Abstract
Since the discovery that deletion of the NF-κB subunits p50 and p52 causes osteopetrosis in mice, there has been considerable interest in the role of NF-κB signaling in bone. NF-κB controls the differentiation or activity of the major skeletal cell types – osteoclasts, osteoblasts, osteocytes and chondrocytes. However, with five NF-κB subunits and two distinct activation pathways, not all NF-κB signals lead to the same physiologic responses. In this review, we will describe the roles of various NF-κB proteins in basal bone homeostasis and disease states, and explore how NF-κB inhibition might be utilized therapeutically.
Keywords: osteoclast, osteoblast, chondrocyte, arthritis, osteoporosis
Introduction
Despite its solid appearance, bone is a dynamic organ whose constituent cells communicate constantly to maintain both structural and metabolic functions and to respond to external stimuli such as mechanical loading, inflammation or hormones. Communication between bone cells also takes place during development, where long bones for example are initially fashioned from a cartilaginous template. Following vascular invasion, osteoblasts (OBs) enter and replace chondrocytes, while osteoclasts (OCs) also enter and create a bone marrow cavity. As bones grow, OBs lay down an organic matrix that is then mineralized by deposition of calcium and phosphate in the form of hydroxyapatite. Some OBs become surrounded by bone and differentiate into osteocytes, which maintain cellular communications via tiny cannaliculi in order to respond to mechanical forces. OCs play a critical role in modeling bones as they grow, since these cells are capable of resorbing both the organic and inorganic components. In mature bones, chondrocytes remain only on joint surfaces where they maintain articular cartilage. OBs and OCs are also present and are active in mature bone, interacting in bone remodeling units. Throughout life, OCs remove bone and OBs replace it, and in normal homeostasis, their activities are balanced, leading to complete replacement of the skeleton every 10 years. The communication between these cell types is critical to bone health, and disturbances in this relationship are found in many disease states. As we will describe in detail, stimuli for both physiologic and pathologic bone remodeling affect NF-κB signaling.
The primary mode of NF-κB regulation is at the level of subcellular localization; to be active, a transcription factor complex must be located in the nucleus. In resting cells, NF-κB dimers are cytoplasmic due to their interactions with IκBs, which mask nuclear localization signals. Stimulation activates IκB kinases (IKKs), which target IκBs for degradation by the proteosome, releasing NF-κB and allowing its nuclear translocation and DNA binding. There are two main pathways that mediate this process of NF-κB activation – classical (canonical) and alternative (non-canonical) – which differ at many levels from the level of initiating cytokines/receptors down to dimer composition in the nucleus, and subsequent biological effects (Figure 1).
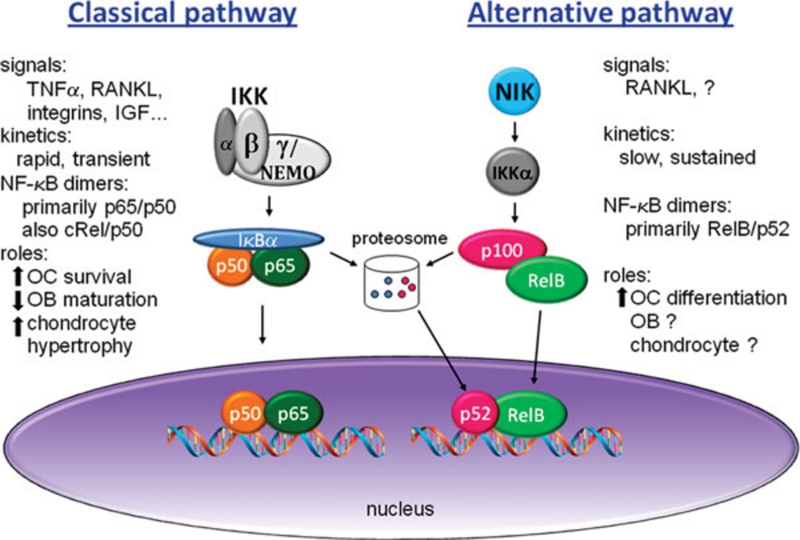
Figure 1.
Classical and alternative NF-κB pathways in skeletal cells. The classical pathway is activated by many extracellular signals, and requires IKKβ and IKKγ/NEMO to phosphorylate IκBα, leading to its degradation by the proteosome. The nuclear translocation of NF-κB, predominantly p65/p50 dimers, has rapid (minutes) and transient kinetics. The effects of classical pathway activation, discussed in subsequent sections of this Review, are to increase OC survival, decrease OB maturation and function, and increase chondrocyte hypertrophy. In skeletal cells, the only established activator of the alternative NF-κB pathway is RANKL. The regulatory kinase is NIK, which activates IKKα, leading to processing of p100 to p52 via the proteosome. The nuclear translocation of RelB/p52 dimers is slow (hours) and sustained, and contributes to OC differentiation.
The classical pathway is activated by virtually all stimuli that affect NF-κB, including RANKL, the master osteoclastogenic cytokine, as well as TNFα and other inflammatory mediators. The key regulatory point for this pathway is the IKK complex – whose critical components are IKKβ and NEMO/IKKγ – which targets IκBα for degradation. Activation of the classical pathway leads primarily to the activation of dimers containing p65 or cRel. Since one of the earliest transcriptional targets of this pathway is IκBα itself, a potent negative feedback loop generates a transient pattern of activation.
