔ94 mutant cell size were normal (Fig. 2C), as has been observed with other dfoxo alleles (Junger et al., 2003), suggesting that dFOXO does not act cell-autonomously to restrict cell proliferation or growth. Hence, the effects of the dfoxoΔ94 mutation on growth must occur via nonautonomous mechanisms. Similar to other dfoxo allelic combinations, homozygous dfoxoΔ94 females were shorter lived than their controls (Fig. 2B) and also laid fewer eggs (Fig. 2C).
The dfoxoΔ94 deletion was then combined with several mutations or genetic manipulations that reduce IIS in Drosophila including a dilp2-3,5 triple mutant (Gronke et al., 2010), median neurosecretary cell (mNSC) ablation (Broughton et al., 2005), chico1 mutants (Bohni et al., 1999), and LnkDel29 mutants (Slack et al., 2010). All resulted in preadult lethality when combined with homozygosity for dfoxoΔ94. Interestingly, the lethality of chico1; dfoxoΔ94 homozygotes was rescued by the expression of a UAS-dfoxo transgene within the MNCs, suggesting that a dFOXO-dependent transcriptional response specifically within the MNCs is both necessary and sufficient for the viability of chico1 homozygotes.
Viable flies were obtained when the dfoxoΔ94 mutant was combined with either overexpression of a dominant negative form of the Drosophila insulin receptor (UAS-InRDN) under the control of the ubiquitous and constitutive daughterless-GAL4 driver (daGAL) or late ablation of the mNSCs by the expression of UAS-reaper (UAS-rpr) under the control of the InsP3-GAL4 driver (InsP3GAL). In addition, we examined adult-onset ubiquitous expression of UAS-InRDN as well as adult-onset ubiquitous expression of a catalytically inactive, dominant negative form of PI3 kinase (UAS-Dp110DN) using the inducible daughterless-GeneSwitch (daGS) driver. daGS only drives transgene expression in the presence of the RU486 steroid drug. Treatment with RU486 had no effect on the lifespan, fecundity, or stress resistance of daGS/+ flies themselves (Supplementary Fig. S2).
IIS-mediated longevity requires dFOXO activity
In the presence of dfoxo, daGAL > UAS-InRDN females lived significantly longer than controls, with a 10–15% increase in median lifespan and 6–10% increase in maximum lifespan (Fig. 3A). In a dfoxo− background, all groups were shorter lived compared with their wild-type counterparts, yet daGAL > UAS-InRDN dfoxo− flies showed an age-related increase in survival compared with both genetic controls (daGAL/+ dfoxo− and UAS-InRDN/+ dfoxo−) (Fig. 3A). When all flies were considered in the analysis, daGAL > UAS-InRDN dfoxo− flies were significantly longer lived than both genetic controls (P <0.001; log-rank test). However, no significant differences in survival were apparent between groups during the first 50 days of the experiment (P = 0.55; log-rank test), whereas survival at ages beyond 50 days was increased in daGAL > UAS-InRDN dfoxo− flies compared with both controls (P <0.0001; log-rank test) (Fig. 3A). Hence, maximum lifespan, calculated as the median age of the oldest 10% of the population to die, was significantly increased by approximately 8% (P <0.0001; log-rank) for daGAL > UAS-InRDN dfoxo− flies (66 days, n = 14) relative to both genetic controls (54 days, n = 11 for daGAL/+ dfoxo− flies and 59 days, n = 16 for UAS-InR-DN dfoxo− flies). This apparent age-related difference in survival was observed in a second, independent experiment, suggesting that ubiquitous and constitutive expression of UAS-InRDN can increase survival later in life and hence extend maximum lifespan even in the absence of dFOXO activity.
Fig. 3.

Effects of dfoxo removal on the survivorship of IIS-compromised flies. (A) Survival curves of female flies overexpressing a dominant negative version of the insulin receptor (daGAL > UAS-InRDN) and their genetic controls (daGAL/+ and UAS-InRDN/+) in both wild-type and dfoxo− backgrounds (representative of two independent experiments). In a wild-type background: for daGAL > UAS-InRDN median survival = 71 days, maximum survival = 82 days, n = 93; for daGAL/+ median survival = 61 days, maximum survival = 77 days, n = 114; for UAS-InRDN/+ median survival = 64 days, maximum survival = 75, n = 115. The survival of daGAL > UAS-InRDN flies was significantly different from each of the controls (P <0.0001; log-rank test). No significant difference in survival was detected between the two controls (P = 0.6, log-rank test). In a dfoxo− background: for daGAL > UAS-InRDN median survival = 53 days, maximum survival = 64 days, n = 114; for daGAL/+ median survival = 49 days, maximum survival = 59 days, n = 109; for UAS-InRDN/+ median survival = 49 days, maximum survival = 57 days, n = 106. The survival of daGAL > UAS-InRDN dfoxo− flies is significantly different from each of the controls (P <0.001; log-rank test). No significant difference in survival was detected between the two controls (P = 0.5, log-rank test). (B) Survival curves of female flies with late ablation of the median neurosecretary cells (InsP3GAL > UAS-rpr) and their genetic controls (InsP3GAL/+ and UAS-rpr/+) in both wild-type and dfoxo− backgrounds. In a wild-type background: for InsP3GAL > UAS-rpr median survival = 82 days, maximum survival = 100 days, n = 99; for InsP3/+ median survival = 60 days, maximum survival = 82 days, n = 94; for UAS-rpr/+ median survival = 62 days, maximum survival = 82 days, n = 93. The survival of InsP3GAL > UAS-rpr flies was significantly different from each of the controls (P <10−12, log-rank test). No significant difference in survival was detected between the two controls (P = 0.8, log-rank test). In a dfoxo− background: for InsP3GAL > UAS-rpr median survival = 44 days, maximum survival = 58 days, n = 94; for InsP3/+ median survival = 41 days, maximum survival = 56 days, n = 108; for UAS-rpr/+ median survival = 44 days, maximum survival = 59 days, n = 95. The survival of InsP3GAL > UAS-rpr flies was not significantly different from either of the controls (P>0.2, log-rank test). (C) Survival curves of female daGS > UAS-InRDN flies induced to ubiquitously express the dominant negative insulin receptor by feeding RU486-containing food from day 3 of adulthood (wild-type +RU486: median survival = 83 days, maximum survival = 95 days, n = 94; dfoxo− +RU486: median survival = 46 days, maximum survival = 70 days, n = 81) compared with uninduced controls (wild-type −RU486: median survival = 67 days, maximum survival = 90 days, n = 97; dfoxo−−RU486: median survival = 44 days, maximum survival = 65 days, n = 79). The survival of wild-type daGS > UAS-InRDN +RU486 was significantly different from the −RU486 control (P <10−6, log-rank test). The survival of dfoxo− daGS > UAS-InRDN +RU486 was not significantly different from the −RU486 control (P = 0.2, log-rank test). (D) Survival curves of female daGS > UAS-dp110DN flies induced to ubiquitously overexpress a dominant negative form of Dp110 by feeding RU486-containing food from day 3 of adulthood (wild-type +RU486: median survival = 80 days, maximum survival = 97 days, n = 91; dfoxo− +RU486: median survival = 51 days, maximum survival = 65 days, n = 95) compared with uninduced controls (wild-type −RU486: median survival = 75 days, maximum survival = 92 days, n = 103; dfoxo−−RU486: median survival = 54 days, maximum survival = 65 days, n = 97). The survival of wild-type daGS > UAS-dp110DN +RU486 was significantly different from the −RU486 control (P<0.001, log-rank test). The survival of dfoxo− daGS > UAS-dp110DN +RU486 was not significantly different from the −RU486 control (P = 0.4, log-rank test). Representative of two independent experiments.
