Abstract
NF-κB proteins are a family of transcription factors that are of central importance in inflammation and immunity. NF-κB also plays important roles in other processes, including development, cell growth and survival, and proliferation, and is involved in many pathological conditions. Reactive Oxygen Species (ROS) are created by a variety of cellular processes as part of cellular signaling events. While certain NF-κB-regulated genes play a major role in regulating the amount of ROS in the cell, ROS have various inhibitory or stimulatory roles in NF-κB signaling. Here we review the regulation of ROS levels by NF-κB targets and various ways in which ROS have been proposed to impact NF-κB signaling pathways.
Keywords: reactive oxygen species (ROS), NF-κB, oxidative stress, antioxidants
NF-κB
NF-κB proteins are a family of transcription factors and are of central importance in inflammation and immunity 1, 2. NF-κB transcription factors also regulate the expression of hundreds of genes that are involved in regulating cell growth, differentiation, development, and apoptosis. The mammalian NF-κB proteins consist of five different related family members that bind as homodimers or heterodimers to 10-base pair κB sites. All of these family members have a Rel-homology (RHD) domain that is essential for DNA binding and dimerization. The three Rel members of the family, RelA (also known as p65), RelB, and cRel, have a C-terminal transcription activation domain (TAD) that serves to positively regulate gene expression. The two other mammalian NF-κB proteins are synthesized as larger p105 and p100 precursor proteins, which have C-terminal ankyrin repeats that inhibit DNA binding until partially processed by proteasome to the smaller p50 and p52 products, respectively 1, 2, 3. In addition, these proteins lack a TAD and therefore do not generally activate transcription unless paired as a heterodimer with one of the Rel proteins. All NF-κB proteins are capable of homodimerization or heterodimerization with the other NF-κB proteins with the exception of RelB, which can only form heterodimers. Though most NF-κB dimer combinations result in the regulation of similar sets of genes, the ability to interact in various homo- and hetero- dimer configurations contributes to their ability to bind with varying affinities to κB sites in distinct DNA sequences, and they thus regulate unique, as well as overlapping, gene sets.
Given this diversity, it is therefore not surprising that a substantial variety of mechanisms have arisen to regulate the DNA binding activity of various NF-κB homo- and hetero- dimers. In general, NF-κB activity is principally regulated by the IκB proteins, which, like p100 and p105, possess ankyrin repeats and are generally inhibitory of DNA binding. Three of these proteins are considered “typical” IκBs, namely IκBα, IκBβ, and IκBɛ. These proteins bind to NF-κB proteins and mask their DNA binding domains. They also possess potent nuclear export signals (NES) and generally remove NF-κB proteins from the nucleus, and are thus inhibitory in multiple ways. Two other IκBs, IκBζ and Bcl-3, are considered “atypical”. They are found in the nucleus, are inducibly expressed, bind only to p50 and p52 homodimers, and under certain circumstances may act to repress or to activate these homodimers. The activity of the typical IκBs is controlled through phosphorylation by upstream IκB kinases (IKKs).
Although there are many ways of activating NF-κB, two main signaling pathways have been described that lead to the activation of NF-κB target genes. These are referred to as the canonical (or classical) and noncanonical (or alternative) pathways 1, 2, 4, 5. These two pathways may usually be distinguished by whether the p50 product of p105 (canonical) or p52 product of p100 (noncanonical) is involved. Since p50 is frequently associated with RelA and p52 is frequently associated with RelB, the regulation of these two NF-κB heterodimers has been the most studied and they are considered the prototypical heterodimers for the canonical and noncanonical pathways, respectively.
The canonical NF-κB pathway is activated mostly by the stimulation of proinflammatory receptors, such as the TNF Receptor superfamily, the Toll-Like receptor family (TLRs), and by cytokine receptors for the Interleukins. It is also activated by genotoxic agents as well. Upstream receptors that activate the canonical signaling pathway typically activate an IKK complex consisting of IKKα and IKKβ, which are the catalytic kinases, and IKKγ (also known as NEMO), which acts as a regulatory subunit. Recruitment and activation of this IKK complex is usually dependent on various TNF Receptor Associated Factor (TRAF) family proteins and sometimes on RIP kinases. The p105 (nfκb1 gene product) is constitutively processed by the proteosome into p50, which is held inactive as a heterodimer with RelA (or c-Rel) by its interaction with the inhibitory IκB proteins. (IκBα has been the most studied). Phosphorylation of IκBα on serines 32 and 36 by the IKK complex (primarily IKKβ) targets it for ubiquitination. Subsequently the ubiqutinated IκBα is degraded by the proteosome and this unmasks the DNA binding activity of the p50/RelA heterodimer and also allows it to translocate to the nucleus where it can bind to κB sites and activate gene transcription 1, 2, 4, 5.
Noncanonical NF-κB activation is stimulated by specific TNF receptor family members that signal through the recruitment of TRAF2 and TRAF3. These include LTβR, CD40, CD27, CD30, BAFF-R, RANK, and others 6, 7, 8. The upstream kinase in the noncanonical pathway is the NF-κB-inducing kinase (NIK). Continual degradation of NIK in resting cells prevents constitutive activation of the noncanonical NF-κB pathway 9. Degradation of NIK is mediated by a complex between TRAF3, TRAF2, and cIAPs 1 and 2, which ubiquitinates NIK, targeting it for proteosomal degradation, and the degradation of TRAF2 or TRAF3 by receptor-stimulated processes prevents NIK degradation by this complex and results in NIK stability 10, 11. Stabilization of NIK results in the stimulation of downstream noncanonical signaling events.
In contrast to the canonical pathway, p100 is processed to p52 only after the noncanonical pathway is stimulated. The ankyrin-repeat inhibitory portion of unprocessed p100 therefore acts like a negative regulator by inhibiting DNA binding and nuclear localization of the NF-κB heterodimer. Thus p100 processing is a critical step in the noncanonical NF-κB signal pathway. Processing of p100 is triggered by its phosphorylation, which, unlike in canonical signaling, is dependent on an IKK complex made of homodimeric IKKα. IKKα is in turn activated by its phosphorylation by the recently stabilized NIK. The phosphorylation of p100 by IKKα on its C-terminus targets it for ubiquitination and partial degradation by the proteosome 6, 7, 12, thus freeing p52 and its hetero- or homo-dimeric partner to bind to DNA in the nucleus and affect transcription.
