Abstract
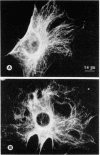
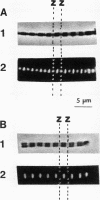
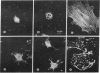
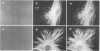
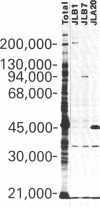
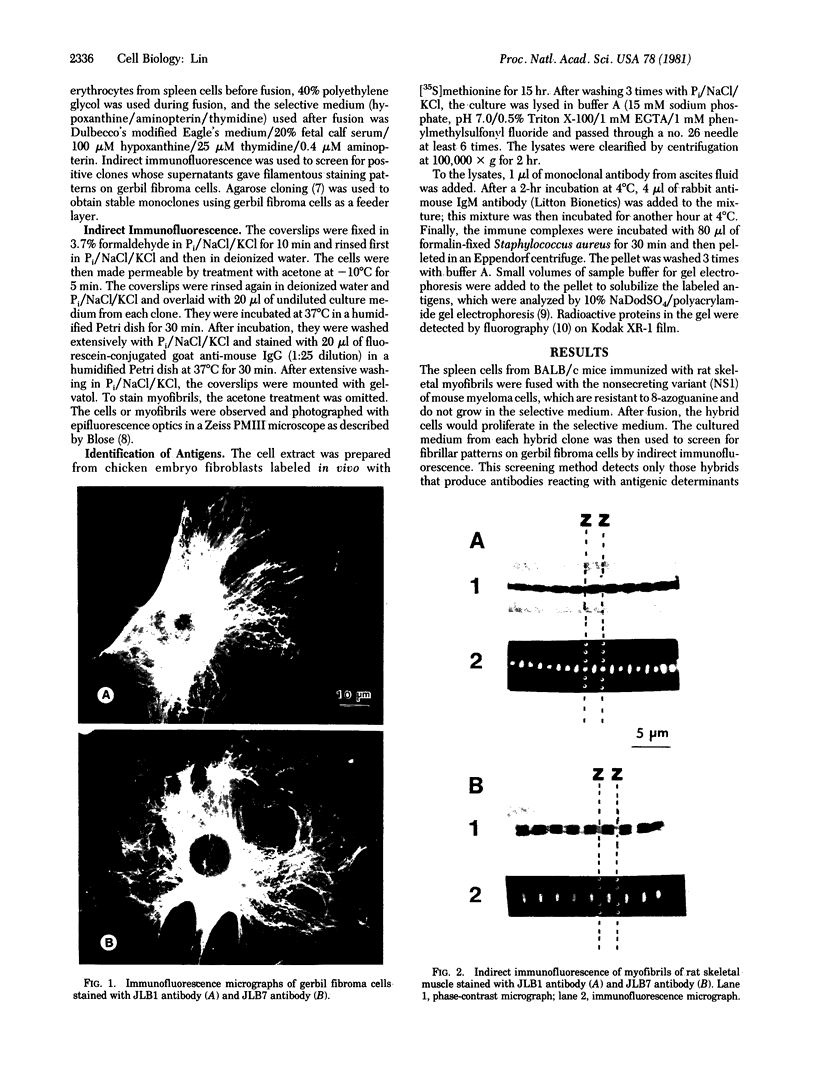
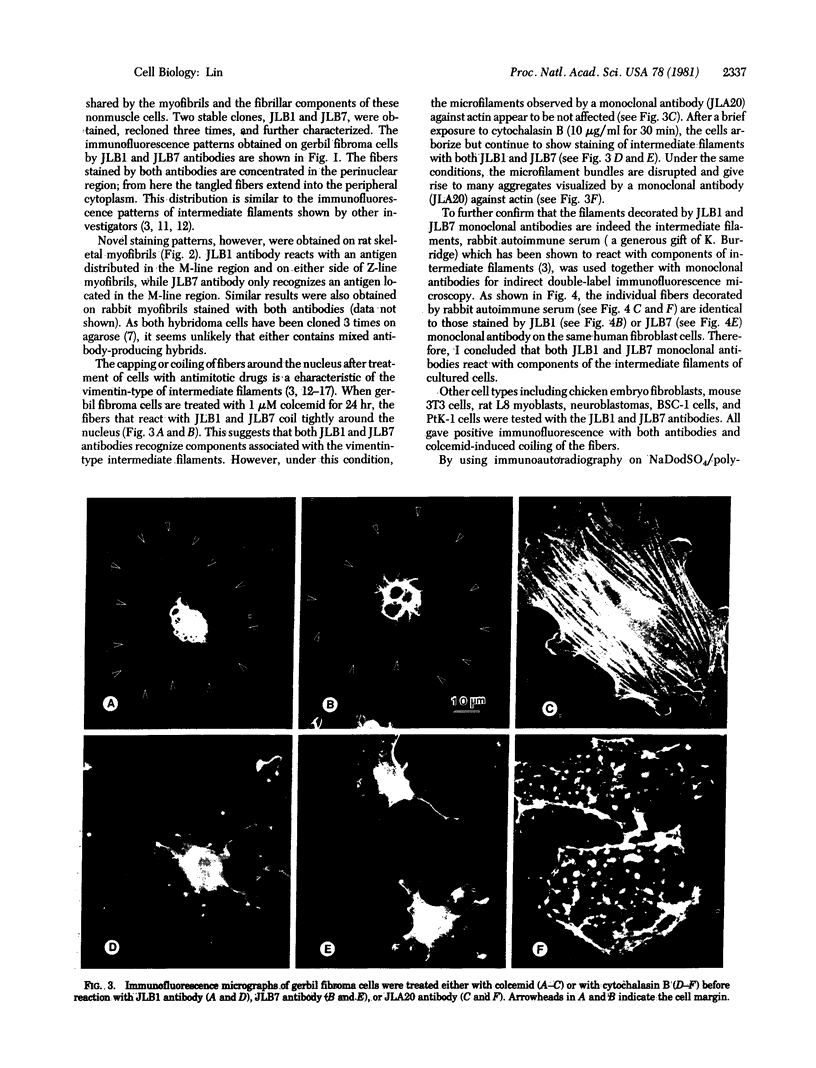
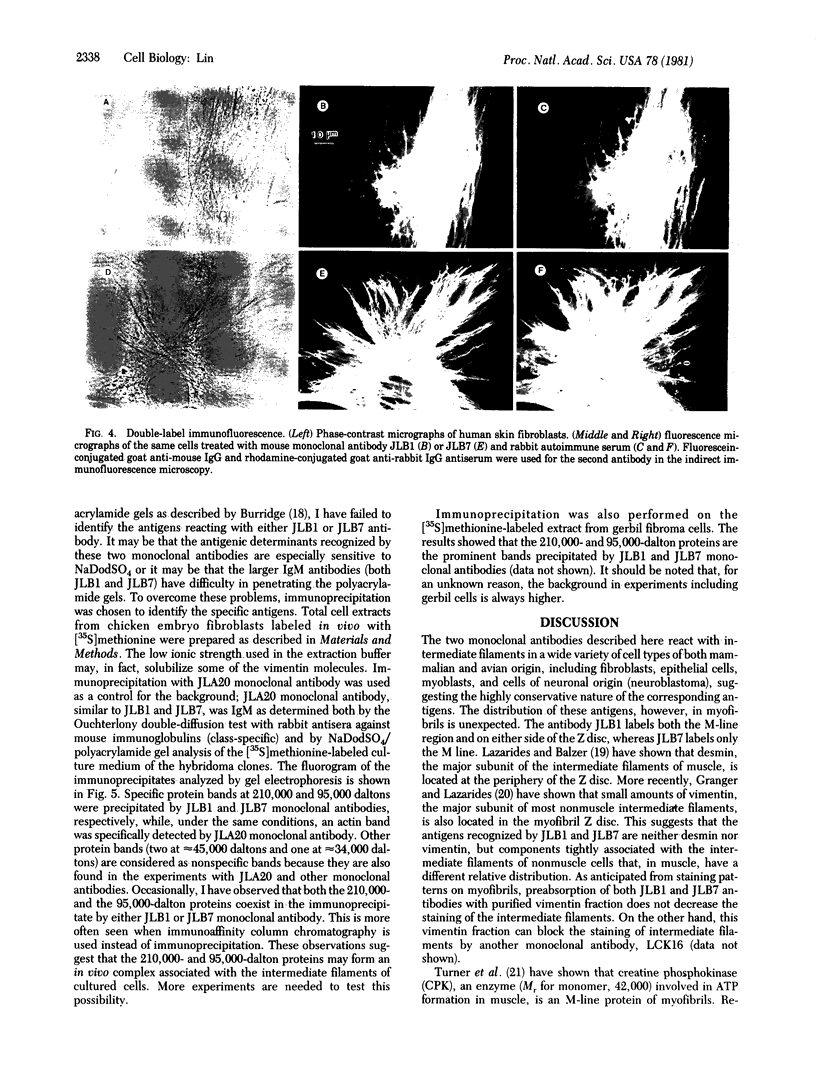
Monospecific antibodies were produced in vitro by fusing mouse myeloma cells with spleen cells from a BALB/c mouse immunized with rat skeletal myofibrils. After cloning 3 times on agarose, two stable clones were obtained and chosen for further characterization. The first clone, JLB1, produced an antibody that recognizing an antigen distributed in the M-line region and on either site of the Z line of myofibrils. The second clone, JLB7, produced an antibody reacting only with an antigen located at the M-line region of myofibrils. Both JLB1 and JLB7 antibodies decorate the typical intermediate filaments of a variety of cultured cells. Colcemid treatment of cells before reaction with both antibodies resulted in the coiling or capping (or both) of the fibers around the nucleus. Brief treatment of cells with cytochalasin B did not affect the integrity of the fibers stained by both antibodies whereas, under the same conditions, microfilament bundles visualized by another monoclonal antibody (JLA20) against actin were disassembled into many aggregates in