In the alternative NF-κB pathway, the protein NIK (NF-κB-inducing kinase) is the regulatory switch. However, unlike the preformed, stable IKK complex, NIK is constitutively degraded due to its interaction with TRAF3. Upon stimulation with the appropriate cytokine, TRAF3 binds to the cytokine receptor and gets degraded, and NIK protein is stabilized. NIK activates IKKα, which controls the processing of the alternative pathway IκB, p100, to p52, and the subsequent nuclear translocation of RelB/p52 complexes. Only a subset of TNF family cytokines, including RANKL but not TNFα, can activate this pathway due to the ability of their corresponding receptors to bind TRAF3.
Interest in the NF-κB pathways in the context of bone biology was spurred by the observation that mice lacking both p50 and p52, and thus globally deficient in NF-κB activation, could not form OCs 1, 2. The resulting phenotype was osteopetrosis, in which dense, misshapen bones are highly susceptible to fracture and teeth fail to erupt. Given the ubiquity of NF-κB in normal cellular physiology, as well as its association with a wide variety of diseases from autoimmunity to cancer, it is not surprising that the study of NF-κB in bone has now spread beyond the OC to include the OB and the chondrocyte. We will first discuss the role of various NF-κB subunits and upstream regulators in each of these cell types and then describe the involvement of NF-κB in several pathophysiologic contexts, including inflammatory arthritis, osteoporosis, osteoarthritis (OA) and bone metastasis of tumors. Understanding the many functions of specific NF-κB pathways and how they are impacted by diseases of bone remodeling will be required in order to translate these studies to effective disease therapies.
NF-κB in the OC
The unique ability of OCs to resorb bone makes them critical for both normal homeostasis and pathologic bone loss. OCs are derived from hematopoietic progenitors in the monocyte lineage which depend on M-CSF (CSF-1) for survival. In vitro, OCs can be generated from bone marrow or splenic macrophages expanded in M-CSF, and then cultured in M-CSF and RANKL for several days on plastic or bone substrates. Upon exposure to RANKL, precursors become committed to the OC lineage and upregulate c-fos and NFATc1, two transcription factors needed for differentiation 3, 4. As they differentiate, OC precursors also fuse to become multinucleated. Mature OCs are highly polarized cells that attach to the bone surface via αvβ3 integrins, forming a tight sealing zone into which acid and proteases are secreted to form a resorption pit 5. RANKL is required for all stages of differentiation beginning with commitment to the lineage, and also stimulates the resorptive activity of mature cells. Mice lacking RANKL or its receptor RANK make no OCs. Osteoclastogenesis also requires a RANKL costimulatory signal derived from receptors coupled to ITAM adaptors DAP12 and FcRγ. Although the combination of M-CSF, RANKL and ITAM signals to a plethora of intracellular pathways, including Ca/calcineurin, MAPKs, PI3K and Src, NF-κB activation is a critical component, without which OCs fail to develop. Many additional factors such as inflammatory cytokines have potent effects on OC differentiation, largely via their ability to activate NF-κB.
RANKL-induced NF-κB
RANK, a member of the TNFR superfamily, has a long cytoplasmic domain without kinase activity that bears several TRAF-binding domains. Early deletion analysis identified a TRAF6-binding domain as the key mediator of NF-κB activation, although the readouts were nonspecific κB reporter assays in non-OC lineage cell lines 6. Mutational analysis performed in authentic OC precursors using a chimeric receptor strategy showed that although a proximal TRAF6-binding site mediated NF-κB activation at the level of IκBα phosphorylation, two more distal sites, which also bind to TRAF2 and 5, could activate classical NF-κB as well 7. Autoubiquitination of TRAF6 and interaction between aPKC and p62 are required for sustained NF-κB activation and OC differentiation 8, 9. CYLD is a negative regulator of TRAF6 ubiquitination and NF-κB activation, and its deletion enhances RANKL-mediated osteoclastogenesis 10.
Global loss of NF-κB signaling, defined by the loss of both p65- and RelB-containing dimers, prevents osteoclastogenesis. Mice with deletion of both the nfkb1 and nfkb2 genes, which ablates the expression of p105/p50 and p100/p52, have severe osteopetrosis 1, 2, but deletion of either nfkb1 or nfkb2 causes no detectable bone phenotype, and in vitro OC formation is intact 11. IKKβ-floxed mice with deletion by Mx1 or CD11b Cre have fewer OCs and increased bone mass 12, 13. As expected, loss of IKKβ blocks RANKL-induced IκBα phosphorylation. Although processing of p100 to p52 is intact, expression of RelB is decreased and RelB nuclear translocation is blunted along with p65 and cRel. As in other systems, OC lineage IKKβ deficiency impairs cell survival, and TUNEL-positive OCs are increased in vivo. In vitro, IKKβ−/− precursors fail to form OCs in response to RANKL, and blockade of IKKβ activity with a NEMO-binding domain (NBD) peptide blocks osteoclastogenesis as well 14, 15.
The subunits specifically controlled by IKKβ-mediated IκBα degradation are p65 and cRel. Although cRel is activated by RANKL, this subunit is dispensable for OC formation and function (D Novack and S Vaira, unpublished observations). In contrast, p65 plays an important role in the OC. Mice lacking p65 globally show embryonic lethality, but radiation chimeras with p65-deficient bone marrow are viable. These p65−/− chimeras have fewer OCs at baseline and a significantly blunted osteoclastogenic response to RANKL injection 16. Unlike in IKKβ-deficiency, p65−/− precursors show normal activation of cRel and RelB by RANKL. Even on a TNFr1−/− background, these p65−/− cells are sensitive to RANKL-induced apoptosis, showing caspase-3 activation and DNA fragmentation. As a consequence, p65−/− bone marrow macrophages (BMMs) form OCs inefficiently in vitro. Blockade of apoptosis with the caspase inhibitor ZVAD restores OC formation and generates functional, resorptive OCs. This pro-survival role is specific for p65, since relB-deficient cells do not undergo RANKL-induced apoptosis, and overexpression of RelB is not able to rescue the apoptotic phenotype of p65-deficient OC precursors.
