Abstract
The IκB kinase/NF-κB signaling pathway has been implicated in the pathogenesis of several inflammatory diseases. Increased activation of NF-κB is often detected in both immune and non-immune cells in tissues affected by chronic inflammation, where it is believed to exert detrimental functions by inducing the expression of proinflammatory mediators that orchestrate and sustain the inflammatory response and cause tissue damage. Thus, increased NF-κB activation is considered an important pathogenic factor in many acute and chronic inflammatory disorders, raising hopes that NF-κB inhibitors could be effective for the treatment of inflammatory diseases. However, ample evidence has accumulated that NF-κB inhibition can also be harmful for the organism, and in some cases trigger the development of inflammation and disease. These findings suggested that NF-κB signaling has important functions for the maintenance of physiological immune homeostasis and for the prevention of inflammatory diseases in many tissues. This beneficial function of NF-κB has been predominantly observed in epithelial cells, indicating that NF-κB signaling has a particularly important role for the maintenance of immune homeostasis in epithelial tissues. It seems therefore that NF-κB displays two faces in chronic inflammation: on the one hand increased and sustained NF-κB activation induces inflammation and tissue damage, but on the other hand inhibition of NF-κB signaling can also disturb immune homeostasis, triggering inflammation and disease. Here, we discuss the mechanisms that control these apparently opposing functions of NF-κB signaling, focusing particularly on the role of NF-κB in the regulation of immune homeostasis and inflammation in the intestine and the skin.
Keywords: NF-κB, signal transduction, inflammation, mouse models of human disease, epithelial homeostasis
Introduction
The NF-κB family of transcription factors consists of five members in mammals, namely p65/RelA, c-Rel and RelB and the p50 and p52 proteins that are produced by regulated proteolytic processing of the p105 and p100 precursors, respectively 1. NF-κB proteins form homo- or heterodimers that can bind to consensus DNA sequences on the regulatory regions of target genes and regulate their transcription. In resting cells, NF-κB dimers are kept inactive by association with inhibitory proteins of the IκB family, which includes IκBα, IκBβ, IκBε, the p100 and p105 precursor proteins and the atypical members Bcl-3, IκBζ and IκBNS 1. Upon stimulation, the IκB kinase (IKK) phosphorylates IκB proteins on specific serine residues, triggering their ubiquitination and proteasomal degradation, which allows NF-κB dimers to accumulate in the nucleus and activate gene transcription. The IKK consists of two catalytic subunits, named IKK1/IKKα and IKK2/IKKβ, and the NEMO/IKKγ regulatory protein. The activation of NF-κB is tightly controlled by negative feedback inhibition through the NF-κB-dependent expression of IκBα and also of the deubiquitinating enzymes A20 and Cyld that negatively regulate IKK activation 2. Two distinct NF-κB activation pathways have been identified. The canonical NF-κB pathway is induced mainly by proinflammatory cytokine receptors and pattern recognition receptors (PRRs), and mediates the degradation of predominantly IκBα and the nuclear translocation of p50, p65 and c-Rel containing dimers that activate the transcription of proinflammatory and prosurvival genes. Activation of canonical NF-κB signaling requires NEMO and is mediated mainly by IKK2, although in IKK2-deficient cells IKK1 can partly compensate for the loss of IKK2 by inducing IκBα phosphorylation and degradation. The alternative NF-κB signaling pathway is activated mainly by receptors regulating lymphoid organogenesis and lymphocyte development and requires the IKK1-dependent, but NEMO- and IKK2-independent, processing of p100 to p52 and the nuclear accumulation of p52/RelB dimers 3.
The canonical NF-κB signaling pathway induces the expression of a large number of genes that have important functions in the regulation of immune and inflammatory responses, including cytokines, chemokines, adhesion molecules and other immunoregulatory proteins. In addition, canonical NF-κB controls the expression of proteins with antiapoptotic, proproliferative and antioxidant activities. The dual capacity of canonical NF-κB signaling to induce inflammatory responses and at the same time to protect cells from the potentially damaging effects of inflammation is best exemplified in TNFR1 signaling. Binding of TNF to TNFR1 induces the activation of NF-κB and MAPK proinflammatory signaling, and also caspase-8 activation and apoptosis. NF-κB activation protects cells from TNFR1-induced apoptosis by inducing the expression of proteins with antiapoptotic and antioxidant functions 4, 5, 6, 7, 8, 9. Thus, NF-κB activation determines the response of cells to TNF stimulation, which induces proinflammatory signaling in NF-κB-competent cells, but kills NF-κB-deficient cells. Due to its dual role as a potent inducer of inflammation and also a critical regulator of cell survival, canonical NF-κB signaling has been implicated in the pathogenesis of a number of inflammatory diseases. In this review, we focus on the function of canonical NF-κB signaling in the pathogenesis of inflammatory diseases in epithelial tissues such as the skin and the intestine.
The epithelial layers covering the skin, the gastrointestinal tract, the lungs and the urogenital tract together have the daunting task to protect our body over a surface of several hundreds of square meters against threats that constantly arise from the outside world. Potentially dangerous insults challenging the epithelial lining of the body include UV light, mechanical and chemical stresses and most importantly trillions of microbes, most of which are harmless and rather beneficial natural inhabitants of epithelial surfaces and the intestinal lumen, but can cause disease under permissive conditions. Multiple mechanisms are in place to regulate immune homeostasis in epithelial tissues by integrating microbial and other stress inputs into immune regulatory circuits that ensure the maintenance of a healthy immune balance, facilitating effective host defense and at the same time preventing excessive and potentially dangerous inflammatory responses. While these regulatory mechanisms prevent disease pathogenesis in most healthy individuals, a large number of patients suffer from chronic inflammation in epithelial tissues, including inflammatory bowel diseases (IBD) and inflammatory skin diseases such as psoriasis. These diseases are believed to arise from the deregulation of immune homeostasis in the respective tissue, often associated to an abnormal response to microbial components.
Owing to its well-established proinflammatory functions, NF-κB is regarded primarily as a potentially pathogenic factor that is harmful to the host when excessively or improperly activated. However, a number of in vivo studies in genetic mouse models over the past years have revealed that NF-κB inhibition can also trigger chronic inflammatory conditions. This function of NF-κB appears to be particularly important at epithelial surfaces, where NF-κB activity in epithelial cells is required for the maintenance of immune homeostasis 10. Therefore, proper regulation of NF-κB activation at epithelial interfaces is crucial for the maintenance of physiological tissue homeostasis and for efficient host defense against environmental insults. In this review, we discuss the current knowledge on how NF-κB regulates immune homeostasis at epithelial interfaces and how deregulated NF-κB activation triggers inflammation in the respective tissues, with particular focus on the intestine and the skin.