Reactive oxygen species (ROS)
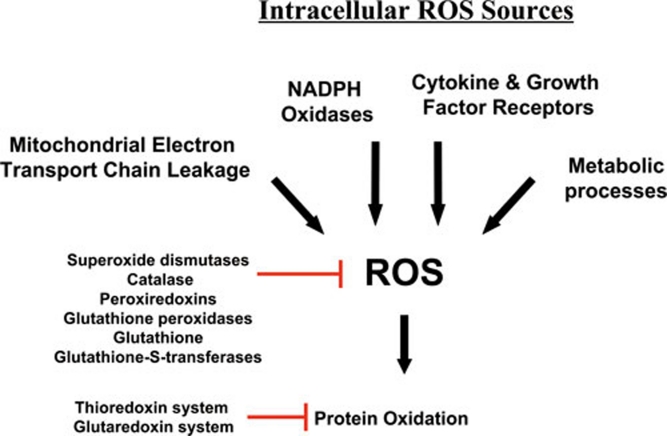
There are many cellular sources of reactive oxygen species (ROS) within a cell (Figure 1). These sources can be broadly divided into two main categories. Firstly, there are those biological processes that release ROS as a byproduct, or a waste product, of various other necessary reactions and secondly, there are those processes that generate ROS intentionally, either in molecular synthesis or breakdown, as part of a signal transduction pathway, or as part of a cell defense mechanism.
Figure 1.
Intracellular Sources of ROS. The mitochondria are a major source of ROS, especially through electron leakage from Complexes I and III. ROS are also produced by NAD(P)H oxidases, sometimes in response to cytokines and other growth factor receptors, which may also utilize other pathways to produce ROS for use in their signaling pathways. Lastly, metabolic enzymes often create ROS as side products or through nonspecific reactions.
In the first category, the mitochondria are in a large measure the greatest source of ROS, since the reactions that occur during oxidative phosphorylation processes frequently lose electrons during their transfer between electron transport chain complexes. These electrons react with molecular oxygen to produce ROS. In consequence of this, the toxic buildup of ROS and cellular oxidation is usually alleviated by enzymes such as the superoxide dismutases, catalase, and peroxiredoxins, as well as systems of antioxidants and their associated enzymes, such as the thioredoxin and glutathione systems (Figure 1) 13, 14, 15. These systems not only serve to repair oxidative damage, but also contribute to the overall response of the cell to ROS by acting as oxidative sensors in signal transduction pathways. For instance, thioredoxin-1 oxidation has been proposed to serve in translating information on the redox state of the cell into ASK1 kinase activity through various mechanisms 16, 17, 18, 19, 20.
In the second category of ROS sources are many enzymes that generate ROS for diverse purposes, although none more robustly than the phagocytic NADPH oxidase, NOX2 (gp91), which uses NADPH to reduce molecular oxygen, thus producing superoxide 21, 22. This superoxide is typically used as a defense tool against infectious pathogens, and is converted in phagosomal compartments by superoxide dismutase and myeloperoxidase to hypochlorous acid (HOCl), which is a potent microbicidal compound 23. However, during this process, some leakage of ROS from the phagosome occurs; and they enter the cytosol, contributing to the oxidative stress of the cell. In addition to NOX2, other NADPH oxidases of this family have been characterized in many cell types 21, 22, though the family members typically produce ROS less robustly. In addition to their cell defense function, local recruitment of NADPH oxidases, including non-phagocytic oxidases, has more recently been implicated in the production of ROS by growth factors and cytokines, though some of these may produce ROS by other means 24. Consequently, these ROS are generated intentionally by the cell to function specifically within signaling pathways. Likewise, other ROS-generating enzymes, such as lipoxygenases and cyclooxygenases. create ROS to function within specific catabolic or anabolic processes, and typically produce substantially less ROS than NADPH oxidases. Thus, there are many different potential intracellular sources of ROS (Figure 1), much of which is capable of influencing, or being influenced by, NF-κB activity.
NF-κB: Protecting from ROS
As mentioned previously, ROS are toxic in cells at certain levels, due to the oxidative stress they exert by their reaction with proteins, lipids, and nucleic acids. The correct cellular response to ROS production is consequently critical in order to prevent further oxidative damage, and to maintain cell survival. However, when too much cellular damage has occurred, it is to the advantage of a multicellular organism to remove the cell for the benefit of the surrounding cells. Reactive oxygen species can therefore trigger both apoptotic and necrotic cell death depending on the severity of the oxidative stress 25, 26, 27. Although there are a few exceptions where NF-κB contributes to cell death 28, in most cases the expression of NF-κB target genes typically promotes cellular survival. Therefore it is not surprising that ROS would modulate an NF-κB response and that NF-κB target genes would attenuate ROS to promote survival. One of the main signaling pathways that intersects with NF-κB with regard to ROS and cell death is the crosstalk that occurs between NF-κB and JNK. Crosstalk from NF-κB to JNK is known to prevent sustained JNK activation and thus prevents cell death through both apoptosis and necrosis 29, 30, 31. This extensive crosstalk occurs in multiple ways, and has been reviewed elsewhere 32, 33, 34, 35, 36.
Antioxidant NF-κB targets
One of the most important ways in which NF-κB activity influences ROS levels is via increased expression of antioxidant proteins (Figure 2). Here we discuss a few of the known as well as proposed NF-κB targets that may contribute to protection from ROS.
Figure 2.
Activation of NF-κB and regulation of downstream transcriptional antioxidant and pro-oxidant targets. NF-κB is activated primarily by two pathways: the canonical pathway (shown on left) and the noncanonical pathway (shown on right). Downstream binding of the NF-κB proteins to DNA regulates downstream transcriptional targets. Shown are many potential antioxidant and pro-oxidant targets that have been proposed in the literature.
Manganese Superoxide Dismutase (MnSOD, or SOD2) is perhaps the most famous of NF-κB targets with antioxidant activity, due to numerous studies 37, 38, 39, 40. MnSOD is a mitochondrial enzyme that protects cells from oxidative stress by converting .O2− into H2O2. Mice lacking MnSOD die perinatally after birth due to massive oxidative stress 41. It is down-regulated in many oxidative diseases 42, and may be up-regulated in some cancers 43.
Likewise, its cytoplasmic relative, Copper-Zinc Superoxide Dismutase (Cu,Zn-SOD, or SOD1) has been shown to be an NF-κB target in at least one study 44. It catalyzes a similar reaction, causing the dismutation of .O2− into H2O2. SOD1-deficient mice have a shortened life span, have persistent oxidative damage and develop hepatocellular carcinoma 45.