Because RANK, unlike TNFR1, lacks a death domain, the mechanism for RANKL-induced apoptosis was unknown. JNK had been identified as a mediator of TNF-induced apoptosis in p65-deficient cells 17, 18, and is activated by RANKL. Two negative regulators of JNK – MKP5 and GADD45β – are not upregulated by RANKL in p65−/− preOCs, and JNK phosphorylation is also increased in these cells 16. Similarly, JNK activation is increased in IKKβ−/− precursors, and inhibition of JNK in either p65−/− or IKKβ−/− cultures restores osteoclastogenesis 13, 16. One proposed effector of JNK-mediated apoptosis is Bid 19, which we were able to target with shRNA in p65−/− BMMs, preventing apoptosis and rescuing OC formation 16. In summary, the classical NF-κB pathway, via p65, has a central role in blocking a RANKL-induced pro-apoptotic pathway mediated by JNK, Bid and caspase3, but is not required for OC differentiation or resorptive activity.
The alternative NF-κB pathway plays an important role in the OC. RANK binds TRAF3 more strongly than other TNFR family members 20, leading to NIK stabilization. NIK−/− mice have a mild but significant increase in trabecular bone volume at baseline, but have a severely blunted OC response to RANKL administration in vivo 21, 22. In the absence of NIK or IKKα, p100 cannot be processed to p52, and accumulated p100 acts as a global IκB over time 23. Thus, in such cases although RANKL-naïve BMMs have normal IκBα degradation and p65 activation acutely, preOCs differentiated in RANKL for 48 hours do not have significant levels of p65, p50, RelB or p52 in the nucleus. In vitro, NIK−/−, as well as NIK-defective aly/aly, OC precursors cannot differentiate in response to RANKL 21, 24, 25. Furthermore, expression of a non-degradable p100 in wild-type BMMs prevents RANKL-mediated differentiation. Removal of p100 by deletion of the nfkb2 gene in NIK−/− precursors restores osteoclastogenesis in vitro. Thus, the IκB function of p100 modulates osteoclastogenesis.
The nuclear translocation of RelB is uniquely regulated by p100 26, making this subunit the most responsive to alternative pathway activation downstream of NIK. Like NIK−/− mice, relB−/− mice have only a small increase in bone mass, with a normal number of TRAP+ OCs at baseline 22. RelB-deficient precursors form OCs very poorly in vitro with RANKL stimulation, and fail to upregulate markers of differentiation such as DC-STAMP and cathepsin K. Induction of c-fos is blunted while upregulation of NFATc1 is absent in relB−/− precursors treated with RANKL (Davis and Novack, unpublished observations), suggesting that the RelB may be the key subunit regulating OC differentiation. This function is specific to RelB, since p65-deficient precursors can upregulate NFATc1 normally, and overexpression of p65 in relB−/− cells cannot rescue osteoclastogenesis. Further supporting RelB as the dominant subunit downstream of NIK, overexpression of RelB but not p65 in NIK−/− BMMs can rescue OC differentiation and bone resorption 22.
Another study demonstrating the importance of NF-κB in osteoclastogenesis is that by Otero et al. 27, who show that expression of a constitutively active IKKβ (IKKβ-SS/EE) is capable of inducing differentiation of BMMs into resorptive OCs in the absence of RANK or RANKL. Nuclear translocation of p65 and RelB is very strong in these cells, although RelB is dispensable in this case, perhaps due to prolonged p65 activation compared to RANKL-mediated differentiation. Given that RANKL induces activation of several pathways shown to be necessary for osteoclastogenesis in vitro, including JNK and ERK, which presumably are not activated by IKKβ-SS/EE, it is quite intriguing that this constitutive activation of NF-κB is sufficient to mediate OC differentiation.
TNFα effects in the OC
Inflammatory bone loss is a significant clinical component in rheumatoid arthritis (RA), periodontal disease and orthopedic implant loosening, and in all of these cases, TNFα is implicated as a primary mediator. Thus, the effects of TNFα on OC differentiation and bone resorption have been studied extensively. There is a strong consensus that TNFα and RANKL can act synergistically to induce osteoclastogenesis 28, 29, 30, but it has been more controversial whether or not TNFα alone is sufficient 30, 31. Some of the discrepancies may lie in the use of different mouse strains and culture conditions, differences between human and rodent biology, and/or timing of TNFα stimulation 28, 30, 32. Furthermore, TNF-generated OCs do not resorb bone unless stimulated with IL-1β 31, 33.
TNFα is a potent activator of classical NF-κB downstream of TNFR1, relying on TRAF2/5 and cIAP1/2 for activation of IKKβ (Figure 2). Due to the presence of a death domain in TNFR1, TNFα stimulates pro-apoptotic pathways in addition to the pro-survival pathways that are downstream of classical NF-κB. Thus, the addition of TNFα to OCs or their precursors causes apoptosis only when classical NF-κB is blocked. In vivo, deletion of TNFR1 in IKKβ−/− mice increased OC numbers, but did not correct osteopetrosis. However, in an acute inflammatory condition, bone loss was similar to TNFr1−/− controls 12. Furthermore, TNFα can support the survival of mature OCs when RANKL is withdrawn 31. Thus, NF-κB signaling downstream of TNFα supplies a strong survival signal to OC lineage cells. TNF can also induce the expression of c-fos and NFATc1, in a p50/p52-dependent manner, albeit at lower levels than RANKL 34, indicating a pro-differentiation role for TNFα as well. Furthermore, addition of TNFα with RANKL significantly rescues the ability of NIK- or IKKα-deficient precursors to form OCs in vitro, despite the fact that p100 levels are elevated 12, 23, 35, suggesting that the differentiation signal is independent of alternative NF-κB activation. However, in vivo, the osteoclastogenic response of relB−/− mice was limited in response to TNFα 22. Thus, the mechanisms and in vivo relevance of these effects are not yet clear.