Role of NF-κB at the intestinal epithelial interface
Maintaining homeostasis within the intestinal mucosal immune system is particularly challenging, considering that trillions of bacteria live in the intestinal lumen. Most of these commensal bacteria are found in the large intestine or colon, and are beneficial for the host by helping in food digestion; however, they can cause damage if they cross the epithelial barrier and enter the mucosa. Indeed, inappropriate immune responses to commensal bacteria are thought to contribute to the development of IBD 11, 12, 13. Therefore, both the intestinal epithelium and the underlying mucosal immune cells need to remain quiescent to the luminal flora while being able to effectively mount protective immune responses upon translocation of these bacteria into the mucosa, or upon colonization of the intestine by pathogenic bacteria. In addition to constituting a mere physical barrier separating the gut luminal contents from the mucosal immune system, the single-cell-layered intestinal epithelium is increasingly acknowledged for actively regulating gut immune responses. Intestinal epithelial cells (IECs) express several PRRs, including TLRs, both at their basolateral and their apical cell membrane 14. On encountering their microbial ligands, these receptors initiate signaling cascades, leading to the activation of NF-κB and other proinflammatory pathways. Therefore, commensal bacteria are believed to regulate the level of NF-κB activity at the intestinal epithelial interface and thereby affect the mucosal immune balance 14, 15. Moreover, multiple cytokines also influence epithelial NF-κB activity, especially during ongoing inflammatory responses. Therefore, proper regulation of NF-κB activity at the intestinal epithelial interface is important in steady-state conditions as well as during activation of mucosal immune responses.
Detrimental role for NF-κB activation in the intestine
Multiple lines of evidence suggest that NF-κB activation actively contributes to the development and maintenance of intestinal inflammation. NF-κB was found to be activated in mucosal cells of IBD patients 16, while pharmacological inhibition of NF-κB activity ameliorated intestinal inflammation in mouse models of colitis. For instance, administration of antisense oligonucleotides to p65 or a peptide that binds to NEMO and inhibits IKK activation reduced the severity of colon inflammation in both chemical-induced models and in the Il-10−/− mouse model of colitis 17, 18, 19. These studies suggested that excessive NF-κB activation contributes to intestinal inflammation and that NF-κB inhibition could have therapeutic effects in IBD. However, pharmacological inhibition could not address whether the pathogenic effect of NF-κB was due to NF-κB activation in epithelial or in mucosal immune cells. In the Il-10−/− model of colitis, NF-κB activation is thought to contribute to intestinal inflammation by acting in mucosal immune cells rather than in epithelial cells. Using an elegant NF-κB-driven EGFP reporter gene transgenic mouse model, Karrasch et al. 20 showed that upregulation of NF-κB activity during colitis onset in Il-10−/− mice follows distinct kinetics in IECs versus mucosal immune cells. Colonization of germ-free Il-10−/− mice, which do not develop colitis 21, with colitogenic bacteria induced colitis associated with rapid and transient activation of NF-κB in IECs, while lamina propria immune cells displayed delayed but more persistent NF-κB activation 20. NF-κB inhibition by conditional ablation of IKK2 in IECs did not alter colitis incidence or severity, while IKK2 ablation in myeloid cells diminished colitis occurrence in Il-10−/− mice 22, demonstrating that NF-κB activation in myeloid cells has an important role for the induction of colon inflammation in this model. Taking into account the facts that germ-free conditions as well as MyD88 deficiency prevent colitis development in Il-10−/−mice 21, 23, these studies collectively suggest that bacteria-induced MyD88-mediated NF-κB activation in myeloid cells drives colitis development in Il-10−/− mice.
A number of genetic mouse models support the notion that increased NF-κB activation contributes to intestinal inflammation. Mice lacking CYLD show increased sensitivity to dextran sodium sulfate (DSS)-induced colitis, presumably due to an exacerbated response of CYLD-deficient immune cells to the destruction of the epithelial barrier by the DSS treatment 24. Similarly, mice lacking single immunoglobulin IL-1R-related (SIGIRR) develop more severe colon inflammation after DSS treatment 25, 26, 27. Although both CYLD and SIGIRR are negative regulators of NF-κB activity, CYLD deubiquitinates essential NF-κB signaling proteins such as NEMO and thereby limits NF-κB activation upon various stimuli 28, whereas SIGIRR selectively inhibits NF-κB activation induced by members of the TLR/IL-1R family 29. This suggests that TLR/IL-1R-induced hyperactivation of NF-κB could be sufficient for promoting intestinal inflammation and thus points to an important role for bacteria in this process. Moreover, complementation of Sigirr−/− mice with a transgene expressing SIGIRR specifically in IECs rescued their DSS-sensitive phenotype, showing that an epithelial-specific function of SIGIRR protects mice from DSS-induced colitis 27. In line with this observation, IEC-specific deletion of the essential NF-κB negative feedback regulator A20 was recently shown to sensitize mice to DSS-induced intestinal inflammation 30. Although abolishing the antiapoptotic effects of A20 may have contributed to the colitis-prone phenotype of these mice, the studies on A20 and SIGIRR deficiency together suggest that impaired negative regulation of NF-κB activity in IECs leads to exacerbated epithelial cell responses and more severe inflammation in the DSS model of colitis. Thus, studies in several mouse models of colitis have shown that excessive NF-κB activation, regardless of whether it originates from mucosal immune cells or from IECs, can promote severe intestinal inflammation.