Ferritin Heavy Chain (FHC) is the second-most well-known NF-κB target that protects from oxidative damage 46. An iron storage protein, FHC does not directly scavenge ROS, but protects the cell from oxidative damage by preventing iron-mediated generation of highly reactive .OH radicals from H2O2 (Fenton reaction). Thus FHC may synergize with MnSOD to rid the cell of ROS by preventing the generation of more highly reactive species (.O2− and .OH) and promoting the breakdown of H2O2 into water by peroxidases and catalases 47.
Little evidence exists for the regulation of Catalase by NF-κB. However, one report suggests that this is the case 48, and one other study suggests that catalase could be the target of inhibitory p50 homodimers, since its promoter is bound by p50 in unstimulated cells and catalase is down-regulated when canonical NF-κB activation occurs 49.
While the evidence is not yet overwhelming, both thioredoxins, Thioredoxin-1 (Trx1) and Thioredoxin-2 (Trx2), two of the most important cellular antioxidants in the cell, have been reported to be regulated by NF-κB 38, 39. Thioredoxins protect from oxidative stress by means of their 2-cysteine active site that reacts with ROS and is also able to reduce oxidized proteins. They also serve as hydrogen donors to the thioredoxin-dependent peroxide reductases. Trx1 is expressed in the cytoplasm and nucleus. Inactivation of Trx1 in mice also results in early embryonic lethality 50. Trx2 is localized within the mitochondria and is also indispensable for cell survival 51, 52. Deletion of Trx2 causes massive apoptosis due to the accumulation of intracellular ROS, resulting in early embryonic lethality in homozygous mice 52.
Glutathione S-transferase pi (GST-pi) is up-regulated by oxidative stress through NF-κB 53. GST-pi is a phase II enzyme that catalyzes the reaction of the GSH thiolate to toxic electrophilic compounds, thus allowing highly reactive carcinogens or radicals to be eliminated by excretion machinery 54, 55. It also is proposed to contribute to the repair of damage from oxidative stress. Disruption of the gene encoding GST-Pi in HCT116 cells showed that GSTP1 protects HCT116 cells from oxidative stress and resultant apoptosis under growth-limiting conditions 56.
Metallothionein-3 (MT3) has been shown to be an NF-κB target in keratinocytes and fibroblasts 57. Metallothioneins are low-molecular-weight, cysteine-rich proteins which bind to many different metals 58. In addition to regulating metal toxicity, the cysteine residues in metallothioneins can scavenge .O2− and .OH radicals 59.
NAD(P)H dehydrogenase [quinone] 1 (NQO1) is an NF-κB target that is activated in response to the DNA crosslinking agent mitomycin C 60. This FAD-binding protein is a cytoplasmic 2-electron reductase that reduces quinones to hydroquinones. Since it is a 2-electron reductase, its enzymatic activity prevents the one electron reduction of quinones that produces radical species 61. Interestingly, NQO1 deletion also prevents the activation of NF-κB 62.
HO-1 is a heme oxygenase that is up-regulated by NF-κB 63, 64, 65 and other transcription factors in response to oxidative stress and hypoxia. Heme oxygenase-1 catalyzes heme degradation, resulting in the formation of carbon monoxide and biliverdin, which is subsequently reduced to bilirubin by biliverdin reductase 66. Since bilirubin is a potent antioxidant, it is thought that HO-1 is therefore protective from oxidative stress.
Glutathione peroxidase-1 (Gpx1) is an abundant cytoplasmic enzyme that catalyzes the conversion of H2O2 into water using glutathione as a substrate 67. It is one of the most important members of antioxidant proteins. Although it prefers H2O2 as a substrate, it also can reduce lipid peroxides 67, as well as peroxynitrite 68. In skeletal muscles cells, glutathione peroxidase is up-regulated by NF-κB in response to oxidative stress. The glutathione peroxidase promoter is bound by all five of NF-κB subunits in U937 cells in response to LPS, signifiying an important NF-κB target 49.
Dihydrodiol dehydrogenase (DDH1 or AKR1C1) is one of many dehydrogenase enzymes regulated by NF-κB 69. DDH1 is a phase-2 aldoketo reductase and oxidizes transdihydrodiols of polycyclic aromatic hydrocarbons. Like many phase-2 enzymes that activate toxic compounds to eliminate them from the body, the reactive products downstream of its reaction have been associated with induction of ROS 70. However, ectopic expression of DDH1 has been shown to lower the basal levels of ROS in some cell types, suggesting that DDH1 can act as a protective enzyme 71.
Pro-ROS NF-κB targets
Since NF-κB is important in inflammation, some enzymes that promote the production of ROS are also regulated as its targets, especially in cells of the immune system. Below, we discuss a few involved in the generation of ROS.
During the inflammatory process, expression of the phagocytic NADPH oxidase NOX2 (gp91 phox) is dependent on, and induced by, NF-κB 72. As mentioned previously, NADPH oxidase enzymes are specifically devoted to the production of ROS. NADPH oxidases use NADPH to produce superoxide, which is used in immune defenses, and also is used for cell signaling.
An enzyme that exists in two intraconverible forms that catalyze either reduction or oxidation reactions, Xanthine Oxidase/Dehydrogenase (XOR, or Xanthine Oxidoreductase) is regulated by NF-κB 73. The dehydrogenase form is the most dominant form in vivo, however it may be converted to the oxidase form through the oxidation of its protein sulphydryl groups 74. XOR typically catalyzes the interconversion of Xanthine and Urate with NAD+ and water as cofactors. However, the enzyme has very low specificity, and transfer of electrons to O2 instead of NAD+ results in the generation of superoxide and hydrogen peroxide 75. ROS production from XOR is implicated in several pathological conditions, including heart failure 75.
The Inducible Nitric Oxide Synthase, or iNOS (NOS2) is heavily upregulated by NF-κB 76, 77, 78, 79 and Neuronal Nitric Oxide Synthase, or nNOS (NOS1) is also an NF-κB target 80, 81. While technically nitric oxide synthases actually produce a reactive nitrogen species (i.e., nitric oxide, or NO) and not reactive oxygen species, we mention them here because NO is often produced where it can react with superoxide leading to formation of the highly reactive peroxynitrite. While peroxynitrite itself is highly reactive as both an oxidant and nitrating agent, it also reacts with CO2 to form Nitrosoperoxycarbonate (ONOOCO2-), which then homolyzes to form carbonate (CO3.-) and nitrogen dioxide radicals (NO2.). Peroxynitrite can cause various kinds of cellular damage, including damage to DNA, and can activate cell death pathways 82. At low levels, peroxynitrite, and its resultant radicals may participate in signal transduction pathways, in a large part by tyrosine nitration 83. Thus the expression of nitric oxide synthases can potentiate ROS damage as well as signaling.