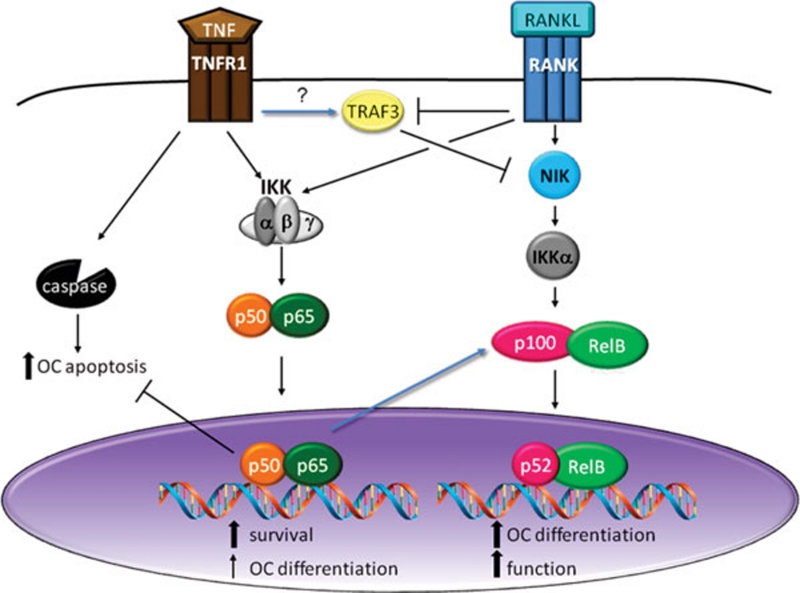
Figure 2.
Crosstalk between RANKL- and TNF-induced NF-κB signaling in OCs. RANKL activates the alternative NF-κB pathway by blocking TRAF3-mediated NIK degradation, leading to nuclear translocation of RelB/p52 dimers. Transcriptional targets of this pathway provide signals for OC differentiation and function. RANKL also activates the classical pathway downstream of the IKK complex, supporting OC lineage cell survival downstream of p65/p50. TNF also activates the classical NF-κB pathway, which blocks apoptosis, upregulates p100/RelB expression, and may support OC differentiation. TNF also stabilizes TRAF3, which can inhibit NIK.
In contrast to the well-studied effects on classical NF-κB, the impact of TNF on the alternative pathway is less defined. RelB and p100 are both transcriptionally upregulated in response to TNFα, due to the presence of κB sites in their promoters 36. The enhanced cytoplasmic pool of RelB/p100 allows a cell to respond to RANKL with robust alternative pathway activation, providing one mechanism for TNFα/RANKL synergism. Despite the preponderance of evidence that TNFα synergizes with RANKL in vivo, some groups have found that co-treatment with TNFα and RANKL reduces OC differentiation compared to treatment with RANKL alone, in vitro, via modulation of alternative NF-κB 37. Although TNFR1 has not been shown to bind TRAF3, TNFα appears to stabilize TRAF3, contributing to NIK degradation 37. Supporting a negative effect of TNFα on alternative NF-κB in OCs via p100, mice lacking p100 are highly sensitive to TNFα-induced bone loss in vivo, and their OC precursors readily differentiate into OCs with TNFα in vitro even in the absence of RANK/RANKL. However, the biological significance of TRAF3- or p100-mediated negative effects of TNFα on OCs is not clear. Indeed, since TNFα induces the expression of RANKL by OBs and other stromal cells 38, OC lineage cells virtually always see TNFα and RANKL together, and in vivo the net effect of TNFα exposure is osteolytic.
NF-κB in OBs
OBs synthesize the organic matrix that forms a scaffold for mineralization, and thus are critical for the establishment and maintenance of bone structure. In addition, OBs express RANKL and M-CSF, which couples the resorptive activity of OCs to the synthetic activity of OBs. OBs are derived from mesenchymal stem cells in the bone marrow stroma and share a common precursor with adipocytes and chondrocytes. In contrast to OC differentiation, in which a single cytokine, RANKL, is the primary driver of maturation, OB differentiation seems to be guided by several extracellular signaling factors, including fibroblast growth factors, parathyroid hormone-related protein, bone morphogenic proteins (BMPs), transforming growth factor β (TGFβ), Wnts and members of the growth hormone/IGF family. As a consequence, a wide array of intracellular signaling pathways has been implicated in osteoblastogenesis, including SMADs, protein kinases A and C, MAPK, and β-catenin. The primary transcription factors required for commitment to the OB lineage are Runx2/Cbfa1, along with its downstream target osterix (Osx), but exactly how all of the extracellular factors that control OB differentiation contribute to their regulation is not yet understood. As OBs mature, they must adhere to the bone surface, via β1 integrins, in order to begin their directional secretion of matrix proteins toward the bone-forming front. Signals emanating from liganded integrins support expression and activation of Runx2, contributing to further differentiation and secretion of matrix components. The proliferative and synthetic response of OBs to mechanical loading is most likely mediated via integrins, along with Ca2+ channels. Historically, NF-κB has not been considered as a key mediator of OB signaling, but several recent studies have now shown that NF-κB inhibits both OB differentiation and activity.