Beneficial role for NF-κB activation in the intestine
While the results discussed above revealed the deleterious effect of increased NF-κB activation in intestinal inflammation, a number of studies showed that inhibition of NF-κB activation specifically in the intestinal epithelium causes severe intestinal inflammation. Mice lacking NEMO specifically in IECs developed severe chronic colitis characterized by epithelial ulceration, elevated expression of proinflammatory mediators and infiltration of immune cells 31. While IEC-specific ablation of IKK1 or IKK2 alone did not cause spontaneous intestinal pathology, mice lacking both IKKs in the intestinal epithelium developed severe colitis similar to the NEMOIEC-KO mice 31. Therefore, complete abrogation of canonical NF-κB activity in the intestinal epithelium, achieved by ablation of NEMO or by combined deficiency of both IKK1 and IKK2, caused severe colon inflammation, demonstrating that IKK/NF-κB signaling performs essential homeostasis-preserving functions in the colonic epithelium 31. This notion is supported by subsequent studies in mice lacking TAK1 in IECs, which showed that TAK1IEC-KO mice also spontaneously developed intestinal inflammation 32. However, whereas NEMOIEC-KO mice develop inflammation only in the colon and most of them survive for several months, TAK1IEC-KO mice also suffer from small intestinal inflammation and die shortly after birth. Since TAK1 acts upstream of the IKK complex, the additional lack of IKK-independent functions of TAK1, such as its involvement in MAPK activation, could explain the more severe phenotype of TAK1IEC-KO mice. Both NEMOIEC-KO and TAK1IEC-KO mice displayed increased levels of IEC apoptosis, suggesting that ablation of NEMO or TAK1 sensitized IECs to apoptosis 31, 32, a notion consistent with the well-appreciated prosurvival function of NF-κB. Crossing into a TNFR1-deficient genetic background inhibited colitis development in the NEMOIEC-KO mice, while it only delayed the appearance of intestinal inflammation in TAK1IEC-KOmice, suggesting that TNFR1 signaling plays an important, but not always indispensable, role in the pathogenesis of intestinal inflammation in these models 31, 32. The mechanisms by which TNF induces disease in the NEMOIEC-KO and TAK1IEC-KO mice are unclear at present. Since NF-κB protects cells from TNF-induced apoptosis, it is possible that TNF induces the death of NF-κB-deficient IECs, compromising the epithelial barrier and in this way triggering colitis. However, TNF is also likely to play a crucial role in coordinating the intestinal inflammatory response by acting in non-epithelial cells such as myeloid or endothelial cells. Moreover, NF-κB is known to protect cells from a wide variety of cell death triggers; therefore, the mechanisms by which TNF contributes to intestinal inflammation in these models await further clarification. For instance, TNF-independent epithelial cell death resulting from accumulation of reactive oxygen species was recently suggested to contribute to the development of intestinal inflammation in TAK1IEC-KO mice 33.
The crucial role of NEMO-dependent NF-κB signaling in the maintenance of intestinal homeostasis is also supported by studies in patients carrying hypomorphic NEMO mutations. These patients usually suffer from severe immunodeficiency and developmental skin defects, but some of these patients also develop colitis 34, 35, 36, 37. Interestingly, hematopoietic stem cell transplantation (HSCT) is effective in treating the immunodeficiency, but does not improve the colitis phenotype. On the contrary, HSCT often worsens pre-existing colitis or even triggers colon inflammation in patients who did not suffer from it before transplantation 36, 37, suggesting that impaired NF-κB signaling in non-hematopoietic cells is responsible for colitis development. In light of the findings in NEMOIEC-KO mice, it is reasonable to assume that NF-κB deficiency in the intestinal epithelium is responsible for the pathogenesis of colitis in patients with NEMO mutations. Therefore, NEMO-dependent IKK signaling in epithelial cells controls intestinal immune homeostasis in both mice and humans.
In contrast to NEMOIEC-KO and TAK1IEC-KO mice, RelAIEC-KO as well as IKK2IEC-KO and IKK1IEC-KO mice did not develop spontaneous intestinal inflammation 31, 38, 39. Since lack of only one of the IKKs or the NF-κB subunits does not completely abrogate NF-κB activation due to compensatory effects of the remaining subunits, it appears that low thresholds of NF-κB activation in IECs are sufficient to maintain intestinal immune homeostasis under basal conditions. However, both IKK2IEC-KO and RelAIEC-KO mice are hypersensitive to colitis induced by DSS 22, 38, 39, suggesting that the intestinal epithelium needs to be capable of raising NF-κB activity in order to cope with tissue damage. Interestingly, Zaph et al. 40 found that IKK2IEC-KO mice showed deregulated immune responses to infection with the intestinal parasite Trichuris muris, and suggested that epithelial NF-κB orchestrates mucosal immune responses by regulating the production of essential immunomodulatory cytokines such as TSLP by IECs. This suggests that epithelial NF-κB activation, in addition to its cell-intrinsic effects, also has crucial paracrine effects on cells of the mucosal immune system. Taken together, genetic mouse models completely or partially impairing NF-κB activation, specifically in IECs, have clearly demonstrated that epithelial NF-κB activation has an essential function for the maintenance of physiological immune homeostasis and the elicitation of protective host defence responses in the intestinal mucosa. In fact, at least the latter part of this paradigm also holds true in the stomach, since mice lacking IKK2 in gastric epithelial cells were recently shown to suffer from more severe Helicobacter-induced gastric inflammation than wild-type mice 41.
Mechanisms underlying the dual role of intestinal NF-κB activation
The studies described above highlighted the dual role of NF-κB in the maintenance of intestinal immune homeostasis and the pathogenesis of intestinal inflammation. Similarly, studies in mice revealed that signaling through MyD88 has a comparable dual role in the intestine. MyD88 deficiency was shown to prevent colitis in Il-10−/− as well as NEMOIEC-KO mice 23, 31, demonstrating that MyD88 signaling exerts detrimental colitogenic effects in these models. In contrast, Myd88−/− mice are hypersensitive to DSS-induced colitis 42, 43, indicating that MyD88 also serves an essential protective role in the intestine. Being an essential adaptor protein for TLR-induced NF-κB activation, it seems likely that the opposing effects of bacteria-induced MyD88 signaling reflect the colitogenic and protective functions of NF-κB activity in the intestine. This hypothesis also raises the possibility that the opposite functions of NF-κB activation in the gut might be mediated by differential signaling induced by bacteria in distinct cell types of the intestinal epithelial interface.