Cyclooxygenase-2 (COX-2, also known as Prostaglandin G/H synthase 2) is a well-known NF-κB target involved in inflammation 84, 85 that converts arachidonic acid into prostaglandin H2 (PGH2) by a free radical mechanism involving a protein tyrosyl radical generated by cooperation from a heme prosthethic group 86. During the second step of the reaction that produces PGH2, superoxide is also generated 86. Thus, superoxide is a side product of this reaction, and may contribute to oxidative stress as well as signaling.
Other enzymes, in addition to COX-2, that generate ROS during arachidonic acid metabolism have also been reported to be NF-κB targets. Among these are arachidonate 12-lipoxygenase (LOX-12, or ALOX12) 87 and arachidonate 5-lipoxygenase (LOX-5, or ALOX5) 88. As with COX-2-mediated reactions, oxidized metabolites and byproducts of these enzymatic reactions contribute to ROS within the cell 89, 90. In addition, the metabolic products of LOX-12 and LOX-5 , 12(S)- hydroxyeicosatetranoic acid and leukotriene B4, respectively, have been shown to activate and induce NADPH oxidases 91.
Cytochrome p450 enzymes, which are phase I enzymes that detoxify toxic compounds, have long been known to produce ROS when uncoupled, particularly H2O2 and hydroxyl radicals 92, 93, 94, 95. Cyp2E1, Cyp2C11, and Cyp7b are all known to have NF-κB promoter elements. Cyp2E1 and Cyp2C11 are both down-regulated by pro-inflammatory cytokines 96, 97, suggesting a negative regulation by NF-κB, while Cyp7b is up-regulated 98. Both Cyp2E1 and Cyp2C11 are known to be able to produce ROS through uncoupled reactions 95, 99, 100, 101.
Influence of ROS on NF-κB activation
Having examined some of the transcriptional targets of NF-κB that affect ROS amounts within the cells, let us now turn to the ways in which ROS affect the activity of NF-κB (Figure 3). A difficulty in defining ROS contributions to signaling is that ROS can often function in multiple places (i.e. upstream or downstream) within a given pathway, and sometimes in opposing ways (i.e. inhibitory or stimulatory). Such seems to be the case with regards to ROS functions in the NF-κB pathway. For instance ROS often stimulates the NF-κB pathway in the cytoplasm, but inhibits NF-κB activity in the nucleus 102. Thus, overexpression of the antioxidant protein TRX1 was shown to diminish NF-κB activation by inhibiting IκB degradation 103, but others have shown that TRX1 translocation to the nucleus during TNF or PMA stimulation serves to enhance NF-κB DNA binding 104, 105. ROS have been reported to both activate and to repress NF-κB signaling. While many of the differences noted in the literature are probably due to the use of different methodology, many of the differences are also attributed to the study of different upstream pathways and cell-specific differences.
Figure 3.
Crosstalk of ROS with NF-κB signaling pathways. ROS interacts with NF-κB at various places within the signaling pathway. Many of these interactions occur in a cell type-specific manner. ROS has been proposed to both activate and inactivate the IKK complex leading to an effect on the downstream targets. Often ROS has been shown to activate NF-κB through alternative IκBα phosphorylation, which may or may not result in the degradation of IκBα. Lastly, ROS may influence the DNA binding properties of the NF-κB proteins themselves. Oxidation of p50 on its DNA binding domain has been shown to prevent its DNA binding, and must be reversed in the nucleus by a Trx1-dependent process involving Ref-1. On the other hand, the phosphorylation of RelA that is influenced by ROS-dependent processes leads to greater NF-κB activation.
What are the targets of ROS in the NF-κB pathway? The best characterized way in which ROS affects signaling is through its reaction with cysteine, especially at an enzyme's catalytic sites, where the cysteine has a low pKa and exists in the thiolate form 106. A primary example is that of protein tyrosine phosphatases, which have been shown to be inactivated by ROS through oxidation of catalytic cysteines 107, 108, 109. ROS has also been shown to inactivate dual specificity phosphatases 110, which dephosphorylate phospholipids, in addition to both phospho-tyrosine and phospho-serine/threonine residues. In the absence of phosphatase activity, prolonged phosphorylation stimulates the activity of kinases and other enzymes within the cell.
Initial oxidation of cysteines, resulting in sulfenic acid is usually reversible by the cellular antioxidant machinery, but further oxidation to sulfinic and then to sulfonic acids results in irreversible inactivation of the phosphatases by ROS 107, 111. Sulfenic acid is usually unstable and may react with cellular glutathione to form a disulfide bond resulting in an S-glutathionated protein. While a S-glutathionated enzyme is often still inactive, it may then be reduced to its normal state by glutaredoxin in the cytoplasm. If another cysteine instead of glutathione is in close proximity to a recently oxidized cysteine in the form of sulfenic acid, an intramolecular disulfide bond can form, possibly leading to a change in protein conformation and thus preventing or initiating protein activity.
Direct regulation of NF-κB heterodimers by ROS
Direct oxidation of NF-κB by ROS inhibits its DNA binding ability 112. A specific cysteine of p50 is especially sensitive to oxidation. This cysteine, Cys-62 is in the RHD and therefore its oxidation inhibits DNA binding 113, 114, 115. This oxidation is probably followed by an S-glutathionylation event since glutathionated NF-κB has been shown to have less transcriptional activity 116, 117. This oxidation may be selectively reduced and the p50 DNA binding restored by a nuclear enzyme that is associated with base excision repair called Ref-1 (also called APE1) 118, apparently in part through its direct interaction with TRX1 104, 119. This same cysteine may likewise be S-nitrosylated by NO 120, which is produced by the iNOS protein that is up-regulated as an NF-κB target, thus acting as a negative feedback loop 121.
There are other more indirect ways that ROS influences DNA binding of NF-κB proteins. The phosphorylation of RelA on Ser-276 is required for expression of a subset of NF-κB-dependent genes 122. Phosphorylated Ser-276 is necessary for the interaction of RelA with CBP/300 123, as well as the positive transcription elongation factor b 122. PKAc mediates phosphorylation of Ser-276 123, 124, and this event is thought to be dependent on ROS based on a variety of reasons 125, among which is the finding that anti-oxidant treatment inhibits Ser-276 phosphorylation and CBP/300 binding 126.