The connection between NF-κB and OBs stems from initial observations that inflammation, and in particular TNFα, inhibits bone formation in vivo and in vitro 39, 40. Li et al. 41 then showed that TNFα- and TNFR1-deficient mice have increased basal bone mass in vivo and increased OB differentiation in vitro. Furthermore, these effects were dependent on classical NF-κB since NBD peptide blocked the effects of TNFα, while overexpression of p65/p50 mimicked TNFα effects. Reporter assays indicated that NF-κB interferes with SMAD activity downstream of BMP2 or TGFβ. Similarly, Saos2 osteosarcoma cells showed increased OB differentiation and BMP2 responsiveness when NF-κB was blocked by IκBαDN 42. Yamazaki et al. 43 further demonstrated that p65 can interact with the Smad1-Smad5 complex in the nucleus and disrupt its binding to target promoters. Interestingly, TNFα-mediated inhibition of Runx2 was NF-κB dependent 41, but inhibition of Osx, a downstream target of Runx, was shown to be independent of NF-κB 44. Overall, classical NF-κB activation downregulates OB differentiation (Figure 3).
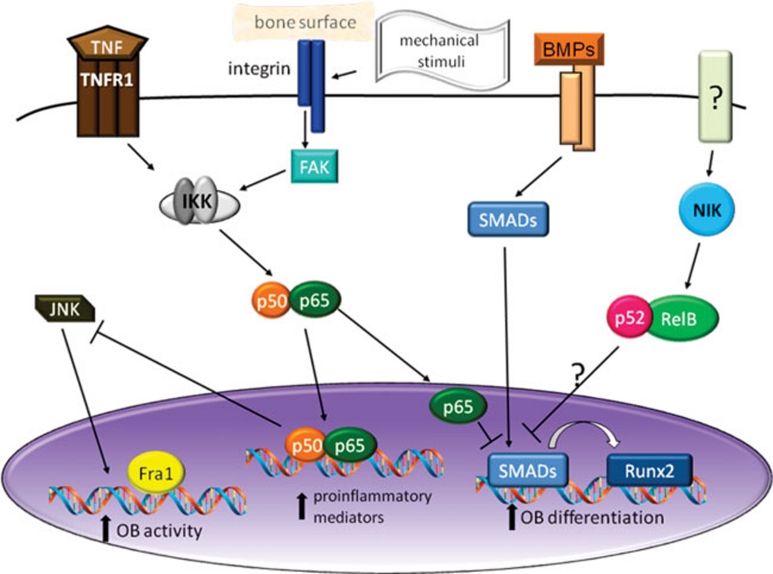
Figure 3.
Mechanisms of NF-κB-mediated OB inhibition. Mechanical stimuli, working through integrins and FAK, can activate classical NF-κB, increasing the expression of proinflammatory mediators, including TNF, which also activates classical NF-κB. Nuclear p65 inhibits OB differentiation by blocking induction of Runx2 by SMAD and interferes with OB activity by reducing JNK activity and Fra1 expression. The alternative NF-κB pathway may also inhibit OB differentiation/function but the mechanisms are not established.
In order to examine effects of NF-κB on mature OBs, separate from the effects on differentiation, Chang et al. 45 generated transgenic mice expressing a dominant negative form of IKKγ (NEMO) using the Bglap2 (osteocalcin) promoter. Young (2-4 weeks old) Bglap2-IKK-DN mice had normal numbers of OBs but increased trabecular bone mass, bone formation rates and expression of OB marker genes, although this effect was lost as mice aged. Expression of the IKK-DN transgene enhanced JNK activation and increased the expression of fos-related antigen (Fra1), a protein that was required for the OB-stimulatory effects of NF-κB blockade.
The fact that basal bone mass can be affected by manipulation of NF-κB raises the question of the identity of the physiological stimulus for NF-κB in OBs. One candidate is mechanical stimulation, which is known to activate the classical NF-κB pathway in OBs and related cells 46. NF-κB activation depends on the degree of the stimulus, with low tensile changes inhibiting and high stresses activating the pathway. There may also be differences depending on the type of stress – tonic vs oscillatory – and on whether the cultures are two or three dimensional 47, 48. The downstream NF-κB targets include COX2 and eNOS as well as TNFα and IL-1β. Fluid shear stress-induced NF-κB in OBs depends on focal adhesion kinase (FAK), a component of the focal adhesion complex that mediates signaling downstream of integrin engagement with extracellular matrix 49. Since mechanical forces play an important role in the maintenance of bone mass, this NF-κB-mediated response to sheer stress might be an important component.
All of the previously discussed studies on the role of NF-κB in the OB have focused only on the classical pathway. Two recent papers 24, 50 addressed the alternative pathway by describing the bone phenotype of aly/aly mice, which have a mutation in NIK that prevents processing of p100 to p52. Both groups found that these mice have increased numbers of OBs and increased bone formation rates. These mice also have decreased numbers and activity of OCs, so it is unlikely that the enhanced bone formation is secondary to the OC defect, since normal OC-OB coupling would be expected to lead to decreased OB function. However, OB differentiation and function were not further examined, and it remains to be determined whether the alternative NF-κB pathway plays a significant role in OB biology.
NF-κB in osteocytes
Osteocytes are OB lineage cells that become completely surrounded by bone matrix and thus reside in lacunae. These cells have long dendritic processes that are important in sensing mechanical forces (mechanotransduction) and that allow them to communicate with each other via interconnecting canaliculi 51. There are relatively few studies on intracellular signaling in osteocytes due to a relative paucity of in vitro model systems. However, genetic deletion of β1 integrins reduces the strain-induced bone formation 52. Although NF-κB is likely to be downstream of integrin engagement in osteocytes, as well as OBs, to our knowledge there is no data on NF-κB activation specifically in osteocytes. Given that osteocytes and OBs do not always respond in the same way to identical stimuli 53, potential roles of NF-κB in osteocytes will have to be determined empirically.