A recent study attempted to distinguish the intestinal cell types responsible for the beneficial and detrimental effects of MyD88 signaling using Helicobacter hepaticus-induced colitis as a model 44. Bone marrow transfer experiments showed that MyD88 signaling in hematopoietic cells mediated intestinal inflammation induced by H. hepaticus. Since both donor and acceptor mice in these experiments were RAG2-deficient, these results indicate that the proinflammatory effects of MyD88 in this colitis model reside in innate immune cells 44. This is in accordance with the fact that colitis development in Il-10−/− mice relies on MyD88 signaling and NF-κB activation in myeloid cells 22, 23. These studies together suggest that the detrimental effects of NF-κB activation in the gut are, at least in part, mediated by MyD88-induced signaling in myeloid cells. In contrast, because irradiated Rag2−/−/Myd88+/+ mice reconstituted with Rag2−/−/Myd88−/− bone marrow cells did not display the spontaneous lethality observed in Rag2−/−/Myd88−/− mice 44, the authors concluded that MyD88 signaling in epithelial cells is critical for host survival in the absence of adaptive immunity. Although reciprocal bone marrow transfer experiments could not be performed in this study, and although necessary experiments with IEC-specific MyD88 deficiency are missing, several indications support the hypothesis that MyD88 signaling in IECs indeed serves beneficial purposes in the gut immune system. For instance, increased susceptibility of mice lacking MyD88 or TLR4 to DSS-induced colitis is associated with increased levels of IEC apoptosis 45, suggesting the presence of cell-intrinsic cytoprotective effects of TLR-induced MyD88 signaling in IECs. In this respect, TLR4-induced expression of COX2 was proposed to play an important role in protecting IECs from cell death during DSS colitis 45. In addition, since both Myd88−/− and Tlr4−/− mice show increased bacterial translocation after DSS administration 45, MyD88-mediated expression of antimicrobial peptide genes could be a second possible IEC-intrinsic beneficial effect of MyD88 signaling in the gut. Indeed, IEC-derived antimicrobial factors often are induced by MyD88 signaling and are important for protecting the host against bacteria threatening to invade the mucosa 46, 47. Furthermore, TLR2 was shown to regulate the expression of tight junction and cell adherens proteins in IECs 48, suggesting that MyD88 signaling could prevent excessive intestinal inflammation by regulating the formation of a tight epithelial barrier. In addition, apical TLR9 stimulation was proposed to prevent excessive NF-κB activation in epithelial cells, and TLR9-deficient mice showed increased sensitivity to DSS-induced colitis, further supporting the existence of beneficial TLR functions in the intestinal epithelium 49. Finally, although Myd88−/− mice showed impaired regenerative IEC proliferation after DSS-induced epithelial injury 42, 45, it is not clear whether this effect is due to defective MyD88 signaling in epithelial cells themselves or in other mucosal cells. Indeed, the proliferative IEC response after DSS injury is thought to rely on MyD88-dependent positioning of activated macrophages and COX2-producing stromal cells near the crypt base 50, 51. However, IEC-intrinsic MyD88 signaling could still play a role in these events, because bone marrow transfer experiments have suggested that recruitment and activation of macrophages depends on TLR4 signaling in IECs 52. Taking the above observations together and bearing in mind the colitis-prone phenotypes of mice with IEC-specific defects in NF-κB activation, it is reasonable to hypothesize that MyD88-dependent NF-κB activation in IECs has beneficial effects in the maintenance of intestinal immune homeostasis. In fact, a recent study describing spontaneous small intestinal inflammation in mice expressing a dominant-negative mutant MyD88 protein selectively in IECs further supports this hypothesis 53.
Conversely, the above observations in Myd88−/− mice suggest that the beneficial effects of NF-κB activation in IECs are largely induced by bacterial triggering of TLRs. However, potentially differential signaling induced by different TLRs complicates this hypothesis. For instance, Tlr5−/− mice spontaneously develop colitis that is crucially mediated by TLR4 signaling 54, pointing to beneficial and detrimental effects of signaling induced by TLR5 and TLR4, respectively. Despite these overall beneficial effects of TLR5 signaling, administration of TLR5 agonists in DSS-injured colons aggravates colitis, indicating that TLR5 signaling can also be deleterious in the intestine 55, 56. Cell type specificity of TLR5 signaling likely plays a role in its differential outcomes for the host. Similarly, hypersensitivity of Tlr2−/− mice to Citrobacter rodentium-induced colitis is rescued by additional TLR4 deficiency 57. Interestingly, bone marrow transfer experiments performed in this study showed that TLR4 signaling in hematopoietic cells causes lethality, while TLR2 signaling in tissue-resident cells mediates mucosal healing during infection. Since Myd88−/− mice are also hyper-susceptible to Citrobacter rodentium-induced colitis 58, these experiments suggest that MyD88-dependent beneficial effects in this model might be mediated by epithelial TLR2 signaling toward NF-κB activation. These observations indicate that the differential effects of individual TLRs in distinct cell types confound our understanding of TLR signaling function in intestinal immune homeostasis. Moreover, the fact that certain TLRs completely or partially rely on TRIF for activating NF-κB further obscures our understanding of TLR-induced NF-κB signaling in the intestine. In sharp contrast to Myd88−/− mice, mice lacking TRIF were recently found to be more resistant to DSS-induced colitis than wild-type mice 59. Although abrogation of TRIF-dependent type I Interferon responses could be implicated in this phenotype, this observation suggests that TRIF-mediated NF-κB activation might have deleterious effects during intestinal inflammation. Delineating the contrasting effects of MyD88- and TRIF-dependent signaling during DSS-induced colitis would be required in order to fully comprehend the divergent roles of TLR-induced NF-κB activation in the intestine.
One important aspect of MyD88 signaling that needs to be taken into account is that this adaptor is not only involved in TLR signaling but also in signaling induced by members of the IL-1 family of cytokines. These cytokines have been implicated in the regulation of intestinal inflammation, since mice that lack essential inflammasome components and therefore cannot produce biologically active IL-1β and IL-18 are hypersensitive to DSS-induced colitis 60, 61, 62, 63 Although impaired responses to either IL-1β or IL-18 were shown to enhance susceptibility to DSS-induced colitis 64, 65, 66, IL-18 was identified as the crucial inflammasome-derived cytokine that protects mice from intestinal inflammation 61, 63. Even though the cellular target of IL-18 is not clear, these observations raise the possibility that the increased susceptibility of Myd88−/− or IEC-specific NF-κB-deficient mice to DSS-induced colitis might, at least in part, be caused by impaired IL-18 signaling.
Taken together, the observed parallels between the dual roles for intestinal NF-κB activation on the one hand and the opposing effects of MyD88-mediated signaling in the intestine on the other hand make it tempting to speculate that TLR signaling at least partially underlies both detrimental and beneficial effects of NF-κB activation in the intestine. However, given the multitude and the complexity of the cellular interactions that regulate intestinal homeostasis and inflammation, studies using mice that allow cell type-specific manipulation of TLR signaling will be needed in order to dissect the distinct effects of TLR-induced NF-κB activation in maintaining the mucosal immune balance at the intestinal epithelial interface.