InsP3GAL > UAS-rpr females also lived significantly longer than both genetic controls (InsP3GAL/+ and UAS-rpr/+) in the presence of dfoxo with approximately 30% increase in median and approximately 20% in maximum lifespan (Fig. 3B). Again, all groups were shorter lived in a dfoxo− background, but in contrast to daGAL > UAS-InRDN flies, InsP3GAL > UAS-rpr were not significantly longer lived than their genetic controls in a dfoxo− background (Fig. 3B).
In both daGS > UAS-InRDN and daGS > UAS-Dp110DN flies, induction of transgene expression by RU486 increased lifespan in both genotypes, with median lifespan increased by 24% and 7%, respectively, and maximum lifespan increased by 5% for both genotypes compared with uninduced flies of the same genotypes (Fig. 3C,D). No extension of lifespan was observed in either daGS > UAS-InRDN and daGS > UAS-Dp110DN flies in a dfoxo− background upon RU486-induced transgene expression compared with uninduced controls (Fig. 3C,D).
Taken together, these data show that loss of dFOXO activity is sufficient to almost completely inhibit the longevity of IIS mutants in Drosophila. By contrast, treatment of dfoxo− females with the TOR kinase inhibitor, rapamycin, significantly increased median lifespan by approximately 10%, as was observed in rapamycin-treated wDahomey control females (Supplementary Fig. S3). Furthermore, dfoxo− females also showed an increase in lifespan under dietary restriction (Supplementary Fig. S4 and Table S1). Thus, loss of dFOXO activity specifically abrogates the increase in lifespan from reduced IIS.
Loss of dFOXO activity fails to rescue IIS-mediated fecundity defects
IIS plays a complex role during oogenesis in Drosophila: autonomous IIS within the germline directly controls germline cyst development, vitellogenesis, and the rate of GSC divisions (LaFever & Drummond-Barbosa, 2005; Hsu et al., 2008; Hsu & Drummond-Barbosa, 2009), while IIS via dilps indirectly controls the proliferation of the follicle cells (LaFever & Drummond-Barbosa, 2005). We therefore examined the effects of dfoxo removal on egg laying in IIS-compromised females.
daGAL4 > UAS-InRDN females showed reduced egg laying compared with both daGAL/+ and UAS-InRDN/+ controls in both wild-type and dfoxo− genetic backgrounds (Fig. 4A). Similar results were observed for InsP3GAL > UAS-rpr females: egg laying was reduced compared with both InsP3GAL/+ and UAS-rpr/+ controls in both wild-type and dfoxo− backgrounds (Fig. 4B). Ubiquitous adult-specific induction of UAS-InRDN or UAS-Dp110DN expression also decreased female fecundity and, again, this decrease in female egg laying was still observed in a dfoxo− background (Fig, 4C,D). Taken together, these data show that removal of dfoxo is not sufficient to rescue the reduced fecundity of IIS-compromised females. Furthermore, in a dfoxo− mutant background, InsP3GAL > UAS-rpr females laid significantly fewer eggs than their dfoxo− genetic controls (P <0.05, t-test) and both daGS > InRDN dfoxo− and daGS > Dp110DN dfoxo− females treated with RU486 laid significantly fewer eggs than their uninduced controls (P <0.001, anova), suggesting that loss of dFOXO activity and reduced IIS in these flies acted additively to reduce egg laying.
Fig. 4.

Effects of dfoxo removal on the female fecundity. (A–D) Average number of eggs laid per 7-day-old female or after 7 days of RU486 treatment. Data are presented as the mean number of eggs laid per female over a 24-h period ± SEM. Eggs were counted from ten separate vials, and each vial contained ten females. (A) Females with constitutive and ubiquitous expression of the dominant negative insulin receptor. In both wild-type and dfoxo− backgrounds, daGAL > UAS-InRDN females laid significantly fewer eggs than both daGAL/+ and UAS-InRDN/+ controls (**P <0.05, t-test). There was no significant difference between daGAL > UAS-InRDN and daGAL > UAS-InRDN dfoxo− flies. (B) Females with late ablation of the median neurosecretary cells. In both wild-type and dfoxo− backgrounds, InsP3GAL > UAS-rpr laid significantly fewer eggs than both InsP3GAL/+ and UAS-rpr/+ controls (**P <0.05, t-test). (C) Females induced to ubiquitously overexpress the dominant negative insulin receptor by feeding RU486-containing food from day 3 of adulthood. In both wild-type and dfoxo− backgrounds, daGS > UAS-InRDN females induced with RU486 (+RU486) laid significantly fewer eggs uninduced controls (−RU486) (**P <0.05, t-test). (D) Females induced to ubiquitously overexpress dominant negative Dp110 by feeding RU486-containing food from day 3 of adulthood. In both wild-type and dfoxo− backgrounds, daGS > UAS-Dp110DN females induced with RU486 (+RU486) laid significantly fewer eggs uninduced controls (−RU486) (**P <0.05, t-test). (E) dFOXO protein expression in the germline. Western blot analysis of dFOXO protein expression in ovaries overexpressing two independent dFOXO transgenes (dfoxo-p8 and dfoxo-p13) within the germline under the control of the mataGAL4 driver. Blots were probed with anti-tubulin as a control for protein loading. Ovaries from these females overexpressing dFOXO protein in the germline look structurally wild-type, but egg production is significantly reduced. Eggs were collected from 7-day-old females over a 24-h period and counted. Data are presented as the mean number of eggs laid per female over these 24-h periods ± SEM (**P <0.05, anova). (F) Quantitative RT-PCR analysis of yolkless mRNA expression in female flies of the indicated genotypes normalized to actin5C. Data are presented as means ± SEM (n = 5). yolkless expression is upregulated in daGAL > UAS-InRDN females compared with controls (**P <0.05, t-test) in both wild-type and dfoxo− backgrounds.
