Abstract
The prevalence of the overactive bladder (OAB) symptom complex increases with age. Older people also appear to experience more severe incontinence syndromes, including OAB, than their younger counterparts. Older patients are more likely than younger individuals to ask for medication for bladder problems and to require higher doses of medication. Conventional treatment for OAB with conservative and lifestyle measures in combination with antimuscarinic pharmacotherapy is effective in older people. Although there is a theoretical potential for cognitive impairment with antimuscarinic agents, the newer antimuscarinics are cognitively safe in cognitively intact older people.
The overactive bladder (OAB) symptom complex, much like all lower urinary tract symptoms, is increasingly prevalent in association with increasing age.1,2 This brief review outlines the epidemiology of these symptoms in later life and discusses the particular changes that occur in the aging lower urinary tract. In addition, the use of antimuscarinic agents in older patients is reviewed, including a discussion of the potential for the antimuscarinic effects to have a detrimental impact on cognitive function.
Changes in the urinary tract associated with aging
Natural changes in the urinary tract may account for some of the apparent increase in lower urinary tract symptoms with increasing age. The sensation of bladder filling is reduced and bladder capacity falls, leading to an older person having less time to respond to the call to urinate and perhaps explaining why many older people complain of severe urge, rather than urgency, as defined by the International Continence Society.3 However, older people also experience a more severe degree of incontinence, including OAB, than their younger counterparts.2,4 For older sufferers with OAB, bladder sensation appears to be heightened, bladder capacities much lower, and urethral resistance rises in association with the onset of the condition, although the mechanism underlying this is unknown.
Underlying mechanisms
Urinary urgency in older people may be a result of both bladder and intracerebral lesions. Severity of urinary urgency and of incontinence per se have been associated with increased white matter hyperintensities (WMH) on MRI scanning of older people, suggesting that increasing ischemic insults in association with aging may impair higher centres of influence on the maintenance of continence, manifesting as severe urge, urgency and urgency incontinence.5,6
Treatment of OAB in older patients
Conventional treatment for OAB with conservative and lifestyle measures in combination with antimuscarinic pharmacotherapy is effective in older people. In direct contradiction to the usual geriatric paradigms of prescribing, older people: are more likely than young to require, and ask for, medication for their bladder problem;7 appear to require higher doses of medication;8,9 and, despite higher reported rates of adverse events, are more adherent to their medication than younger users.10
Antimuscarinics: efficacy
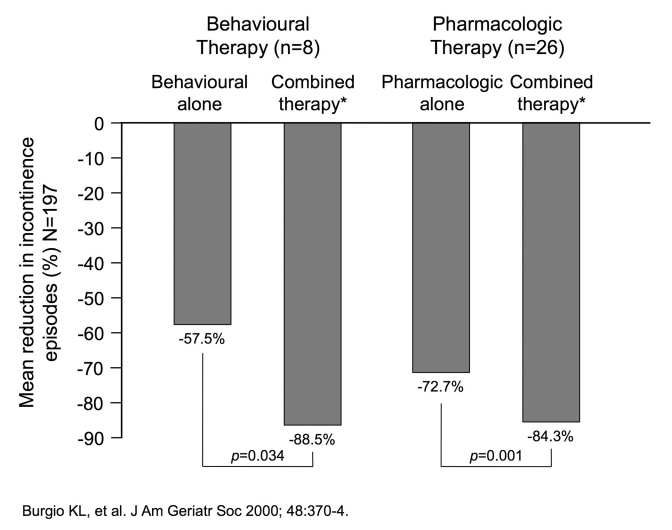
In a study published in 2000, investigators examined the effects of combining behavioural treatment and drug treatment for urge incontinence in community-dwelling older women.11 A total of 197 ambulatory, nondemented, community-dwelling women (age 55 years or older) with persistent urge urinary incontinence were enrolled. Of these, 34 participated in combined treatment. Eight patients started on conservative measures (biofeedback-assisted behavioural training) and then had eight weeks of combined behavioural training and drug treatment with oxybutynin, titrated from 2.5 mg to 15 mg daily. The second group received the antimuscarinic therapy first, followed by eight weeks of this drug therapy combined with behavioural training. As shown in Figure 1, the combination of behavioural training and pharmacotherapy was superior to either element alone. Although a small group, this finding is worthy of further exploration.
Fig. 1.
Benefits of combined pharmacologic and behavioural therapy in older people. *Behavioural therapy and pharmacotherapy (oxybutynin 2.5–15 mg daily)
Several antimuscarinic agents have subsequently been evaluated in older patients, although these are mostly subgroup or pooled analyses from more inclusive study populations.