Role of NF-κB at the skin epithelial interface
The skin constitutes the primary interface between the body and its environment. In addition to providing a watertight epithelial barrier, it fulfills multiple complex functions ranging from temperature homeostasis to sensory functions and immune surveillance against environmental pathogens and stresses. The skin is formed by two main compartments, the epidermis and the dermis, which are separated by the basement membrane 67. The epidermis consists of keratinocytes, which proliferate in the basal layer and differentiate while moving towards the outer layers to form a stratified epithelium that provides the skin barrier. The dermis contains mainly fibroblasts and a large number of immune cells, and also structures important for skin function such as blood vessels, nerves, hair follicles and glands. An intense crosstalk between the different cell types of the skin is critical for the regulation of skin homeostasis. Of particular interest for this review, the skin contains numerous resident immune cells, including macrophages, dendritic cells and T cells, most of which are found in the dermis. In contrast, the epidermis normally contains only Langerhans cells and the γδT-cell receptor-bearing dendritic epidermal T cells, which are present in mice but not in humans 68, 69. The skin immune cells are critical for host defense in response to pathogen invasion and for wound healing under physiological conditions, and are also critical mediators for the pathogenesis of inflammatory skin diseases. Numerous skin pathologies such as psoriasis, atopic dermatitis or contact dermatitis are associated with unbalanced skin immune responses leading to chronic inflammation. The role of immune cells in inflammatory skin diseases has been extensively studied both in mouse models and in humans 70. In contrast, the role of epidermal keratinocytes as active players in the regulation of skin immune homeostasis and in the pathogenesis of inflammatory skin diseases is less well understood. Initially considered to be simply structural components of the skin, keratinocytes were recently proposed to have important functions in the regulation of skin homeostasis and in the pathogenesis of inflammatory skin diseases. Epidermal keratinocytes are constantly challenged by multiple environmental stimuli including UV radiation, chemical and mechanical factors capable of inflicting epidermal injuries, and also by potentially pathogenic microbes. Therefore, keratinocytes have the challenging task to integrate environmental stimuli into the network of cellular interactions that control skin homeostasis in order to maintain a healthy skin and elicit well-controlled antimicrobial and wound-healing responses. As a critical pathway for the regulation of cellular responses to multiple stress-inducing factors, NF-κB signaling is expected to play a major role in epidermal physiology. Indeed, a number of recent studies in genetic mouse models revealed an important but complex function of NF-κB in epithelial cells in the regulation of skin immune homeostasis.
Increased NF-κB activation induces skin inflammation
The first in vivo experimental evidence suggesting that increased NF-κB activation triggers skin inflammation was obtained from mice lacking IκBα, the main member of the IκB family of inhibitory proteins that control NF-κB activation by binding and sequestering NF-κB dimers in the cytoplasm. IκBα-deficient mice were born normally but shortly after birth developed severe multi-organ inflammation affecting the skin, resulting in the death of the animals within ∼10 days 71, 72. The inflammatory skin phenotype of these mice is characterized by increased keratinocyte proliferation, epidermal hyperplasia and immune cell infiltration in the dermis and in the epidermis. IκBα-deficient mice crossed into the Rag2−/− background did not develop skin inflammation, demonstrating that the presence of T- or B lymphocytes is essential for skin lesion pathogenesis. Genetic deficiency in TNFR1, TNF or LTα alone reduced skin inflammation, while combined knockout of TNF, LTα and LTβ could completely prevent the onset of inflammatory skin lesions in IκBα knockout mice 73. The development of skin inflammation in IκBα−/− mice could be fully prevented by epidermis-specific ablation of p65, showing that unrestricted activation of NF-κB dimers containing p65 in keratinocytes mediates the pathogenic effects. Interestingly, epidermis-specific ablation of IκBα caused epidermal hyperplasia but did not induce skin inflammation. The inflammatory skin phenotype of IκBα−/− animals was fully recapitulated when IκBα was ablated simultaneously in both epidermal keratinocytes and T cells, suggesting that the development of inflammatory skin lesions is induced by impaired negative regulation of NF-κB activation in both epithelial cells and T lymphocytes 73.
Mice overexpressing IKK2 in epidermal keratinocytes under the control of the keratin 5 promoter were recently reported to develop an inflammatory skin disease resembling interface dermatitis starting at around 2 weeks of age 74. K5-IKK2 mice displayed a thickened, hyperpigmented hairless skin, typical of lichenoid inflammation, accompanied by alterations of ectodermal appendages (hair follicles, exocrine glands, teeth). The inflammatory skin lesions in these mice were characterized by infiltration of macrophages and T cells in the dermis; however, the lesions also developed in K5-IKK2 skin transplanted onto NOD/SCID mice, suggesting that adaptive immune cells were not required. These results further supported a proinflammatory function of elevated NF-κB activation in epidermal keratinocytes.
Keratinocyte-restricted inhibition of NF-κB induces skin inflammation
The discovery that NEMO mutations cause Incontinentia Pigmenti (IP) provided the first evidence that inhibition of IKK/NF-κB signaling induces skin inflammation 75. Incontinentia Pigmenti (IP) is an X-linked genetic disorder characterized by early male lethality and the development of multiple abnormalities in heterozygous females, which show mosaic presence of NEMO-deficient and wild-type cells due to random X chromosome inactivation. The most characteristic feature of IP is the development of skin lesions, which follow four distinct but often overlapping stages. At or shortly after birth the skin of IP patients displays erythematous and bullous skin lesions containing loose keratinocytes, eosinophilic granulocyte infiltrates and apoptotic cells. These lesions subsequently become hyperkeratotic followed by the development of hyperpigmented patches, due to the accumulation of melanin in the dermis. In the last stage, phagocytes clear up the free melanin, leaving areas of dermal scarring with atrophic skin that also displays lack of skin appendages such as hair follicles or sweat glands 76.