NF-κB in chondrocytes
Long bones develop by endochondral ossification, in which chondrocytes form a cartilaginous template, which is then transformed into bone. Soon after mesenchymal cells differentiate into chondrocytes in the embryo, these cells become organized into growth plates with zones of proliferation, hypertrophy/differentiation and maturation. Terminally differentiated chondrocytes undergo apoptosis, and the cartilage is invaded by blood vessels, bringing in OBs to lay down bone matrix. The master chondrocyte transcription factor is Sox9, which is expressed at highest levels in the proliferating chondrocytes, promoting differentiation. Interestingly, at later stages, Sox9 seems to inhibit differentiation. BMP2 plays an important role in chondrocyte maturation by driving Sox9 expression. However, BMP2 also drives terminal differentiation, supporting the expression of collagen X.
NF-κB mediates the regulation of growth plate chondrogenesis by IGF-1, which stimulates longitudinal bone growth by inducing chondrocyte proliferation and maturation and inhibiting apoptosis. The IGF-1 receptor is a receptor tyrosine kinase that activates an array of intracellular signaling pathways, and recent studies have shown that the major effects of IGF-1 on the growth plate appear to be mediated by p65 54, 55. Chemical inhibition of NF-κB blocks the stimulatory effects of IGF-1 on chondrocyte proliferation and hypertrophy in rat metatarsal explant cultures and its inhibitory effect on apoptosis in cultured chondrocytes. Experiments with p65 siRNA and overexpression in cultured chondrocytes showed similar results, and suggest that p65 is the critical NF-κB subunit mediating these effects. Furthermore, BMP-2 expression is controlled by p65 in growth plate chondrocytes. p65-deficient mice on a TNFr1−/− background are smaller than their littermates (DVN and S Vaira, unpublished), but a growth plate phenotype has not yet been specifically examined. p50/p52 double-deficient mice also have reduced growth and shortened long bones 1, but it has not been determined whether this is due to growth plate defects or is secondary to the failure of OC differentiation. In sum, the classical NF-κB pathway seems to participate in chondrocyte maturation downstream of IGF-1 and upstream of BMP-2, but the detailed mechanisms of regulation are not yet known.
Mice lacking IKKα, a critical component of the alternative NF-κB pathway, also appeared to have skeletal defects 56, 57, but further studies demonstrated that these were secondary to failed epidermal differentiation, which disrupted epidermal-mesenchymal interactions 58. To date, no other studies examining a possible role for alternative NF-κB in chondrocytes have been presented.
In mature vertebrates, the major role for chondrocytes is at the articular surface where they play an important role in maintaining proper joint function. Articular chondrocytes are thought to be relatively quiescent cells, and NF-κB is not known to play a significant role in their homeostasis. However, damage to articular cartilage is the hallmark OA, and the role of NF-κB in this disease and others is discussed below.
Inflammatory arthritis
Because of the well-studied role of NF-κB activation in inflammation, many different groups have examined NF-κB in the context of inflammatory arthritis. Rheumatoid arthritis (RA) is an autoimmune disease in which autoreactive T and/or B lymphocytes initiate joint-centered inflammation, including the participation of neutrophils and macrophages. These infiltrating cells, along with synovial cells, secrete a variety of cytokines (TNFα, IL-1β, IL-6, IL-17) that have significant effects on the bone, both around the joint and systemically. Perhaps most prominent is periarticular osteolysis, due to recruitment of OCs. However, all of the cells within inflamed joints respond to the inflammatory cytokines in a manner largely dependent on NF-κB, and NF-κB activation is readily detectable in disease human samples and mouse models 59.
Many groups have demonstrated that inhibition of classical NF-κB, either genetically (deletion of IKKβ) or pharmacologically (NBD peptide, TAT-IκB-super repressor, IKKβ inhibitor), can ameliorate inflammation, and thus the subsequent bone erosion, in models of inflammatory arthritis 12, 14, 15, 60, 61. Unfortunately, the resulting lack of inflammation prevents interpretation of in vivo results with respect to the role of NF-κB specifically in bone cells. We have found that radiation chimeras bearing p65−/−/TNFr1−/− bone marrow develop as much inflammation in the serum transfer model of arthritis as p65+/+TNFr1−/− controls, but have fewer OCs and are resistant to osteolysis around inflamed joints 16. Thus, classical NF-κB blockade limits joint inflammation in several arthritis models and prevents OC recruitment.
Very few have addressed the role of the alternative NF-κB pathway in inflammatory arthritis. TH17 cells are a specific subset of T cells thought to be particularly important in autoimmune diseases, and NIK is one factor controlling their differentiation 62. We found that NIK−/− mice are resistant to antigen-induced arthritis due to defective T-cell responses 35, suggesting that the alternative pathway may be involved in the development of RA. Peripheral joint inflammation in the lymphocyte-independent serum transfer model does not depend on NIK, but the osteolytic response is severely blunted in NIK−/− mice, indicating that OC recruitment involves alternative NF-κB 35.
In addition to localized osteolysis, patients with RA have a global loss in bone mass and increased risk of fractures 63. This osteoporotic effect appears to be due to circulating cytokines, as TNFα blockade can improve systemic bone density 64. As discussed above, TNFα has potent effects on OC differentiation, which depend on NF-κB.