In a 12-week planned placebo-controlled study of darifenacin in 400 older patients (≥ 65 years) with OAB, the active therapy did not achieve a statistically significant separation from placebo for the primary endpoint of change in weekly urge urinary incontinence episodes.12 However, a significantly greater proportion of darifenacin-treated patients achieved at least a 50% reduction in episodes compared to placebo (70% vs. 58%, respectively, p = 0.021). This was accompanied by significant differences between groups in reductions in micturition frequency (−25.3% with darifenacin vs. −18.5% placebo; p < 0.01). QoL assessments revealed significant improvements in the OAB-q, Patient Perception of Bladder Condition, and patient and physician assessments of treatment benefit.
In an a priori study presented at the 2011 European Association of Urology congress, fesoterodine demonstrated a significantly significant improvement in the primary endpoint of change in daily urgency episodes (−3.47, vs. −1.92 with placebo, p < 0.0001).13
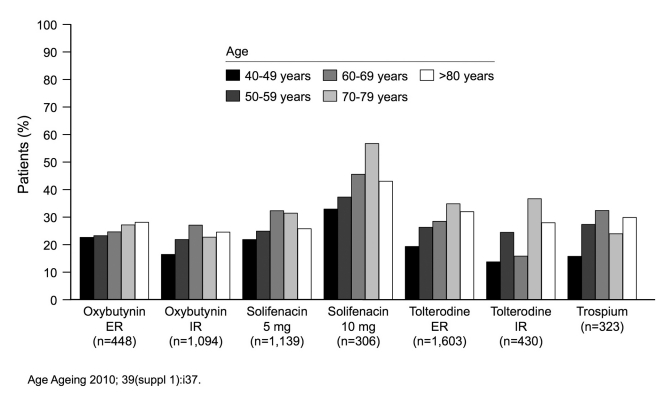
Notably, studies with both fesoterodine and solifenacin have demonstrated that a large proportion of elderly patients require higher doses for optimal efficacy.8,9 Older people do complain of more adverse events from therapy but, as previously noted, older patients appear to be more adherent to their therapies than younger patients (Figure 2).10
Fig. 2.
Elderly patients are more likely to be persistent with therapy.
Antimuscarinics: effect on cognition
Many clinicians are wary of prescribing antimuscarinic medication to older people and are fearful of adverse events, particularly cognitive impairment. This has perhaps led to the vast underuse of antimuscarinic medications reported for the elderly. Are such concerns about cognition without foundation? Theoretically, no; the aging brain is, overall, deficient in cholinergic neurotransmission and muscarinic mechanisms are required for a number of cognitive processes, including short-term memory. M2 receptors in particular, are known to mediate cognitive processes.
It is well-established that the use of medications with antimuscarinic properties (e.g., antidepressants, antipsychotics) is associated with impairment of cognition in community-dwelling older people.14,15 Cholinergic mechanisms are implicated in the neuropathology of Alzheimer’s disease and the continued use of anticholinergic medication over a four-year period appears to be associated with cognitive impairment and an increased risk of incident dementia.16,17 In the 3-City Study, 520 of the 6,912 participants (7.5%) were taking anticholinergic drugs at baseline. In this study, women who were taking anticholinergic drugs at baseline showed greater decline over 4 years in verbal fluency scores and global cognitive functioning than women not using anticholinergic drugs. In men, anticholinergic drugs were associated with a decline in visual memory. There was also a trend towards a reduction in executive function with anticholinergic medication in men.
As far as bladder medications are concerned, immediate release oxybutynin has been shown to adversely affect cognition in the cognitively intact elderly. The newer antimuscarinics for OAB – darifenacin, fesoterodine, solifenacin, tolterodine, and the quaternary ammonium compound, trospium chloride – do not appear to adversely affect cognition.18–22 Oxybutynin transdermal gel similarly appears to have no demonstrable adverse effect on cognition.23
There are, as yet, no data on the use of these medications in older people who are cognitively at risk, those with mild cognitive impairment, dementia, Parkinson’s disease and the like.24 These patients should be only given medication if conservative measures fail or are impractical, and then started at the lowest possible dose and carefully monitored.
The risk of delirium is often cited as a reason not to give these medications. Delirium appears to be an idiosyncratic reaction and uncommon. In a randomized controlled study of extended-release oxybutynin in nursing-home residents with mild to severe dementia, there was no incidence of delirium over the duration of the study.25
Conclusions
The frequency and severity of urinary symptoms increases with age. OAB in older people may be characterized more by a severe, irresistible “urge,” rather than urgency. It is thought that one of the key underlying pathologic mechanisms in the generation symptoms is the accrual of white matter lesions in the brain. Evidence gathered to date has shown that pharmacotherapy is effective in improving urinary symptoms. Paradoxically, older people tend to require higher doses of medication and are more likely to adhere to therapy than younger people. And although there is a theoretical potential for cognitive impairment with antimuscarinic agents, the newer antimuscarinics are cognitively safe in cognitively intact older people.
Footnotes
Competing interests: Dr. Wagg has received speaker fees or honoraria from Astellas, Orion, Pfizer and SCA.
This paper has been peer-reviewed.