NEMO-deficient mice developed a phenotype closely resembling the features of IP 77, 78. These mice displayed male embryonic lethality, while heterozygous females developed skin abnormalities recapitulating the four-stage disease observed in IP patients. Histologically, the skin of heterozygous NEMO females exhibited epidermal hyperplasia accompanied by infiltration of granulocytes in the epidermis and massive apoptosis of keratinocytes. Remarkably, keratinocyte-restricted deletion of NEMO (NEMOEKO) also resulted in the development of inflammatory skin lesions, demonstrating that NEMO deficiency in keratinocytes triggered skin inflammation 79. NEMOEKO mice displayed a normal epidermis at birth; however, starting from postnatal day 2 the animals showed a characteristic lack of pigmentation and ultimately developed inflammatory skin lesions leading to death by postnatal day 6. NEMOEKO mice bred into the RAG1-deficient background developed skin lesions demonstrating that the development of skin inflammation in this model does not require the presence of lymphocytes 79. Crossing into the TNFR1-deficient genetic background prevented the development of inflammatory skin lesions early in life in NEMOEKO mice; however, most double NEMOEKO/Tnfr1−/− mice developed skin lesions later in life, usually between 4 and 6 months. Therefore TNFR1 signaling is essential for the early stages of the disease, but TNFR1-independent mechanisms induce skin inflammation in later stages 79.
Epidermal keratinocyte-specific ablation of IKK2 caused the development of a strong inflammatory skin disease that shared some similarities with human psoriasis. IKK2EKO mice showed a normal epidermis at birth, but starting from postnatal day 4, they developed inflammatory skin lesions that led to death of the animals by postnatal days 7 to 9 80. Histologically, the disease presents features similar with the phenotype observed upon deletion of NEMO in the epidermis, such as epidermal hyperplasia, thickening of the epidermis and presence of granulocyte abscesses in the epidermis. However, in contrast to NEMOEKO mice, IKK2EKO animals do not display hyperpigmentation and increased keratinocyte apoptosis. The immune mechanisms involved in the inflammatory skin diseases triggered by epidermal deficiency of NEMO or IKK2 appear to be similar but not identical. IKK2EKO mice crossed into a TNFR1-deficient genetic background do not develop skin lesions and maintain a healthy skin for the entire durations of their lives, demonstrating that TNFR1-mediated signaling is essential for triggering skin inflammation in this model 80. In contrast, IKK2EKO mice crossed into an IFNγ-deficient background or in a TCRα-deficient background, developed inflammatory skin lesions showing that neither IFNγ nor the presence of αβ T cells is required for skin inflammation in this model 80, 81. Similarly, the presence of neutrophil infiltration of the skin was not necessary for the development of skin lesions, as shown in IKK2EKO mice crossed into a CD18-deficient background 81. In contrast, local elimination of macrophages using subcutaneous injection of clodronate liposomes dramatically reduced inflammation in IKK2EKO mice, showing that the development of inflammatory skin lesions in this model is largely driven by macrophages 81.
Other models studying inhibition of the NF-κB pathway in the epidermis took advantage of transgenic expression of a degradation-resistant IκBα 'super-repressor' (IκBαSR). The selective expression of IκBαSR under the control of the keratin 5 promoter induced skin inflammation and epidermal hyperproliferation in mice 82. However, in contrast to the IKK2EKO mice, these animals survived to adulthood, but developed subsequently spontaneous squamous cell carcinomas. Both inflammation and tumor development in K5IκBαSR mice were dependent on TNFR1, but not on IL-1R signaling 83. In contrast, mice with epidermis-specific ablation of p65/RelA did not display spontaneous skin inflammation 73, presumably due to compensation of p65 function by other NF-κB subunits. Indeed, RelA/c-Rel double-deficient epidermis developed TNF-dependent skin inflammation and epidermal hyperplasia after grafting onto Rag1-deficient animals, suggesting that RelA and c-Rel shared a redundant function in keratinocytes that is essential for the maintenance of skin immune homeostasis 84.
More recently, mice with epidermal-specific ablation of TAK1 (TAK1EKO), a kinase critical for both NF-κB and MAPK activation, were also shown to develop an inflammatory skin disease closely resembling the skin lesions observed in NEMOEKO mice. TAK1EKO epidermis is hyperkeratotic, hyperproliferative and displays a high level of apoptosis accompanied by the presence of intraepidermal granulocyte microabscesses 85, 86. Similar to NEMOEKO and IKK2EKO mice, the phenotype of TAK1EKO mice was also rescued by crossing into a TNFR1-deficient background. The proposed mechanism for the development of skin inflammation in this model was a JNK-dependent increase in ROS production by keratinocytes 87.
The studies discussed above convincingly demonstrate that IKK/NF-κB signaling in epidermal keratinocytes has a critical function in the regulation of skin immune homeostasis. However, the molecular mechanisms leading to skin inflammation upon NF-κB inhibition in keratinocytes remain incompletely understood. Given the well-described antiapoptotic function of NF-κB, increased death of NF-κB-deficient epidermal keratinocytes could constitute a trigger for the development of skin inflammation. This hypothesis is supported by the analysis of the skin of NEMOEKO and TAK1EKO animals, which display increased numbers of apoptotic keratinocytes 79, 85. However, neither the IKK2EKO nor K5-IκBα-SR mice showed increased keratinocyte apoptosis during the early stages of the disease, arguing against the hypothesis that increased sensitivity of NF-κB-deficient keratinocytes to death is the trigger for the development of skin inflammation. Interestingly, animals with impaired NF-κB activation in epidermal keratinocytes appear normal at birth, and only start developing skin lesions 2-4 days after birth. The postnatal development of the disease suggests that environmental factors could be implicated in triggering the lesions. Moreover, using inducible models, skin inflammation could also be induced after genetic ablation of NEMO or IKK2 in adult skin 79, 88, suggesting that the development of skin lesions is not triggered by developmental processes occurring in the early postnatal skin.
The important role of TNF in the pathogenesis of inflammatory skin lesions in mice with deregulated NF-κB signaling in the skin is consistent with the well-established role of TNF in human psoriatic skin inflammation. Psoriasis is a chronic inflammatory skin disease characterized by a hyperproliferative, poorly differentiated epidermis and infiltration of the skin by immune cells (T cells, macrophages, neutrophils). Recently, a genome-wide screening approach revealed association of psoriasis with genes encoding regulators of the NF-κB-pathway 89. Although the ultimate underlying cause of psoriasis remains unclear, both genetic and environmental factors seem to be involved. TNF production was increased mostly in myeloid cells invading the dermis in psoriatic patients; therefore, TNF expression could be a secondary effect of perturbed epidermal homeostasis. Interestingly, reconstitution of lethally irradiated K5-IκBαSR/Tnfr1−/− mice with Tnfr1+/− bone marrow failed to induce skin inflammation 83, suggesting that TNFR1 signaling is required in non-hematopoietic, radiation-resistant cells for triggering of skin inflammation in this model. The respective contribution of epithelial and immune cell abnormalities as a primary cause in the development of psoriatic lesions remains one of the most debated topics in dermatology. Although the initiating mechanisms remain, in most cases, to be poorly understood, more and more evidence points to a potentially critical role for keratinocytes themselves as regulators of immune homeostasis in the skin 70. Collectively, the different models presented here highlight the role of NF-κB as a master regulator of skin immune homeostasis. Despite differences between mouse and human skin, the inflammatory lesions developing in these mouse models share many similarities with human inflammatory skin diseases. Therefore, understanding the mechanisms by which NF-κB controls skin immune homeostasis in the mouse models will help to understand the mechanisms controlling the pathogenesis of human inflammatory skin diseases, possibly opening the way to the development of new therapeutic approaches.