Mutation of dfoxo can reverse the reduction in GSC proliferation caused by the loss of chico function, demonstrating that dFOXO is required in the GSCs for at least some of the effects of lowered IIS on reproduction (Hsu et al., 2008). To further examine the effects of dFOXO activity more specifically within the germline, we generated flies overexpressing a dFOXO transgene in the GSCs, using the maternal GAL4 driver, mata-GAL4. Despite significant overexpression of dFOXO protein in the ovaries of these females, they were still fertile with no overall gross morphological defects in ovarian structure (Fig. 4E). However, egg laying by these females was reduced by approximately 30% (Fig. 4E), demonstrating that increased dFOXO activity specifically within the germline is sufficient to reduce egg production.
In daGAL4 > UAS-InRDN females, the dominant negative insulin receptor is expressed in all somatic cells but not in the germline. Therefore, the reduction in egg laying in these females must be mediated via indirect responses of the germline to somatic signals. IIS can indirectly affect egg production through the regulation of yolk protein uptake into the oocycte during vitellogenesis (Richard et al., 2005). It is therefore possible that dFOXO transcriptional activity is required for the expression of yolk protein transcripts themselves. However, we found no significant difference in the expression of yolk protein 2 (YP2) in daGAL4 > UAS-InRDN females, in either a wild-type or dfoxo− background, indicating that YP2 expression is unresponsive to both IIS itself and dFOXO activity. In contrast, transcription of the yolk protein receptor, yolkless, whose expression is normally restricted to the oocyte, was significantly increased in daGAL4 > UAS-InRDN females compared with controls (Fig. 4F). However, this increase in yolkless expression was still present in dfoxo− mutants (Fig. 4F) and so occurs independently of dFOXO-mediated transcriptional regulation.
dFOXO is not required for IIS-mediated oxidative stress resistance
Genetic interventions that inhibit IIS often result in enhanced resistance to various stresses including oxidative stress (Clancy et al., 2001; Broughton et al., 2005). We therefore examined the effects of paraquat, an intracellular ROS generator, on the survival of daGAL4 > UAS-InRDN, InsP3GAL > UAS-rpr and daGS > Dp110DN flies. In a wild-type background, daGAL4 > UAS-InRDN, InsP3GAL > UAS-rpr and daGS > Dp110DN flies all survived for significantly longer on food supplemented with 20 mm paraquat compared with their respective controls (Fig. 5A,B,C). Interestingly, we still observed a small but significant and proportionally similar increase in the survival of daGAL4 > UAS-InRDN dfoxo−, InsP3GAL > UAS-rpr dfoxo− and daGS > Dp110DN dfoxo− flies over their respective controls (Fig. 5A,BC), showing that in the absence of dFOXO activity, reductions in IIS can still increase resistance to paraquat treatment.
Fig. 5.

Functions for dFOXO during IIS-mediated oxidative stress resistance. (A) Survival curves in response to 20 mm paraquat of female flies overexpressing a dominant negative version of the insulin receptor (daGAL > UAS-InRDN) and their genetic controls (daGAL/+ and UAS-InRDN/+) in both wild-type and dfoxo− backgrounds (representative of two independent experiments). In a wild-type background (top panel): for daGAL > UAS-InRDN median survival = 3.5 days, maximum survival = 4.9 days, n = 100; for daGAL/+ median survival = 1.5 days, maximum survival = 1.5 days, n = 90; for UAS-InRDN/+ median survival = 1.5 days, maximum survival = 4.9, n = .80. The survival of daGAL > UAS-InRDN flies was significantly different from each of the controls (P <10−6; Log-rank test). In a dfoxo− background (bottom panel): for daGAL > UAS-InRDN median survival = 1.9 days, maximum survival = 3.5 days, n = 49; for daGAL/+ median survival = 1.5 days, maximum survival = 2.9 days, n = 80; for UAS-InRDN/+ median survival = 1.5 days, maximum survival = 2.4 days, n = 70. The survival of daGAL > UAS-InRDN dfoxo− flies was significantly different from each of the controls (P <0.05; log-rank test). (B) Survival curves in response to 20 mm paraquat of female flies with late ablation of the median neurosecretary cells (InsP3GAL > UAS-rpr) and their genetic controls (InsP3GAL/+ and UAS-rpr/+) in both wild-type and dfoxo− backgrounds. In a wild-type background (top panel): for InsP3GAL > UAS-rpr median survival = 2.1 days, maximum survival = 3.9 days, n = 95; for InsP3GAL/+ median survival = 1.5 days, maximum survival = 2.1 days, n = 88; for UAS-rpr/+ median survival = 2.1 days, maximum survival = 1.9, n = 99. The survival of InsP3GAL > UAS-rpr flies was significantly different from each of the controls (P <10−15; log-rank test). In a dfoxo− background (bottom panel): for InsP3GAL > UAS-rpr median survival = 2.1 days, maximum survival = 2.5 days, n = 78; for InsP3GAL/+ median survival = 1.5 days, maximum survival = 2.1 days, n = 77; for UAS-Rpr/+ median survival = 1.5 days, maximum survival = 2.1 days, n = 76. The survival of InsP3GAL > UAS-rpr dfoxo− flies was significantly different from each of the controls (P <10−12; log-rank test). (C) Survival curves in response to 20 mm paraquat of female flies induced to overexpress dominant negative Dp110 (daGS > UAS-Dp110DN) using RU486 (+RU486) and their uninduced controls (−RU486) in both wild-type and dfoxo− backgrounds. In a wild-type background (top panel): for +RU486 median survival = 2.6 days, maximum survival = 6.5 days, n = 100; for −RU486 median survival = 1.6 days, maximum survival = 3.6 days, n = 100. The survival of +RU486 flies was significantly different from the uninduced controls (P <10−9; log-rank test). In a dfoxo− background (bottom panel): for +RU486 median survival = 1.3 days, maximum survival = 2.6 days, n = 85; for −RU486 median survival = 1.1 days, maximum survival = 2.2 days, n = 0.84. The survival of +RU486 flies was significantly different from the uninduced controls (P <0.001); log-rank test).