The inflammatory milieu also affects OB-mediated bone formation. Bone surfaces adjacent to inflammation have decreased bone formation rates, associated with lower expression of markers of mature OBs 65, 66. Given the inhibitory effects of TNFα on OB differentiation discussed above, it is likely that classical NF-κB activation is involved in the decreased bone formation. Although repair of bone erosions following RA therapy is uncommon 67, to date, the possibility of enhancing bone formation at sites of arthritic bone erosion using NF-κB blockade has not been explored.
Osteoarthritis
Osteoarthritis (OA) is characterized by loss of cartilage on joint surfaces, and articular chondrocytes play an active role in the pathogenesis of the disease. Chondrocytes express both matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs), which degrade or preserve the extracellular matrix, respectively. In healthy joints, TIMP levels exceed MMP levels to maintain homeostasis, but in OA, MMP levels increase due to mechanical stress, matrix degradation products and age-related advanced glycation end products (AGEs). The AGE receptor RAGE mediates upregulation of MMPs, as well as PGE2 and NO, by activation of classical NF-κB. OA chondrocytes also express TNFα and IL-1β, which also signal through classical NF-κB and serve to amplify the catabolic response. As discussed in the context of OBs, mechanical stimulation can activate NF-κB in chondrocytes 68. Matrix protein fragments, including those from the major cartilage component collagen II, also depend on NF-κB to stimulate expression of MMPs, NO and inflammatory cytokines 69, 70. Since biologic agents that block either TNFα or IL-1β do not block OA progression 71, 72, it is likely that other stimuli for NF-κB activation, in combination with non-cytokine transcriptional targets of NF-κB, cause significant cartilage destruction.
Normal articular chondrocytes are quiescent, having arrested differentiation prior to becoming hypertrophic, and the matrix remains unmineralized. In contrast, during development, the matrix around hypertrophic growth plate chondrocytes mineralizes and is then replaced by bone. Chondrocytes in OA joints often undergo hypertrophy, with subsequent matrix changes that lead to loss of cartilage, leading to the hypothesis that OA pathogenesis is a recapitulation of development 73. NF-κB has been implicated in this process of OA hypertrophy, since the chemokines IL-8 and GROα/CXCL1 are NF-κB target genes induced in chondrocytes in response to the stimuli mentioned above 74. These chemokines increase the activity of transglutaminase TG2, a promoter of hypertrophy 75. BMP-2 is another target that promotes terminal chondrocyte differentiation, and SOX9 and GADD45β have also been proposed to play a role. Since animal models of OA are limited, there are few studies of NF-κB inhibitors reported. Chen et al. 76 were able to demonstrate that p65 siRNA reduced early cartilage damage and synovitis in a rat OA model, but analysis was not carried out for a long term. Thus, although preliminary results indicate that classical NF-κB plays a role in OA, additional insights are needed for its therapeutic targeting.
Postmenopausal osteoporosis
When ovarian production of estrogen drops at menopause, bone resorption by OCs increases out of proportion to bone formation by OBs and overall bone mass falls. Estrogen acts directly on OCs to promote apoptosis 77, 78, and thus estrogen deficiency increases OC lifespan. Many studies have shown that estrogen inhibits NF-κB (reviewed by Kalaitzidis and Gilmore 79), although this has not been demonstrated in OCs. Estrogen receptors (ERs) can inhibit NF-κB either upstream, via non-genomic cytoplasmic actions, or downstream by blocking the interaction between NF-κB dimers and DNA. One of the reports of ERα deletion specifically in the OC lineage showed that the effects on OCs were independent of ERα DNA binding 78, which would be compatible with either mode of NF-κB inhibition. However, Frasor et al. 80 have used microarrays to demonstrate that NF-κB and estrogen can also synergize to increase the transcription of some targets in breast cancer cells. Given the degree to which NF-κB targets vary by cell type and stimulus, it is not possible to conclude whether or not the effects of estrogen on OC survival are mediated by NF-κB inhibition without data obtained with these cells.
Estrogen also modulates OB lineage cells, affecting both their bone forming activity and their secretion of factors that affect the OC lineage. Decreases in estrogen can lead to upregulation of inflammatory cytokines such as TNFα and IL-1β in both rodents and humans 81. These cytokines promote OC differentiation directly, via NF-κB, and indirectly by increasing the expression of RANKL by OBs, via the p38 pathway 82. Ovariectomy increases levels of nuclear p65 in OBs compared to sham-operated mice 45, an effect that is likely mediated by TNFα/cytokines. Furthermore, when mice expressing IKK-DN only in mature OBs were ovariectomized, bone loss appeared attenuated due to enhanced bone formation, without changes in OC activity 45. Thus, it appears that estrogen deprivation reduces bone formation by activation of NF-κB in OBs.
Paget's disease of bone
Patients with Paget's disease (PD) have one or more focal lesions characterized by a mixture of osteolysis and sclerosis. Although PD occurs in older adults and is focal rather than systemic, evidence indicates that PD is an OC-driven disease that is often familial, and mutations in SQSTM1 can be found in patients with both inherited and sporadic forms 83. This gene encodes p62, a scaffold protein with ubiquitin-binding activity, and the most common PD mutations ablate this binding and increase NF-κB activation 84, 85, 86. Another role for p62 is in autophagy, a process by which cytoplasmic proteins, aggregates and organelles can be degraded in a highly regulated manner. How p62 and the autophagy process might interact in the OC in the context of NF-κB signaling is yet to be determined. Knock-in mice bearing a PD p62 mutation (P394L) have OCs with increased responsivity to RANKL and low bone mass, but lack the focal lesions and hypersensitivity to vitamin D3 seen in human PD 87, suggesting that other factors are required for PD. The measles protein, MVNP, when expressed in OC precursors, recapitulates the PD features such as focal lesions and hypersensitivity to vitamin D3, which is not produced by p62 mutation 88, but there is no evidence that these effects are mediated by NF-κB.