Concluding remarks
The studies discussed here have provided ample experimental evidence that NF-κB is a critical regulator of immune homeostasis and inflammation in epithelial tissues (Figure 1). However, the mechanisms determining whether NF-κB activation will have beneficial or pathogenic consequences remain largely elusive. Dissecting the molecular and cellular mechanisms by which NF-κB exerts its opposing functions will be necessary in order to understand the function of this pathway in health and disease. Studies addressing the cell-specific function of the different components of the NF-κB signaling pathway and its upstream and downstream regulators in relevant in vivo disease models will be indispensable in this effort. In particular, clarifying the interactions between microbe-sensing pathways, such as the TLR system, and NF-κB signaling in both immune and non-immune cells will be required in order to better understand the mechanisms by which NF-κB regulates health and disease in epithelial tissues. The NF-κB signaling cascade remains a very attractive target for the therapy of inflammatory diseases, but only when the full complexity of its diverse and often opposing functions has been sufficiently understood at the cellular and molecular level will it be feasible to design safe and effective therapeutic approaches using NF-κB inhibitors.
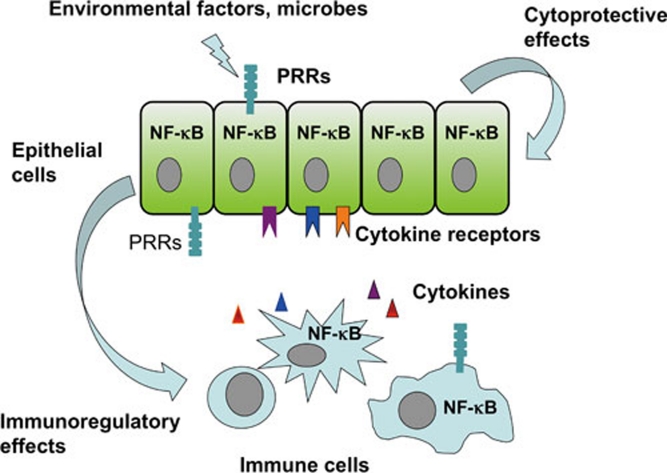
Figure 1.
NF-κB regulates immune homeostasis in epithelial tissues. NF-κB displays critical regulatory functions in epithelial cells, where it controls cellular responses to microbial and other environmental factors. NF-κB regulates the expression of cytokines and chemokines by epithelial cells, which act on immune and other non-epithelial cells to modulate immune responses. NF-κB inhibition sensitizes epithelial cells to stress-inducing stimuli coming either from the environment (e.g., microorganisms) or from immune cells (e.g., cytokines) and compromises their viability resulting in the deregulation of tissue immune homeostasis and triggering inflammation. On the other hand, persistently elevated NF-κB activation induces the expression of proinflammatory chemokines and cytokines by epithelial cells that trigger immune cell activation and inflammation. NF-κB activation in immune cells lining epithelial tissues also plays an important role in the regulation of inflammation. NF-κB activation in response to PRR stimulation induces the expression of proinflammatory mediators by myeloid and other immune cells triggering inflammation. Therefore, finely balanced NF-κB activity in both epithelial and immune cells is critical for the maintenance of immune homeostasis and the prevention of chronic inflammation in epithelial tissues.
Acknowledgments
The work in the authors' laboratory is supported by funding from the University of Cologne, the Deutsche Forschungsgemeinschaft and the European Union.
References
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Wullaert A, Heyninck K, Janssens S, Beyaert R. Ubiquitin: tool and target for intracellular NF-kappaB inhibitors. Trends Immunol. 2006;27:533–540. doi: 10.1016/j.it.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Tsukahara K, Nakao A, Hiraguri M, et al. Tumor necrosis factor-alpha mediates antiapoptotic signals partially via p38 MAP kinase activation in human eosinophils. Int Arch Allergy Immunol. 1999;120 (Suppl 1:54–59. doi: 10.1159/000053596. [DOI] [PubMed] [Google Scholar]
- Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Stehlik C, de Martin R, Kumabashiri I, et al. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Bubici C, et al. Gadd45 beta mediates the NF-kappa B suppression of JNK signaling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signaling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu MT. Toll-like receptor signaling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Shibata W, Maeda S, Hikiba Y, et al. Cutting edge: the IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- Dave SH, Tilstra JS, Matsuoka K, et al. Amelioration of chronic murine colitis by peptide-mediated transduction of the IkappaB kinase inhibitor NEMO binding domain peptide. J Immunol. 2007;179:7852–7859. doi: 10.4049/jimmunol.179.11.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10–/– NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L, Nebelsiek T, Fingerle AA, et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci USA. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Zhang J, Stirling B, Temmerman ST, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116:3042–3049. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Polentarutti N, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci USA. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Veliz T, et al. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- Xiao H, Gulen MF, Qin J, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Courtois G. Tumor suppressor CYLD: negative regulation of NF-kappaB signaling and more. Cell Mol Life Sci. 2008;65:1123–1132. doi: 10.1007/s00018-007-7465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Anders HJ, Mantovani A. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 2009;30:439–446. doi: 10.1016/j.it.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Vereecke L, Sze M, Guire CM, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- Kajino-Sakamoto R, Inagaki M, Lippert E, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajino-Sakamoto R, Omori E, Nighot PK, et al. TGF-{beta}-Activated kinase 1 signaling maintains intestinal integrity by preventing accumulation of reactive oxygen species in the intestinal epithelium. J Immunol. 2010;185:4729–4737. doi: 10.4049/jimmunol.0903587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smahi A, Courtois G, Rabia SH, et al. The NF-kappaB signaling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371–2375. doi: 10.1093/hmg/11.20.2371. [DOI] [PubMed] [Google Scholar]
- Carrol ED, Gennery AR, Flood TJ, Spickett GP, Abinun M. Anhidrotic ectodermal dysplasia and immunodeficiency: the role of NEMO. Arch Dis Child. 2003;88:340–341. doi: 10.1136/adc.88.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JD, Duerst RE, Gelfand EW, Orange JS, Bunin N. Challenges in the use of allogeneic hematopoietic SCT for ectodermal dysplasia with immune deficiency. Bone Marrow Transplant. 2009;43:217–221. doi: 10.1038/bmt.2008.308. [DOI] [PubMed] [Google Scholar]