IIS-dependent xenobiotic metabolism is dFOXO-dependent
In both worms and flies, long-lived IIS mutants show increased expression of genes involved in xenobiotic metabolism (McElwee et al., 2007), and IIS mutants in Drosophila show increased survival in the presence of the xenobiotic toxin, DDT (Gronke et al., 2010). In a wild-type background, daGAL4 > UAS-InRDN, InsP3GAL > UAS-rpr and daGS > Dp110DN flies survived for longer in the presence of DDT compared with their controls (Fig. 6A,B,C). In a dfoxo− background, all experimental and control groups showed increased sensitivity to DDT and unlike with paraquat treatment, daGAL4 > UAS-InRDN dfoxo−, InsP3GAL > UAS-rpr dfoxo− and daGS > Dp110DN dfoxo− flies did not show any increased resistance to DDT over their respective controls (Fig. 6A,B,C). Thus, removal of dFOXO activity not only increased sensitivity to DDT treatment but completely abrogated the increased resistance to DDT of IIS-compromised flies.
Fig. 6.

Functions for dFOXO during IIS-mediated dichlorodiphenyltrichloroethane (DDT) resistance. (A) Survival curves in response to DDT of female flies overexpressing a dominant negative version of the insulin receptor (daGAL > UAS-InRDN) and their genetic controls (daGAL/+ and UAS-InRDN/+) in both wild-type and dfoxo− backgrounds. In a wild-type background: for daGAL > UAS-InRDN median survival = 4.9 days, maximum survival = 4.9 days, n = 76; for daGAL/+ median survival = 2.4 days, maximum survival = 3.5 days, n = 97; for UAS-InRDN/+ median survival = 2.4 days, maximum survival = 3.5, n = 100. The survival of daGAL > UAS-InRDN flies was significantly different from each of the controls (P <10−24; log-rank test). In a dfoxo− background: for daGAL > UAS-InRDN median survival = 1.8 days, maximum survival = 2.4 days, n = 55; for daGAL/+ median survival = 1.8 days, maximum survival = 2.4 days, n = 89; for UAS-InRDN/+ median survival = 1.8 days, maximum survival = 2.1 days, n = 65. The survival of daGAL > UAS-InRDN dfoxo− flies was not significantly different from each of the controls (P>0.1; log-rank test). (B) Survival curves in response to DDT of female flies with late ablation of the median neurosecretary cells (InsP3GAL > UAS-rpr) and their genetic controls (InsP3GAL/+ and UAS-rpr/+) in both wild-type and dfoxo− backgrounds. In a wild-type background: for InsP3GAL > UAS-rpr median survival = 3.6 days, maximum survival = 4.5 days, n = 92; for InsP3GAL/+ median survival = 2.6 days, maximum survival = 3.1 days, n = 83; for UAS-rpr/+ median survival = 2.6 days, maximum survival = 2.6 days, n = 94. In a dfoxo− background: for InsP3GAL > UAS-rpr median survival = 1.9 days, maximum survival = 2.6 days, n = 90; for InsP3GAL/+ median survival = 1.6 days, maximum survival = 2.6 days, n = 90; for UAS-Rpr/+ median survival = 1.6 days, maximum survival = 2.3 days, n = 94. The survival of InsP3GAL > UAS-rpr dfoxo− flies was not significantly different from each of the controls (P >0.08; log-rank test). (C) Survival curves in response to DDT of female flies induced to overexpress dominant negative Dp110 (daGS > UAS-Dp110DN) using RU486 (+RU486) and their uninduced controls (−RU486) in both wild-type and dfoxo− backgrounds. In a wild-type background (top panel): for +RU486 median survival = 3.3 days, maximum survival = 4.0 days, n = 88; for −RU486 median survival = 2.9 days, maximum survival = 4.0 days, n = 99. The survival of +RU486 flies was significantly different from the uninduced controls (P <0.001; log-rank test). In a dfoxo− background (bottom panel): for +RU486 median survival = 2.3 days, maximum survival = 2.9 days, n = 76; for −RU486 median survival = 2.3 days, maximum survival = 2.9 days, n = 85. The survival of +RU486 flies was not significantly different from the uninduced controls (P = 0.6; log-rank test).
dFOXO-independent effects on developmental delay and growth
daGAL > UAS-InRDN females were delayed in egg–adult development time by over 24 h compared with both the daGAL/+ and UAS-InRDN/+ controls (Fig. 7A). They also showed a significant reduction in both body weight and wing size (Fig. 7B). Removal of dfoxo did not rescue either the developmental delay or reduced body size of daGAL > UAS-InRDN females (Fig. 7A,B). InsP3GAL > UAS-rpr females were not delayed in their development time but were significantly smaller than both InsP3/+ and Uas-rpr/+ controls (Fig. 7C). Again, InsP3GAL > UAS-rpr females were still significantly smaller than controls in a dfoxo− mutant background (Fig. 7C). Thus, global removal of dfoxo is not sufficient to rescue the small body size of IIS mutant flies. Interestingly, when we restricted the expression of the dominant negative insulin receptor to the developing eye using eyGAL4 (which produces a smaller eye under wild-type conditions), we observed a complete rescue of growth inhibition in the dfoxoΔ94 mutant background (Fig. 7D). It is therefore possible that dFOXO activity is required for the production of systemic growth factors that are required for proper organismal growth.
Fig. 7.