Aside from the regulation of Ser-276 phosphorylation by ROS, Ser-536 phosphorylation of RelA is induced by NAC through a PI3-kinase-mediated mechanism in a variety of cell lines and this event contributes to its DNA-binding activity 127.
ROS regulation of upstream NF-κB activating pathways.
Exogenously added H2O2 regulates NF-κB activation, and it does so in part through alternative phosphorylation of IκBα (Figure 3). While typically IκBα is usually phosphorylated on serines 32 and 36, which leads to its ubiquitination and degradation, H2O2 affects the phosphorylation of IκBα on Tyr42 or other tyrosine residues, and IκBα may or may not be degraded as part of the process 128, 129, 130, 131. Although IKK is phosphorylated, IKK is not required in this case, and IκBα phosphorylation may be mediated by casein kinase II, possibly downstream of Syk 129, 130. Although this event is observed in response to exogenous H2O2, there is reason to believe that alternative phosphorylation and inactivation of IκBα occurs under physiological conditions because it is also observed under conditions of pervanadate treatment, indicating a possible role for ROS in inhibiting phosphatases, and has also been observed during hypoxia, reoxygenation following hypoxia and in ischemia/reperfusion injury in vivo 132, 133, 134, 135, 136, 137, 138. Degradation of IκBα may not be necessary in this case because Tyr42-phosphorylated IκBα is bound by the SH2 domains of p85α regulatory subunit of PI3K, thus unmasking NF-κB and allowing it to translocate to the nucleus 136. PI3K as well as c-Src has been implicated in alternative tyrosine phosphorylation of IκBα 132, 134, 136.
ROS modification of IκBα has also been shown to lead to inhibition of NF-κB activation. Glutathionylation of IκBα has been detected at cysteine 189, thus preventing phosphorylation events and subsequent degradation 139. However, most of the inhibitory action of ROS on NF-κB with respect to IκBα has been tied to IκBα stability due to the inhibition of the proteasome 140.
IKK is another primary target for ROS in influencing NF-κB signaling. H2O2 has been shown in some cells to inactivate IKK 141, 142, 143, 144. An inhibitory effect may be mediated by ROS oxidation of IKKβ on cysteine 179, since it is found to be S-glutathionated upon exposure to ROS, thus inactivating its kinase activity 142 and leading to a reduction in NF-κB signaling. Deficiency of glutaredoxin 1, which repairs S-glutathionation, leads to reduced nuclear translocation of RelA and substantial loss of NF-κB binding 142. IKK inactivation through oxidation of Cys-179 of IKKβ has also been shown upon arsenite treament 145 and this cysteine residue is also a target of S-nitrosylation by nitric oxide 146. More importantly, Cys-179 is the oxidation target of anti-inflammatory cyclopentone prostaglandins, PGA and 15d-PGJ2 147, suggesting that oxidation of this residue regulates IKK in physiological settings.
Conversely, some studies have shown that ROS, in particular, H2O2, can activate IKKs in some cell types. Dimerization of IKKγ/NEMO was potentiated by H2O2 through formation of disulfide bonds between Cys54 and Cys 347, thus implicating the NEMO subunit in positive regulation by ROS 148, though the other IKK subunits were likely inhibited. Another study reported activation of IKK by H2O2, but in this report NF-κB activation was shown to be inhibited due to lack of IκBα degradation despite its ubiquitination, suggesting that the proteasome was also inhibited 149. One study showed that phosphorylation of both catalytic IKK subunits was potentiated by H2O2 150, which may suggest inhibition of an IKK phosphatase.
Kinases upstream of IKK could be potentially regulated by ROS. MEKK1 was originally suggested to play a role in NF-κB activation. Since it is a redox-sensitive kinase that is inactivated by glutathionylation at C1238, this could potentially be a link to ROS inactivation of IKK in some cells 151. However, an essential role for MEKK1 in IKK activation has been dismissed based on findings in MEKK1-deficient cells 152, and MEKK3, which may be required instead of MEKK1 153, 154, lacks the cysteine that makes MEKK1 a redox-sensitive kinase 155. To our knowledge, TAK1, which has also been suggested to be required for canonical NF-κB activation 154, 156, 157, 158, 159, is not known to be redox-regulated.
NIK, the upstream kinase in the noncanonical pathway is believed to be activated by ROS through inhibition of phosphatases 160. NIK phosphorylation of IKKα is increased upon treatment of H2O2 within a narrow range. However, given that ROS can sometimes inactivate the proteasome, the increased activity of NIK may be in part due to its increased stability as well since it is constitutively degraded by the proteasome.
One potential redox-regulated kinase that affects IKK activation is Akt. Akt positively influences IKKβ-mediated NF-κB activation through the downstream activation of mTOR in association with Raptor 161, 162. Akt has a kinase domain that is subject to oxidation events that inactivate the kinase activity by forming a disulfide bond between Cys-297 and Cys-311 163, and thus ROS could prevent IKK activation by inhibiting Akt. However, not only is Akt itself regulated by ROS, but PTEN, an upstream inhibitor of Akt activation, has a catalytic cysteine that is oxidized by ROS, thus inactivating its phosphatase activity 164. Thus, Akt can be regulated both positively and negatively by ROS.
Conclusion
In summary, ROS interacts with NF-κB signaling pathways in many ways. The transcription of NF-κB-dependent genes influences the levels of ROS in the cell, and in turn, the levels of NF-κB activity are also regulated by the levels of ROS. Depending on the context, ROS can both activate and inhibit NF-κB signaling. A high degree of complexity characterizes ROS interactions with NF-κB pathways owing to the capability for ROS to act in many ways and at numerous places simultaneously. Another complication is that many ROS effects and interactions appear to be cell type-specific. Though we have learned much about the way that ROS influences signaling there is still doubtless a great deal that is yet to be elucidated.