References
- 1.Malmsten UG, Molander U, Peeker R, et al. Urinary incontinence, overactive bladder, and other lower urinary tract symptoms: a longitudinal population-based survey in men aged 45–103 years. Eur Urol. 2010;58:149–56. doi: 10.1016/j.eururo.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306–14. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Collas D, Malone-Lee JG. Age associated changes in detrusor sensory function in patients with lower urinary tract symptoms. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:24–9. doi: 10.1007/BF01895101. [DOI] [PubMed] [Google Scholar]
- 4.Wagg AS, Cardozo L, Chapple C, et al. Overactive bladder syndrome in older people. BJU Int. 2007;99:502–9. doi: 10.1111/j.1464-410X.2006.06677.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuchel GA, Moscufo N, Guttmann CR, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–9. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadic SD, Griffiths D, Murrin A, et al. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. Neuroimage. 2010;51:1294–302. doi: 10.1016/j.neuroimage.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo MS, Song C, Kim JH, et al. Changes in overactive bladder symptoms after discontinuation of successful 3-month treatment with an antimuscarinic agent: a prospective trial. J Urol. 2005;174:201–4. doi: 10.1097/01.ju.0000161597.30736.21. [DOI] [PubMed] [Google Scholar]
- 8.Wagg A, Wyndaele JJ, Sieber P. Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis. Am J Geriatr Pharmacother. 2006;4:14–24. doi: 10.1016/j.amjopharm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Kraus SR, Ruiz-Cerdá JL, Martire D, et al. Efficacy and tolerability of fesoterodine in older and younger subjects with overactive bladder. Urology. 2010;76:1350–7. doi: 10.1016/j.urology.2010.03.097. [DOI] [PubMed] [Google Scholar]
- 10.Wagg AS, Fahey A, Siddiq E. Persistence with oral antimuscarinics used for the treatment of overactive bladder in different age groups of patients in general practice [abstract] Age and Ageing. 2010;39(Suppl 1):i37. [Google Scholar]
- 11.Burgio KL, Locher JL, Goode PS. Combined behavioural and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–4. doi: 10.1111/j.1532-5415.2000.tb04692.x. [DOI] [PubMed] [Google Scholar]
- 12.Chapple C, DuBeau C, Ebinger U, et al. Darifenacin treatment of patients >or= 65 years with overactive bladder: results of a randomized, controlled, 12-week trial. Curr Med Res Opin. 2007;23:2347–58. doi: 10.1185/03007X226294. [DOI] [PubMed] [Google Scholar]
- 13.Wagg A, Khullar V, Marschall-Kehrel D, et al. Assessment of fesoterodine treatment in older people with overactive bladder: Results of SOFIA, a double-blind, placebo-controlled pan European trial [abstract]. Presented at the 2011 Annual Meeting of the European Association of Urology. [Google Scholar]
- 14.Mulsant BH, Pollock BG, Kirshner M, et al. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60:198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 15.Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–7. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 16.Perry EK, Kilford L, Lees AJ, et al. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol. 2003;54:235–8. doi: 10.1002/ana.10639. [DOI] [PubMed] [Google Scholar]
- 17.Carrière I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169:1317–24. doi: 10.1001/archinternmed.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesnes KA, Edgar C, Tretter RN, et al. Exploratory pilot study assessing the risk of cognitive impairment or sedation in the elderly following single doses of solifenacin 10 mg. Expert Opin Drug Saf. 2009;8:615–26. doi: 10.1517/14740330903260790. [DOI] [PubMed] [Google Scholar]
- 19.Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173:493–8. doi: 10.1097/01.ju.0000148963.21096.5d. [DOI] [PubMed] [Google Scholar]
- 20.Kay G, Kardiasmenos K, Crook T. Differential effects of the antimuscarinic agents tolterodine tartrate ER and oxybutynin chloride on recent memory in older subjects [abstract]. Presented at the 2006 Annual Meeting of the International Continence Society. [Google Scholar]
- 21.Herberg KW. Everyday life safety and safety while driving under treatment with incontinence drugs: New examinations regarding safety of anticholinergic drugs. Med Welt. 1999;50:217. [Google Scholar]
- 22.Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. 2010;64:1294–300. doi: 10.1111/j.1742-1241.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- 23.Kay G, Staskin D, MacDiarmid S, et al. Are the effects of oxybutynin on cognition dependent upon the route of administration – topical or oral? A double-blind placebo-controlled study employing sensitive cognitive and psychomotor testing [abstract] Neurourol Urodyn. 2009;28:711–2. [Google Scholar]
- 24.Wagg A, Verdejo C, Molander U. Review of cognitive impairment with antimuscarinic agents in elderly patients with overactive bladder. Int J Clin Pract. 2010;64:1279–86. doi: 10.1111/j.1742-1241.2010.02449.x. [DOI] [PubMed] [Google Scholar]
- 25.Lackner TE, Wyman JF, McCarthy TC, et al. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56:862–70. doi: 10.1111/j.1532-5415.2008.01680.x. [DOI] [PubMed] [Google Scholar]