Effects of dFOXO removal on IIS-mediated developmental delay and growth inhibition. (A) Egg-to-adult development time is delayed in females overexpressing the dominant negative insulin receptor (daGAL > UAS-InRDN) irrespective of the presence or absence of dfoxo. Only the eclosion period of the adult flies is shown. Data are shown as percentage of flies eclosing. (n = 118 for daGAL > UAS-InRDN, n = 110 for daGAL > UAS-InRDN dfoxo−; n = 120 for daGAL/+, n = 102 for daGAL/+ dfoxo−; n = 107 for UAS-InRDN/+, n = 82 for UAS-InRDN/+ dfoxo−). (B) Body weights and wing areas as indicators of adult fly body size in females overexpressing the dominant negative insulin receptor (daGAL > UAS-InRDN). Data are presented as means ± SEM (n = 10 for each measurement). daGAL > UAS-InRDN females in both wild-type and dfoxo− backgrounds are significantly reduced in body size compared with control flies (daGAL/+ and UAS-InRDN/+) (**P <0.05, t-test). (C) Body weights and wing areas as indicators of adult fly body size in females with late ablation of the median neurosecretary cells (InsP3GAL > UAS-rpr). Data are presented as means ± SEM (n = 10 for each measurement). InsP3GAL > UAS-rpr females in both wild-type and dfoxo− backgrounds are significantly reduced in body size compared with control flies (InsP3GAL/+ and UAS-rpr/+) (**P <0.05, t-test). (D) Removal of dFOXO rescued the growth inhibition effects of overexpressing the dominant negative insulin receptor in a tissue-restricted manner. UAS-InRDN was specifically expressed in the developing eye using eyGAL. This resulted in a smaller eye compared with control (middle panel compared with left panel). This tissue-restricted growth inhibition was fully rescued in homozygous dfoxoΔ94 (dfoxo−) mutant flies (right panel).
Candidates for systemic growth factors regulated by dFOXO are the Drosophila insulin-like peptides or dilps. Three of these peptides (dilp-2, dilp-3, and dilp-5) are expressed in the MNCs of the Drosophila brain. Ablation of the MNCs or genetic deletion of all three MNC-expressed dilps produces small flies owing to systemic effects on IIS-mediated growth (Ikeya et al., 2002; Broughton et al., 2005; Gronke et al., 2010). Furthermore, expression of dilp3 has been shown to be dependent on dFOXO activity (Broughton et al., 2008). In dfoxoΔ94 mutants, the expression of all three MNC-expressed dilps was significantly reduced compared with control flies (Fig. 8A).
Fig. 8.

Gene expression changes in the absence of dFOXO activity. (A) Quantitative RT-PCR analysis of dilp-2, dilp-3, and dilp-5 mRNA expression in female heads isolated from flies of the indicated genotypes normalized to actin5C. Expression of all three dilp transcripts is significantly decreased in dfoxo− flies compared with controls. Data are presented as means ± SEM (n = 5; P <0.05, t-test). (B) Quantitative RT-PCR analysis of 4E-BP mRNA expression normalized to actin5C in female flies treated with 20 mm paraquat. In wild-type flies, 4E-BP mRNA expression was significantly upregulated in response to paraquat treatment. In dfoxoΔ94 homozygous mutant flies, 4E-BP expression was upregulated in comparison with wild-type flies but no further increase in 4E-BP expression was observed when dfoxoΔ94 mutants were treated with paraquat. Data are presented as means ± SEM (n = 5; P <0.05, t-test). (C) Quantitative RT-PCR analysis of 4E-BP mRNA expression in female flies of the indicated genotypes normalized to actin5C. In a wild-type background, 4E-BP mRNA expression was significantly upregulated in daGAL > UAS-InRDN females compared with each of the genetic controls (P <0.05, t-test). In dfoxoΔ94 homozygous mutants, 4E-BP mRNA expression is upregulated in both controls and experimental flies (P <0.05, t-test). However, no further increases in expression were detected in daGAL > UAS-InRDN in a dfoxo mutant background over wild-type background.
Effects of dfoxo deletion on dFOXO target gene expression
The translational regulator 4E-BP (encoded by Thor) has been well documented as a direct target of dFOXO (Junger et al., 2003; Puig et al., 2003). 4E-BP expression is upregulated when dFOXO is activated either in response to low IIS or upon exposure to stressors such as paraquat. We therefore examined the effects of the dfoxoΔ94 mutation on 4E-BP expression under various conditions in which dFOXO activity would normally be induced. Thus, 4E-BP expression was upregulated in both daGAL4 > UAS-InRDN flies and wild-type flies exposed to 20 mm paraquat (Fig. 8B,C). Surprisingly, 4E-BP expression was also increased to a comparable level in dfoxoΔ94 homozygous mutants themselves. However, in dfoxoΔ94 homozygotes, no further increases in 4E-BP expression were observed in daGAL4 > UAS-InRDN flies or upon exposure to paraquat (Fig. 8B,C).
Discussion
As a result of the pleiotropic effects of IIS on animal physiology, extension of lifespan by reduced IIS is often accompanied by other phenotypic responses, including reduced or delayed reproduction, growth inhibition, increased stress resistance, and metabolic dysregulation. In C. elegans, all of the phenotypic outcomes of reduced IIS are under a common regulatory mechanism, because they are all dependent on the transcriptional activity of the FOXO transcription factor, DAF-16 (Kenyon et al., 1993; Dillin et al., 2002). In Drosophila and mammals, many of the same physiological traits are affected by reduced IIS, but a requirement for FOXO transcriptional activity in mediating all of the phenotypic responses to reduced IIS, especially lifespan extension, in these other animal models is less well understood. In this study, we have combined a novel deletion mutant of dfoxo that is devoid of dfoxo mRNA expression with several models of reduced IIS in Drosophila to investigate the consequences of dfoxo removal on lifespan, fecundity, development, growth, and stress resistance.
In C. elegans, IIS-mediated lifespan extension is entirely dependent on the activity of DAF-16, and genetic manipulations that reduce IIS cannot extend the lifespan of daf-16 mutant or RNAi-treated worms (Kenyon et al., 1993; Dillin et al., 2002). We have observed similar results in Drosophila in that mutation of dfoxo completely blocked the lifespan extension associated with late ablation of the MNCs as well as adult-specific expression of either a dominant negative form of the Drosophila insulin receptor or a dominant negative form of Dp110. However, we did observe an age-specific increase in survival late in life in dfoxo mutants with ubiquitous expression of the dominant negative insulin receptor during development. In these flies, no differences in survival were observed until after the flies were aged 50 days, after which experimental flies consistently out-lived controls, resulting in a significant extension of their maximum lifespan. While these effects on survival were reproduced in independent replicate experiments, they were not observed with any other IIS manipulation, suggesting that developmental expression of the dominant negative insulin receptor may produce effects that are not necessarily linked to reduced IIS. It is, however, intriguing to note that at least one genetic manipulation that reduces IIS in Drosophila can still increase lifespan in the absence of dFOXO activity. In contrast to its effects in IIS-mediated lifespan extension, dfoxo mutants still showed increased survival in response to treatment with rapamycin, a specific TOR kinase inhibitor, and in response to dietary restriction. Thus, the inhibition of lifespan extension by the removal of dfoxo appears to be specific to the downregulation of IIS.