the cytoplasm. Identical staining patterns of the intermediate filaments are obtained by double-label immunofluorescence microscopy of the same cell stained with these monoclonal antibodies and rabbit autoimmune serum (which has been shown to react with the components of the intermediate filaments). By using immunoprecipitation, protein bands at 210,000 and 95,000 daltons from chicken embryo fibroblasts were identified as the potential antigens recognized by JLB1 and JLB7 monoclonal antibodies, respectively. The widespread occurrence of these antigenic determinants in different cultured cells suggests the highly conservative property of these intermediate-filament components.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blose S. H. Ten-nanometer filaments and mitosis: maintenance of structural continuity in dividing endothelial cells. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3372–3376. doi: 10.1073/pnas.76.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Busch W. A., Stromer M. H., Goll D. E., Suzuki A. Ca 2+ -specific removal of Z lines from rabbit skeletal muscle. J Cell Biol. 1972 Feb;52(2):367–381. doi: 10.1083/jcb.52.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J., Holtzer H. Response of myogenic and fibrogenic cells to cytochalasin B and to colcemid. I. Light microscope observations. J Cell Biol. 1975 May;65(2):271–285. doi: 10.1083/jcb.65.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. L., Pepe F. A. M band protein. Two components isolated from chicken breast muscle. J Cell Biol. 1972 Dec;55(3):681–695. doi: 10.1083/jcb.55.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976 Jan;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon W. E., 3rd, Bushnell A., Burridge K. Characterization of the intermediate (10 nm) filaments of cultured cells using an autoimmune rabbit antiserum. Cell. 1978 Feb;13(2):249–261. doi: 10.1016/0092-8674(78)90194-0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979 Dec;18(4):1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Kundrat E., Pepe F. A. The M band. Studies with fluorescent antibody staining. J Cell Biol. 1971 Feb;48(2):340–347. doi: 10.1083/jcb.48.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Linder E., Virtanen I., Stenman S. Human smooth muscle autoantibodies reacting with intermediate (100 A) filaments. Nature. 1977 Jul 21;268(5617):240–241. doi: 10.1038/268240a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Slesinski R. S., Littlefield J. W. Chemical mutagenesis at the phosphoribosyltransferase locus in cultured human lymphoblasts. Proc Natl Acad Sci U S A. 1972 May;69(5):1244–1248. doi: 10.1073/pnas.69.5.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Wallimann T., Eppenberger H. M. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]