Acknowledgments
The authors' research is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Hatada EN, Nieters A, Wulczyn FG, et al. The ankyrin repeat domains of the NF-kappa B precursor p105 and the protooncogene bcl-3 act as specific inhibitors of NF-kappa B DNA binding. Proc Natl Sci USA. 1992;89:2489–2493. doi: 10.1073/pnas.89.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382(Pt 2):393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- Zarnegar BJ, Wang Y, Mahoney DJ, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem. 2004;279:30099–30105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2000;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Takeda K, Matsuzawa A, et al. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033–37040. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- Li X, Luo Y, Yu L, et al. SENP1 mediates TNF-induced desumoylation and cytoplasmic translocation of HIPK1 to enhance ASK1-dependent apoptosis. Cell Death Differ. 2008;15:739–750. doi: 10.1038/sj.cdd.4402303. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn MT, Ammons MC, Deleo FR.The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction Clin Sci(Lond)20061111–20. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nishio K, Ogawa Y, et al. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 2006;40:619–630. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- Takeda M, Shirato I, Kobayashi M, Endou H. Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron. 1999;81:234–238. doi: 10.1159/000045282. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Tomita T, Matsui H, et al. Hydrogen peroxide-induced apoptosis and necrosis in human lung fibroblasts: protective roles of glutathione. Jpn J Pharmacol. 1999;79:33–40. doi: 10.1254/jjp.79.33. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Reuther-Madrid JY, Kashatus D, Chen S, et al. The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol Cell Biol. 2002;22:8175–8183. doi: 10.1128/MCB.22.23.8175-8183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Tang G, Xiang J, et al. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2002;22:8571–8579. doi: 10.1128/MCB.22.24.8571-8579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Kim YS, Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. Reactive oxygen species in TNFalpha-induced signaling and cell death. Mol Cells. 2010;30:1–12. doi: 10.1007/s10059-010-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Nakano H, Nakajima A, Sakon-Komazawa S, et al. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13:730–737. doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- Papa S, Bubici C, Zazzeroni F, et al. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Kim YS, Liu Z. Lipid rafts and oxidative stress-induced cell death. Antioxid Redox Signal. 2007;9:1471–1483. doi: 10.1089/ars.2007.1658. [DOI] [PubMed] [Google Scholar]
- Jones PL, Ping D, Boss JM. Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Mol Cell Biol. 1997;17:6970–6981. doi: 10.1128/mcb.17.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Javelaud D, Wietzerbin J, Besancon F. NF-kappaB activation prevents apoptotic oxidative stress via an increase of both thioredoxin and MnSOD levels in TNFalpha-treated Ewing sarcoma cells. FEBS Lett. 2004;578:111–115. doi: 10.1016/j.febslet.2004.10.082. [DOI] [PubMed] [Google Scholar]
- Kairisalo M, Korhonen L, Blomgren K, Lindholm D. X-linked inhibitor of apoptosis protein increases mitochondrial antioxidants through NF-kappaB activation. Biochem Biophys Res Commun. 2007;364:138–144. doi: 10.1016/j.bbrc.2007.09.115. [DOI] [PubMed] [Google Scholar]
- Das KC, Lewis-Molock Y, White CW. Activation of NF-kappa B and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Am J Physiol. 1995;269(5 Pt 1):L588–L602. doi: 10.1152/ajplung.1995.269.5.L588. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Salinas M, Martin D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24:7324–7334. doi: 10.1523/JNEUROSCI.2111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]
- Schreiber J, Jenner RG, Murray HL, et al. Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Proc Natl Acad Sci USA. 2006;103:5899–5904. doi: 10.1073/pnas.0510996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Oshima M, Oshima H, et al. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Hosoi F, Yamaguchi-Iwai Y, et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBOJ. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Hu J, Ketterer B, Taylor JB. The organization of the human GSTP1-1 gene promoter and its response to retinoic acid and cellular redox status. Biochem J. 1996;313(Pt 1):155–161. doi: 10.1042/bj3130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado DF, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Curr Protein Pept Sci. 2008;9:325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- Salinas AE, Wong MG. Glutathione S-transferases--a review. Curr Med Chem. 1999;6:279–309. [PubMed] [Google Scholar]
- Dang DT, Chen F, Kohli M, et al. Glutathione S-transferase pi1 promotes tumorigenicity in HCT116 human colon cancer cells. Cancer Res. 2005;65:9485–9494. doi: 10.1158/0008-5472.CAN-05-1930. [DOI] [PubMed] [Google Scholar]
- Hinata K, Gervin AM, Jennifer Zhang Y, Khavari PA. Divergent gene regulation and growth effects by NF-kappa B in epithelial and mesenchymal cells of human skin. Oncogene. 2003;22:1955–1964. doi: 10.1038/sj.onc.1206198. [DOI] [PubMed] [Google Scholar]
- Howells C, West AK, Chung RS. Neuronal growth-inhibitory factor (metallothionein-3): evaluation of the biological function of growth-inhibitory factor in the injured and neurodegenerative brain. FEBS J. 2010;277:2931–2939. doi: 10.1111/j.1742-4658.2010.07718.x. [DOI] [PubMed] [Google Scholar]
- Kumari MV, Hiramatsu M, Ebadi M. Free radical scavenging actions of metallothionein isoforms I and II. Free Radic Res. 1998;29:93–101. doi: 10.1080/10715769800300111. [DOI] [PubMed] [Google Scholar]
- Yao KS, Hageboutros A, Ford P, O'Dwyer PJ. Involvement of activator protein-1 and nuclear factor-kappaB transcription factors in the control of the DT-diaphorase expression induced by mitomycin C treatment. Mol Pharmacol. 1997;51:422–430. [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem. 2006;281:19798–19808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci USA. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Marin-Garcia J, Rogers TB, Lakatta EG, Long X. Phosphorylation and hypoxia-induced heme oxygenase-1 gene expression in cardiomyocytes. J Card Fail. 2004;10:519–526. doi: 10.1016/j.cardfail.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Lin CC, Chiang LL, Lin CH, et al. Transforming growth factor-beta1 stimulates heme oxygenase-1 expression via the PI3K/Akt and NF-kappaB pathways in human lung epithelial cells. Eur J Pharmacol. 2007;560:101–109. doi: 10.1016/j.ejphar.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Prawan A, Kundu JK, Surh YJ. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxid Redox Signal. 2005;7:1688–1703. doi: 10.1089/ars.2005.7.1688. [DOI] [PubMed] [Google Scholar]