In worms, DAF-16 is also required to mediate the reduction in brood size associated with the genetic perturbation of daf-2 or age-1 expression (Tissenbaum & Ruvkun, 1998). However, we have found that in Drosophila, removal of dFOXO activity fails to rescue the reduction in egg laying associated with reduced IIS and consequently the reduced fecundity of IIS-compromised females does not appear to be dFOXO-dependent. In fact, low IIS and removal of dFOXO activity actually had additive effects, causing further reductions in egg laying than reduced IIS alone. The nature of the genetic manipulations used in our study to reduce IIS exclude direct reductions within the germline itself, and so the observed effects on female fecundity must be mediated by disruption of somatic signals to the germline. Our data would suggest that these somatic signals act independently of dFOXO. In support of this, we observed dFOXO-independent effects on the expression of the vitellogenic gene, yolkless, with reduced somatic IIS. Our study also highlights important differences between worms and flies in the timing requirements for IIS during reproduction. In C. elegans, daf-2 RNAi initiated at egg hatching caused a delay in reproduction, whereas daf-2 RNAi during adulthood had no effect (Dillin et al., 2002). Our data have shown that reductions to IIS specifically during adulthood by expression of the dominant negative insulin receptor or dominant negative Dp110 are sufficient to reduce female fecundity.
A role for dFOXO within the germline itself during oogenesis, however, cannot be excluded. Previous studies have shown that the effects of low IIS on GSC proliferation can be reverted by a reduction in dFOXO activity, suggesting that at least some aspects of oogenesis are regulated by dFOXO (Hsu et al., 2008), and we have shown here that overexpression of dFOXO alone specifically within the germline is sufficient to reduce egg laying. Hence, dFOXO-dependent and dFOXO-independent processes may mediate the full effects of reduced IIS on oogenesis. The proliferation of the GSCs in response to diet has been shown to be regulated via both dFOXO-dependent and dFOXO-independent processes (Hsu et al., 2008). Moreover, the Ras-binding domain of Drosophila PI3K is required for maximal PI3K activity during egg laying (Orme et al., 2006). Therefore, signaling via both dFOXO and Ras/Mapk may together mediate the full IIS response during oogenesis. Removal of dFOXO alone would hence be insufficient to rescue IIS-mediated defects in egg laying.
In C. elegans, DAF-16 regulates the expression of several oxidative stress responsive genes such as sod 3, mtl-1, ctl-1, and ctl-2 (Honda & Honda, 1999; Murphy et al., 2003). Furthermore, oxidative stress resistance in daf-2 mutant worms is entirely dependent on the presence of DAF-16 (Honda & Honda, 1999). In contrast, we have found that all of the models of reduced IIS tested in this study showed increased resistance to the intracellular ROS generator, paraquat, even in the absence of dFOXO. dFOXO-independent effects may therefore contribute, at least in part, to the increased survival of IIS-compromised flies in response to paraquat treatment. Thus, IIS-dependent oxidative stress resistance can be uncoupled from IIS-dependent lifespan extension based on their requirements for dFOXO, suggesting that they are mediated via nonoverlapping mechanisms. Hence, oxidative stress resistance is not causal in IIS-mediated lifespan extension. By comparison, increased resistance to the xenobiotic toxin, DDT, was completely abolished in dfoxo mutants, suggesting that it is entirely dependent upon dFOXO activity. Furthermore, dfoxo mutants were more sensitive to DDT treatment than wild-type controls, indicating that dFOXO activity is required for survival in the presence of DDT. Our findings that IIS-dependent DDT resistance and lifespan extension require dFOXO suggests that enhanced xenobiotic metabolism may contribute to longevity in long-lived IIS mutant flies. Interestingly, transcriptome analyses of IIS mutants from worms, flies, and mammals have shown that the regulation of cellular detoxification is an evolutionary conserved function of long-lived IIS mutants in all three model organisms (McElwee et al., 2007).
Perhaps our most surprising observation was that in combination with several IIS mutants, removal of dFOXO caused developmental lethality, for example, chico; foxo double mutants were lethal at prepupal stages. Furthermore, we were able to rescue the lethality of chico; foxo double mutants by the expression of dFOXO within the MNCs. Thus, a dFOXO-dependent transcriptional response specifically within the MNCs is required for the viability of chico mutants. The MNCs express the Drosophila insulin-like peptides dilp-2, dilp-3, and dilp-5, and we have shown that dFOXO is required for the basal expression of all three MNC-expressed dilps because their expression is reduced in dfoxo mutant heads. This raises the possibility that dFOXO activity itself may regulate systemic IIS, supported by our observations that removal of dFOXO activity has nonautonomous effects on growth. Nonautonomous inhibition of both somatic and GSC divisions has also been reported for other dfoxo mutants (Junger et al., 2003; Hsu et al., 2008).
In C. elegans, the group of genes identified as direct targets of DAF-16 and that are differentially expressed in response to IIS are enriched for IIS pathway genes, suggesting that when IIS is low, DAF-16 increases insulin sensitivity by upregulating the expression of IIS pathway genes (Schuster et al., 2010). It is probable that a similar feedback mechanism operates in Drosophila, mediated by dFOXO transcriptional regulation. In a previous study, we identified the dilps among several Drosophila IIS pathway genes that show increased expression in an IIS mutant, suggestive of such transcriptional feedback (Slack et al., 2010).
Several dFOXO target genes have been well characterized in Drosophila, including the translational regulator 4E-BP. We have demonstrated that 4E-BP expression is upregulated in response to both reduced IIS and paraquat exposure in a dFOXO-dependent manner. Interestingly, 4E-BP expression was also increased upon removal of dFOXO itself. It is possible that dFOXO functions to restrict basal 4E-BP expression under normal conditions. Alternatively, the effects on 4E-BP expression upon removal of dFOXO may be indirectly mediated via dFOXO-dependent effects on the activity of other transcription factors. 4E-BP has recently been shown to be a potential target for the Drosophila FoxA transcription factor, forkhead (FKH), in response to low TOR signaling (Bulow et al., 2010). 4E-BP expression is suppressed by the loss of FKH activity and elevated upon FKH overexpression (Bulow et al., 2010). dFOXO and FKH share the conserved forkhead DNA-binding domain, and so it is possible that they compete for binding at the same target genes. The increase in 4E-BP expression observed in dFOXO mutants may therefore occur as a result of FKH-mediated transcriptional regulation. However, increased expression of other potential FKH target genes such as cabut and CG6770 have not been observed in dfoxo mutants (N. Alic, C. Slack and L. Partridge, unpublished data).