- Pai SY, Levy O, Jabara HH, et al. Allogeneic transplantation successfully corrects immune defects, but not susceptibility to colitis, in a patient with nuclear factor-kappaB essential modulator deficiency. J Allergy Clin Immunol. 2008;122:1113–1118. e1. doi: 10.1016/j.jaci.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180:2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- Shibata W, Takaishi S, Muthupalani S, et al. Conditional deletion of IkappaB-kinase-beta accelerates helicobacter-dependent gastric apoptosis, proliferation, and preneoplasia. Gastroenterology. 2010;138:1022–1034. e1021–1010. doi: 10.1053/j.gastro.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Araki A, Kanai T, Ishikura T, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- Asquith MJ, Boulard O, Powrie F, Maloy KJ. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529. 529, e511–512. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Michelsen KS, Eri R, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signaling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Riehl TE, Walker MR, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Hernandez Y, Conduah D, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15:997–1006. doi: 10.1002/ibd.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Xu J, Zhu W, et al. Epithelial-specific blockade of MyD88-dependent pathway causes spontaneous small intestinal inflammation. Clin Immunol. 2010;136:245–256. doi: 10.1016/j.clim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Im E, Riegler M, et al. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102:13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Barnich N, Sauvanet P, et al. Crohn's disease-associated Escherichia coli LF82 aggravates colitis in injured mouse colon via signaling by flagellin. Inflamm Bowel Dis. 2008;14:1051–1060. doi: 10.1002/ibd.20423. [DOI] [PubMed] [Google Scholar]
- Gibson DL, Montero M, Ropeleski MJ, et al. Interleukin-11 reduces TLR4-induced colitis in TLR2-deficient mice and restores intestinal STAT3 signaling. Gastroenterology. 2010;139:1277–1288. doi: 10.1053/j.gastro.2010.06.057. [DOI] [PubMed] [Google Scholar]
- Gibson DL, Ma C, Bergstrom KS, et al. MyD88 signaling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:618–631. doi: 10.1111/j.1462-5822.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Im E, Chung HK, Pothoulakis C, Rhee SH.TRIF mediates Toll-like receptor 5-induced signaling in intestinal epithelial cells J Biol Chem 2010 Sep 20. doi: 10.1074/jbc.M110.158394 [DOI] [PMC free article] [PubMed]
- Allen IC, TeKippe EM, Woodford RM, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Yeretssian G, Doiron K, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Hirota SA, Ng J, Lueng A, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2010. [DOI] [PMC free article] [PubMed]
- Zaki MH, Boyd KL, Vogel P, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeis SL, Powell KR, Merlin D, Sherman MA, Kalman D. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2009;77:604–614. doi: 10.1128/IAI.00907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Kanai T, Okazawa A, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Worschech A, Cardone M, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009;41:1068–1075. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson JM, Sharp LL, Witherden DA, Havran WL. Regulation of skin cell homeostasis by gamma delta T cells. Front Biosci. 2004;9:2640–2651. doi: 10.2741/1423. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2376–2346. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, Chen CH, Rosen CA, Stewart CL. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebholz B, Haase I, Eckelt B, et al. Crosstalk between keratinocytes and adaptive immune cells in an IkappaBalpha protein-mediated inflammatory disease of the skin. Immunity. 2007;27:296–307. doi: 10.1016/j.immuni.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Page A, Navarro M, Garín M, et al. IKKbeta leads to an inflammatory skin disease resembling interface dermatitis. J Invest Dermatol. 2010;130:1598–1610. doi: 10.1038/jid.2010.28. [DOI] [PubMed] [Google Scholar]
- Smahi A, Courtois G, Vabres P, et al. The International Incontinentia Pigmenti (IP) Consortium. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature. 2000;405:466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- Nelson DL. NEMO, NFkappaB signaling and incontinentia pigmenti. Curr Opin Genet Dev. 2006;16:282–288. doi: 10.1016/j.gde.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krähn-Senftleben G, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Bloch W, Courtois G, et al. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell. 2000;5:981–992. doi: 10.1016/s1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- Nenci A, Huth M, Funteh A, et al. Skin lesion development in a mouse model of incontinentia pigmenti is triggered by NEMO deficiency in epidermal keratinocytes and requires TNF signaling. Hum Mol Genet. 2006;15:531–542. doi: 10.1093/hmg/ddi470. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Courtois G, Hafner M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- Stratis A, Pasparakis M, Rupec RA, et al. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J Clin Invest. 2006;116:2094–2104. doi: 10.1172/JCI27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hogerlinden M, Rozell BL, Ahrlund-Richter L, Toftgård R. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-kappaB signaling. Cancer Res. 1999;59:3299–3303. [PubMed] [Google Scholar]
- Lind MH, Rozell B, Wallin RP, et al. Tumor necrosis factor receptor 1-mediated signaling is required for skin cancer development induced by NF-kappaB inhibition. Proc Natl Acad Sci USA. 2004;101:4972–4977. doi: 10.1073/pnas.0307106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugasyan R, Voss A, Varigos G, et al. The transcription factors c-rel and RelA control epidermal development and homeostasis in embryonic and adult skin via distinct mechanisms. Mol Cell Biol. 2004;24:5733–5745. doi: 10.1128/MCB.24.13.5733-5745.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori E, Matsumoto K, Sanjo H, et al. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayama K, Hanakawa Y, Nagai H, et al. Transforming growth factor-beta-activated kinase 1 is essential for differentiation and the prevention of apoptosis in epidermis. J Biol Chem. 2006;281:22013–22020. doi: 10.1074/jbc.M601065200. [DOI] [PubMed] [Google Scholar]
- Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratis A, Pasparakis M, Markur D, et al. Localized inflammatory skin disease following inducible ablation of I kappa B kinase 2 in murine epidermis. J Invest Dermatol. 2006;126:614–620. doi: 10.1038/sj.jid.5700092. [DOI] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, et al. Collaborative Association Study of Psoriasis. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]