- Lei XG, Cheng WH, McClung JP. Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr. 2007;27:41–61. doi: 10.1146/annurev.nutr.27.061406.093716. [DOI] [PubMed] [Google Scholar]
- Sies H, Sharov VS, Klotz LO, Briviba K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. J Biol Chem. 1997;272:27812–27817. doi: 10.1074/jbc.272.44.27812. [DOI] [PubMed] [Google Scholar]
- Ciaccio PJ, Walsh ES, Tew KD. Promoter analysis of a human dihydrodiol dehydrogenase. Biochem Biophys Res Commun. 1996;228:524–529. doi: 10.1006/bbrc.1996.1693. [DOI] [PubMed] [Google Scholar]
- Penning TM, Ohnishi ST, Ohnishi T, Harvey RG. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem Res Toxicol. 1996;9:84–92. doi: 10.1021/tx950055s. [DOI] [PubMed] [Google Scholar]
- Chen J, Adikari M, Pallai R, Parekh HK, Simpkins H. Dihydrodiol dehydrogenases regulate the generation of reactive oxygen species and the development of cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol. 2008;61:979–987. doi: 10.1007/s00280-007-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Xu P, Huecksteadt TP, Hoidal JR. Molecular cloning and characterization of the human xanthine dehydrogenase gene (XDH) Genomics. 1996;34:173–180. doi: 10.1006/geno.1996.0262. [DOI] [PubMed] [Google Scholar]
- Hille R, Nishino T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- Maia L, Vala A, Mira L. NADH oxidase activity of rat liver xanthine dehydrogenase and xanthine oxidase-contribution for damage mechanisms. Free Radic Res. 2005;39:979–986. doi: 10.1080/10715760500210962. [DOI] [PubMed] [Google Scholar]
- Kolyada AY, Savikovsky N, Madias NE. Transcriptional regulation of the human iNOS gene in vascular-smooth-muscle cells and macrophages: evidence for tissue specificity. Biochem Biophys Res Commun. 1996;220:600–605. doi: 10.1006/bbrc.1996.0449. [DOI] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, et al. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Shao L, Du Q, Park KS, Geller DA. Identification of a classic cytokine-induced enhancer upstream in the human iNOS promoter. FASEB J. 2007;21:535–542. doi: 10.1096/fj.06-6739com. [DOI] [PubMed] [Google Scholar]
- Morris KR, Lutz RD, Choi HS, et al. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun. 2003;71:1442–1452. doi: 10.1128/IAI.71.3.1442-1452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S, Tsutsui M, Shimokawa H, et al. Statin treatment upregulates vascular neuronal nitric oxide synthase through Akt/NF-kappaB pathway. Arterioscler Thromb Vasc Biol. 2007;27:92–98. doi: 10.1161/01.ATV.0000251615.61858.33. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao Y, Li G, et al. Regulation of neuronal nitric oxide synthase exon 1f gene expression by nuclear factor-kappaB acetylation in human neuroblastoma cells. J Neurochem. 2007;101:1194–1204. doi: 10.1111/j.1471-4159.2006.04407.x. [DOI] [PubMed] [Google Scholar]
- Ahmad R, Rasheed Z, Ahsan H. Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacol Immunotoxicol. 2009;31:388–396. doi: 10.1080/08923970802709197. [DOI] [PubMed] [Google Scholar]
- Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci. 2009;14:4809–4814. doi: 10.2741/3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WG, Zhu Y, Wu KK. Up-regulation of p300 binding and p50 acetylation in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation. J Biol Chem. 2003;278:4770–4777. doi: 10.1074/jbc.M209286200. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tanabe T. Transcriptional role of the nuclear factor kappa B site in the induction by lipopolysaccharide and suppression by dexamethasone of cyclooxygenase-2 in U937 cells. Biochem Biophys Res Commun. 1998;244:143–148. doi: 10.1006/bbrc.1998.8222. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Nakamura M, Yoshimoto T, Yamamoto S. The transcriptional regulation of human arachidonate 12-lipoxygenase gene by NF kappa B/Rel. FEBS Lett. 1995;363:105–110. doi: 10.1016/0014-5793(95)00293-i. [DOI] [PubMed] [Google Scholar]
- Chopra A, Ferreira-Alves DL, Sirois P, Thirion JP. Cloning of the guinea pig 5-lipoxygenase gene and nucleotide sequence of its promoter. Biochem Biophys Res Commun. 1992;185:489–495. doi: 10.1016/0006-291x(92)91651-6. [DOI] [PubMed] [Google Scholar]
- Schweiger D, Furstenberger G, Krieg P. Inducible expression of 15-lipoxygenase-2 and 8-lipoxygenase inhibits cell growth via common signaling pathways. J Lipid Res. 2007;48:553–564. doi: 10.1194/jlr.M600311-JLR200. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Kim C, Kim JY, Kim JH. Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep. 2008;41:555–559. doi: 10.5483/bmbrep.2008.41.8.555. [DOI] [PubMed] [Google Scholar]
- Gillette JR, Brodie BB, La Du BN. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957;119:532–540. [PubMed] [Google Scholar]
- Thurman RG, Ley HG, Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972;25:420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Nordblom GD, Coon MJ. Hydrogen peroxide formation and stoichiometry of hydroxylation reactions catalyzed by highly purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1977;180:343–347. doi: 10.1016/0003-9861(77)90047-9. [DOI] [PubMed] [Google Scholar]
- Imaoka S, Osada M, Minamiyama Y, et al. Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Cancer Lett. 2004;203:117–125. doi: 10.1016/j.canlet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Abdel-Razzak Z, Garlatti M, Aggerbeck M, Barouki R. Determination of interleukin-4-responsive region in the human cytochrome P450 2E1 gene promoter. Biochem Pharmacol. 2004;68:1371–1381. doi: 10.1016/j.bcp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Li-Masters T, Cheng PY. Mechanisms of cytochrome P450 regulation by inflammatory mediators. Toxicology. 2002;181–182:207–210. doi: 10.1016/s0300-483x(02)00283-4. [DOI] [PubMed] [Google Scholar]
- Dulos J, Kaptein A, Kavelaars A, Heijnen C, Boots A. Tumour necrosis factor-alpha stimulates dehydroepiandrosterone metabolism in human fibroblast-like synoviocytes: a role for nuclear factor-kappaB and activator protein-1 in the regulation of expression of cytochrome p450 enzyme 7b. Arthritis Res Ther. 2005;7:R1271–R1280. doi: 10.1186/ar1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Wu D, Mari M, Bai J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic Biol Med. 2001;31:1539–1543. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Matsui M, Iwata S, et al. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci USA. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Murata M, Sachi Y, et al. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen A, Lemeer S, van der Wijk T, et al. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2005;280:10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- Nakashima I, Kato M, Akhand AA, et al. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid Redox Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- Nakashima I, Takeda K, Kawamoto Y, et al. Redox control of catalytic activities of membrane-associated protein tyrosine kinases. Arch Biochem Biophys. 2005;434:3–10. doi: 10.1016/j.abb.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- den Hertog J, Groen A, van der Wijk T. Redox regulation of protein-tyrosine phosphatases. Arch Biochem Biophys. 2005;434:11–15. doi: 10.1016/j.abb.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci USA. 1991;88:4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano MB, Ghosh D, Trinh F, Leonard WJ. N-terminal DNA-binding domains contribute to differential DNA-binding specificities of NF-kappa B p50 and p65. Mol Cell Biol. 1993;13:852–860. doi: 10.1128/mcb.13.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JR, Kaszubska W, Turcatti G, Wells TN, Hay RT. Role of cysteine62 in DNA recognition by the P50 subunit of NF-kappa B. Nucleic Acids Res. 1993;21:1727–1734. doi: 10.1093/nar/21.8.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Pineda-Molina E, Klatt P, Vazquez J, et al. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- Nishi T, Shimizu N, Hiramoto M, et al. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J Biol Chem. 2002;277:44548–44556. doi: 10.1074/jbc.M202970200. [DOI] [PubMed] [Google Scholar]