Taken together, our data have shown that unlike in C. elegans, where all of the phenotypic effects of reduced IIS are dependent on DAF-16 activity, in Drosophila, several IIS-dependent phenotypes appear to be regulated, at least in part, through dFOXO-independent mechanisms. These results therefore have important implications when analyzing the requirements for IIS in particular phenotypic traits. In worms, the standard protocol would be to remove DAF-16 activity and look for abrogation of the response or phenotype. In flies, and possibly higher organisms, such experiments may prove misleading. For example, dfoxo mutants display a normal response to DR, but overexpression of the dominant negative insulin receptor or removal of dilps-2, -3 and -5 almost completely blocks the DR response (Grandison et al., 2009; Broughton et al., 2010; Gronke et al., 2010). In conclusion, our data suggest that there is evolutionary divergence in the downstream effectors of IIS, and so in higher organisms, additional factors may act in concert with FOXOs to mediate the full response to reduced IIS.
Experimental procedures
Fly stocks and maintenance
The dfoxoΔ94 allele was generated by conventional imprecise excision using P[GT1]foxoBG01018 flies that carry an P[GT1] element transposon in the 5′upstream region of the dfoxo gene, approximately 130 nucleotides upstream of the dfoxo transcriptional start site (Dionne et al., 2006). The 5′ and 3′ breakpoints of the dfoxoΔ94 deletion were mapped to the genomic sequence by PCR and sequencing. UASp-dFOXO transgenic flies for germline expression of dFOXO were generated using standard procedures. The P[GT1]foxoBG01018, daughterless-GAL4 (da-GAL4), UAS-InRDN (K1409A), UAS-Dp110DN (D954A), eyeless-GAL4 (ey-GAL4), and mata-GAL4 stocks were obtained from the Bloomington Stock Centre. daughterless-GeneSwitch (daGS) was kindly provided by Veronique Monnier (Tricoire et al., 2009). InsP3-GAL4 was kindly provided by Michael Pankratz (Buch et al., 2008). All stocks were backcrossed for at least 6 generations into the control whiteDahomey (wDah) stock. wDah was derived by backcrossing white1118 into the outbred wild-type Dahomey background. Flies were raised and maintained on standard sugar/yeast medium (Bass et al., 2007). Stocks were maintained, and experiments were conducted at 25 °C on a 12:12 hours light/dark cycle at constant humidity.
Lifespan
Flies were reared at standard density (50 larvae per vial), allowed to mate for 24 h, sorted by sex, and then transferred to experimental vials at a density of ten flies per vial. Flies were transferred to fresh vials three times a week, and deaths were scored during transferral.
Fecundity assays
For fecundity measurements, eggs were collected over a 24-h period and counted. Data are reported as the mean number of eggs laid per female ± SEM over this 24-h period.
Paraquat and DDT assays
Flies were reared and housed as for lifespan experiments until 7 days of age, and then flies were starved for 5 h on 1% agar before being transferred to fly food containing either 20 mm paraquat or 0.03% (w/v) DDT.
Growth analysis
Body weights of 7-day-old flies (n = 10 for each genotype) were measured using a precision balance. Wing areas were measured as previously described (Bohni et al., 1999). For clonal analysis of growth, clones of dfoxoΔ94mutant cells were induced in the larval wing disks at 24–48 h after egg deposition by heat-shocking larvae of the genotype y, w, hs-flp/w; FRT82, ubi-GFP/FRT82, dfoxoΔ94 for 1 h at 37 °C. Larval wing disks were dissected out, fixed in 4% formaldehyde for 20 min at room temperature, and mounted in Vectashield mounting medium containing DAPI.
Western blots
Western blots were carried out on protein extracts made from whole flies using a TCA-based extraction protocol. Equal amounts of protein as quantified using the Bio-Rad protein assay reagent were loaded onto SDS–PAGE gels and blotted according to standard protocols. Blots were probed with either anti-dFOXO antibody (Giannakou et al., 2007) at a dilution of 1:5000 or anti-tubulin antibody (Sigma, Gillingham, UK) at 1:2500 dilution. Secondary antibodies conjugated to HRP (AbCam, Cambridge, UK) were used, and the signals were detected by chemiluminescence using the Enhanced ECL kit (GE, Amersham, UK).
Quantitative RT-PCR
Total RNA was extracted from ten whole adult flies or 20 adult heads per genotype using standard Trizol (Invitrogen, Paisley, UK) protocols. cDNA was prepared using oligod(T) primer and Superscript II reverse transcriptase according to the manufacturer's protocol (Invitrogen). Quantitative RT-PCR was performed using the PRISM 7000 sequence detection system and Fast SYBR® Green PCR Master Mix (ABI, Warrington, UK). Relative quantities of transcripts were determined using the relative standard curve method and normalized to actin5C. Four or five independent RNA extractions were used for each genotype. Primer sequences are available upon request.
Chromatin immunoprecipitation
Chromatin immunoprecipitations were carried out essentially as described (Slack et al., 2010). For quantitative PCR, a suitable dilution of total chromatin and IP was used for the quantification using the PRISM 7000 sequence detection system and Fast SYBR® Green PCR Master Mix (ABI). For ChIP analysis, relative amounts of the target DNA recovered after ChIP compared with total chromatin were determined using two or three independent biological replicates. The relative proportion of DNA binding was calculated by dividing the proportion of DNA binding in the ChIP for a single region by the average recovered for all regions for that chromatin to normalize for plate–plate differences.
Immunohistochemistry and confocal microscopy
Immunohistochemical analysis of dFOXO protein overexpression in whole mount ovaries of 7-day-old females was performed using the anti-dFOXO antibody at a dilution of 1:250 followed by Alexafluor 488 labeled anti-rabbit secondary antibody (Invitrogen). Nuclei were visualized using DAPI. Images were acquired using a Zeiss LSM 700 confocal microscope and zen (Zeiss, Welwyn Garden City, UK) software.
Statistical analyses
Statistical analyses were performed using jmp (version 7) software (SAS Institute Inc., Cary, NC, USA). Lifespan data were subjected to survival analysis (Log-rank tests). Maximum lifespans were calculated as the median of the last surviving 10% of the population. Other data were tested for normality using the Shapiro–Wilk W test on studentized residuals (Sokal & Rohlf, 1998). One-way analyses of variance (anova) were performed, and planned comparisons of means were made using Tukey–Kramer HSD (Honestly Significant Difference) or Student's t-test.