- Ando K, Hirao S, Kabe Y, et al. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282:30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- Nowak DE, Tian B, Jamaluddin M, et al. RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol. 2008;28:3623–3638. doi: 10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- Gloire G, Piette J. Redox regulation of nuclear post-translational modifications during NF-kappaB activation. Antioxid Redox Signal. 2009;11:2209–2222. doi: 10.1089/ars.2009.2463. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cellular signalling. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Liu J, Yoshida Y, Yamashita U. DNA-binding activity of NF-kappaB and phosphorylation of p65 are induced by N-acetylcysteine through phosphatidylinositol (PI) 3-kinase. Mol Immunol. 2008;45:3984–3989. doi: 10.1016/j.molimm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Schieven GL, Kirihara JM, Myers DE, Ledbetter JA, Uckun FM. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993;82:1212–1220. [PubMed] [Google Scholar]
- Schoonbroodt S, Ferreira V, Best-Belpomme M, et al. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J Immunol. 2000;164:4292–4300. doi: 10.4049/jimmunol.164.8.4292. [DOI] [PubMed] [Google Scholar]
- Takada Y, Mukhopadhyay A, Kundu GC, et al. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- Canty TG, Jr, Boyle EM, Jr, Farr A, et al. Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation. 1999;100(19 Suppl):II361–II364. doi: 10.1161/01.cir.100.suppl_2.ii-361. [DOI] [PubMed] [Google Scholar]
- Fan C, Li Q, Ross D, Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278:2072–2080. doi: 10.1074/jbc.M206718200. [DOI] [PubMed] [Google Scholar]
- Imbert V, Rupec RA, Livolsi A, et al. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425–1430. [PubMed] [Google Scholar]
- Beraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci USA. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Li Q, Zhang Y, et al. IkappaBalpha and IkappaBbeta possess injury context-specific functions that uniquely influence hepatic NF-kappaB induction and inflammation. J Clin Invest. 2004;113:746–755. doi: 10.1172/JCI17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llacuna L, Mari M, Lluis JM, et al. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174:1776–1785. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil IS, Kim SY, Park JW. Glutathionylation regulates IkappaB. Biochem Biophys Res Commun. 2008;373:169–173. doi: 10.1016/j.bbrc.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Wu M, Bian Q, Liu Y, et al. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46:62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos A, Harraz M, Engelhardt JF, Zandi E. Iron-mediated H2O2 production as a mechanism for cell type-specific inhibition of tumor necrosis factor alpha-induced but not interleukin-1beta-induced IkappaB kinase complex/nuclear factor-kappaB activation. J Biol Chem. 2005;280:2912–2923. doi: 10.1074/jbc.M409524200. [DOI] [PubMed] [Google Scholar]
- Reynaert NL, van der Vliet A, Guala AS, et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn SH, Wouters EF, Vos N, Janssen-Heininger YM. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem. 2001;276:35693–35700. doi: 10.1074/jbc.M104321200. [DOI] [PubMed] [Google Scholar]
- Byun MS, Jeon KI, Choi JW, Shim JY, Jue DM. Dual effect of oxidative stress on NF-kappakB activation in HeLa cells. Exp Mol Med. 2002;34:332–339. doi: 10.1038/emm.2002.47. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Takahashi T, Natoli G, et al. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J Biol Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- Reynaert NL, Ckless K, Korn SH, et al. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci USA. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Kapahi P, Natoli G, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Herscovitch M, Comb W, Ennis T, et al. Intermolecular disulfide bond formation in the NEMO dimer requires Cys54 and Cys347. Biochem Biophys Res Commun. 2008;367:103–108. doi: 10.1016/j.bbrc.2007.12.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:769–777. doi: 10.1165/ajrcmb.24.6.4344. [DOI] [PubMed] [Google Scholar]
- Kamata H, Manabe T, Oka S, Kamata K, Hirata H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 2002;519:231–237. doi: 10.1016/s0014-5793(02)02712-6. [DOI] [PubMed] [Google Scholar]
- Cross JV, Templeton DJ. Thiol oxidation of cell signaling proteins: Controlling an apoptotic equilibrium. J Cell Biochem. 2004;93:104–111. doi: 10.1002/jcb.20202. [DOI] [PubMed] [Google Scholar]
- Xia Y, Makris C, Su B, et al. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc Natl Acad Sci USA. 2000;97:5243–5248. doi: 10.1073/pnas.97.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lin Y, Guo Z, et al. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- Blonska M, Shambharkar PB, Kobayashi M, et al. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. J Biol Chem. 2005;280:43056–43063. doi: 10.1074/jbc.M507807200. [DOI] [PubMed] [Google Scholar]
- Ryabinina OP, Subbian E, Iordanov MS. D-MEKK1, the Drosophila orthologue of mammalian MEKK4/MTK1, and Hemipterous/D-MKK7 mediate the activation of D-JNK by cadmium and arsenite in Schneider cells. BMC Cell Biol. 2006;7:7. doi: 10.1186/1471-2121-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HH, Xie M, Schneider MD, Chen ZJ. Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci USA. 2006;103:11677–11682. doi: 10.1073/pnas.0603089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Surabhi RM, Park KJ, et al. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- Li Q, Engelhardt JF. Interleukin-1beta induction of NFkappaB is partially regulated by H2O2-mediated activation of NFkappaB-inducing kinase. J Biol Chem. 2006;281:1495–1505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Dan HC, Cooper MJ, Cogswell PC, et al. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H, Ihara Y, Nakamura H, et al. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]