Acknowledgments
The authors thank N. Alic, S. Gronke and J. Jacobson for critical reading of the manuscript. We also thank N. Alic for generously providing the dFOXO ChIP samples. This work was supported by the Wellcome Trust.
Author contributions
CS participated in the design and coordination of the study, carried out most of the experiments, and drafted the manuscript; MEG participated in the design of the study; AF and MG participated in the experimental work; LP conceived the study, participated in its design and coordination, and drafted the manuscript.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1 (A) Survival curves of female daGS/+ flies +RU486 and −RU486. Experiments were started with 100 flies per condition. No significant difference in survival was detected between treatments by Log-rank test. (B) Average number of eggs laid per female after 7 days of RU486 treatment (+RU486) or untreated (−RU486). Data are presented as the mean number of eggs laid per female over a 24-h period ± SEM. Eggs were counted from ten separate vials and each vial contained ten females. No significant difference was detected between treatments by anova. (C) Survival of daGS/+flies in the presence of 20mM paraquat after 7 days of RU486 treatment (+RU486) or untreated (−RU486). Experiments were started with 100 flies per treatment. No significant difference was detected between treatments by Log-rank test. (D) Survival of daGS/+ flies in the presence of DDT after 7 days of RU486 treatment (+RU486) or untreated (−RU486). Experiments were started with 100 flies per treatment. No significant difference was detected between treatments by Log-rank test.
Fig. S2 (A) Egg to adult development time in males and females of the indicated genotypes. Only the eclosion period of the adult flies is shown. Data are shown as percentage of flies eclosing. The delay in development observed in dfoxoΔ94 homozygotes is also observed in transheterozygotes of dfoxoΔ94/dfoxo25 and hemizygous dfoxoΔ94/Df(3R)ED5624. (Males: wDahomey, n = 114; dfoxoΔ94/+, n = 94; dfoxoΔ94/dfoxoΔ94, n = 106; dfoxo25/+, n = 84; dfoxoΔ94/dfoxo25, n = 112; Df(3R)ED5624/+, n = 98; dfoxoΔ94/Df(3R)ED5624, n = 84. Females: wDahomey, n = 107; dfoxoΔ94/+, n = 106; dfoxoΔ94/dfoxoΔ94, n = 116; dfoxo25/+, n = 114; dfoxoΔ94/dfoxo25, n = 118; Df(3R)ED5624/+, n = 93; dfoxoΔ94/Df(3R)ED5624, n = 99). (B) Body weights and wing areas as indicators of adult fly body size in females of the indicated genotypes. Data are presented as means ± SEM (n = 10 for each measurement). The reduced body size of dfoxoΔ94 homozygotes is also observed in transheterozygotes of dfoxo25/dfoxoΔ94 and hemizygous Df(3R)ED5624/dfoxoΔ94 (**P < 0.05, t-test).
Fig. S3 Effects of rapamycin treatment on the survivorship of dfoxoΔ94 mutants.
Fig. S4 Lifespan and fecundity responses of dfoxoΔ94 mutant females to dietary restriction.
Table S1 Median lifespans and statistical analysis of dfoxoΔ94 mutants and control flies across a food dilution series.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MD. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:562–567. doi: 10.1093/gerona/61.6.562. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl Acad. Sci. USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SJ, Slack C, Alic N, Metaxakis A, Bass TM, Driege Y, Partridge L. DILP-producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell. 2010;9:336–346. doi: 10.1111/j.1474-9726.2010.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7:321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Bulow MH, Aebersold R, Pankratz MJ, Junger MA. The Drosophila FoxA ortholog fork head regulates growth and gene expression downstream of target of rapamycin. PLoS ONE. 2010;5:e15171. doi: 10.1371/journal.pone.0015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 2006;16:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl Acad. Sci. USA. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem. Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell. 2007;6:429–438. doi: 10.1111/j.1474-9726.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 2008;7:187–198. doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol. (Oxf) 2008;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- Gronke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc. Natl Acad. Sci. USA. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev. Biol. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kuningas M, Magi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur. J. Hum. Genet. 2007;15:294–301. doi: 10.1038/sj.ejhg.5201766. [DOI] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, Patel DS, Selman C, Withers DJ, Thornton JM, Partridge L, Gems D. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nielsen MD, Luo X, Biteau B, Syverson K, Jasper H. 14-3-3 Epsilon antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell. 2008;7:688–699. doi: 10.1111/j.1474-9726.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme MH, Alrubaie S, Bradley GL, Walker CD, Leevers SJ. Input from Ras is required for maximal PI(3)K signalling in Drosophila. Nat. Cell Biol. 2006;8:1298–1302. doi: 10.1038/ncb1493. [DOI] [PubMed] [Google Scholar]
- Partridge L, Bruning JC. Forkhead transcription factors and ageing. Oncogene. 2008;27:2351–2363. doi: 10.1038/onc.2008.28. [DOI] [PubMed] [Google Scholar]
- Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, Zhou Y, Partridge L, Harshman LG. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 2005;51:455–464. doi: 10.1016/j.jinsphys.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster E, McElwee JJ, Tullet JM, Doonan R, Matthijssens F, Reece-Hoyes JS, Hope IA, Vanfleteren JR, Thornton JM, Gems D. DamID in C. elegans reveals longevity-associated targets of DAF-16/FoxO. Mol. Syst. Biol. 2010;6:399. doi: 10.1038/msb.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Slack C, Werz C, Wieser D, Alic N, Foley A, Stocker H, Withers DJ, Thornton JM, Hafen E, Partridge L. Regulation of lifespan, metabolism, and stress responses by the Drosophila SH2B protein, Lnk. PLoS Genet. 2010;6:e1000881. doi: 10.1371/journal.pgen.1000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. New York: 1998. Biometry. WH Freeman. [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech. Ageing Dev. 2009;130:547–552. doi: 10.1016/j.mad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Weber K, Johnson N, Champlin D, Patty A. Many P-element insertions affect wing shape in Drosophila melanogaster. Genetics. 2005;169:1461–1475. doi: 10.1534/genetics.104.027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells RJ, Bodmer R. Age-related cardiac deterioration: insights from Drosophila. Front. Biosci. 2007;12:39–48. doi: 10.2741/2047. [DOI] [PubMed] [Google Scholar]
- Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, Lim HY, Elmen L, Hayes M, Oldham S, Bodmer R. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8:542–552. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc. Natl Acad. Sci. USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