Osteolytic bone metastasis and multiple myeloma
The bone microenvironment, with its rich blood supply and wide array of cytokines and growth factors, can provide a fertile soil for the growth of tumor cells. Tumors that preferentially metastasize to bone often develop a reciprocal relationship with bone cells, in which bone cells release tumor growth factors, while tumor cells secrete factors that stimulate OCs and OBs to enhance bone turnover, further increasing levels of tumor growth factors. In most cases of cancer bone metastasis, such as with breast or lung primary tumors, the increase in OC-mediated resorption is not matched by bone formation and thus leads to net bone loss. Supporting a critical role for OCs in tumor growth within bone, their blockade can reduce bone metastasis in osteolytic models and breast cancer patients, while their activation can increase it 89, 90, 91, 92. However, other factors such as the anti-tumor immune response can affect tumor growth in the bone microenvironment (Zhang and Faccio, unpublished).
Many breast cancers have constitutive activation of NF-κB, and RelB has been identified specifically as a factor associated with a more invasive phenotype in ER-negative cancer cells 93, indicating that the alternative NF-κB pathway is a potential target for both tumor and host responses in breast cancer. We used the osteolytic B16 mouse melanoma cell line to show that mice lacking either NIK or RelB do not lose bone in association with the formation of B16 tumors in the bone marrow space 22. Conversely, activation of NIK specifically in OCs enhances both tumor growth and osteolysis caused by B16 melanoma (Yang and Novack, unpublished). Although other OC-defective mice have displayed decreased formation of bone lesions in association with decreased bone loss 94, 95, neither relB−/− nor NIK−/− mice showed any change in tumor burden compared to their wild-type littermate controls, suggesting that the anti-tumor immune response may also be impaired when the alternative NF-κB pathway is blocked. More work will need to be done to clarify possible pro-tumor effects of alternative pathway blockade that could interfere with the beneficial effects of reducing osteolysis by blocking the OC.
Multiple myeloma (MM) is a disease of malignant plasma cells with characteristic osteolytic bone lesions. Similar to the solid tumor metastases discussed above, MM cells interact with the bone microenvironment, relying on stromal/OB cells for survival signals and stimulating OCs to induce bone loss. Studies using MM cell lines and patient samples identified genetic mutations that activate alternative NF-κB, either by inactivating negative regulators such as TRAF3 or overexpressing NIK 96, 97. Furthermore, proteosome inhibitors have emerged as the treatment of choice for MM, in large part due to their ability to block NF-κB activation in the tumor cells. Proteosome inhibitors block osteoclastogenesis 98, 99, although it is not clear whether inhibition of NF-κB is the key factor. Specific inhibition of both classical and alternative NF-κB pathways via IKKβ and IKKα inhibitors has also been shown to reduce the growth of MM cell lines 100, 101. Additionally, MM cells may be able to activate NF-κB via proteosome inhibitor-resistant pathways 102, suggesting that targeting NF-κB in addition to the proteosome might be therapeutically beneficial for targeting both MM and the OC response.
Concluding remarks
The balance between the activities of OCs and OBs is critical for normal bone homeostasis, and its perturbation in many diseases is related to activation of NF-κB. Activation of classical NF-κB, such as in inflammatory arthritis and osteoporosis, increases bone resorption by OCs and may inhibit bone formation by OBs, making the upstream kinase IKKβ an attractive therapeutic target. This pathway also appears to be activated in chondrocytes affected by OA. The alternative NF-κB pathway plays a significant role in pathological bone loss via its effects on the OC, but its function in other bone cells has yet to be established.
The challenge in capitalizing on our increasingly detailed understanding of NF-κB for clinical use in diseases of bone is to maintain homeostatic bone turnover while targeting pathological effects, which may be local or systemic. The diseases we have discussed such as arthritis and osteoporosis are chronic but not often life-threatening, increasing the need for safe and well-targeted agents. The minimal effects of alternative NF-κB pathway blockade on basal bone homeostasis, in conjunction with profound effects on pathological bone loss, make NIK and its downstream effectors attractive options for pharmaceutical development. Particularly in the context of bone metabolism, gaining tools to delineate which NF-κB pathways are active in specific cell types, and at specific phases of disease progression, will be critical for translating NF-κB targets into clinical use.
Acknowledgments
The author thanks Chang Yang, Jennifer Davis, Gabriel Mbalaviele and Roberta Faccio for critical reading of the manuscript. This work was supported by NIH grants AR052705 and EB007568, the Children's Discovery Institute and the Barnes-Jewish Hospital Foundation.
References
- Franzoso G, Carlson L, Xing L, et al. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Nishimura R, Matsubara T, et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest. 2004;114:475–484. doi: 10.1172/JCI19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang Z-Q, Cecchini MG, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Novack DV, Faccio R.Osteoclast motility: Putting the brakes on bone resorption Ageing Res Rev 2009. Sep 27; doi: 10.1016/j.arr.2009.09.005 [DOI] [PMC free article] [PubMed]
- Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem. 1998;273:20551–20555. doi: 10.1074/jbc.273.32.20551. [DOI] [PubMed] [Google Scholar]
- Liu W, Xu D, Yang H, et al. Functional identification of three receptor activator of NF-κB cytoplasmic motifs mediating osteoclast differentiation and function. J Biol Chem. 2004;279:54759–54769. doi: 10.1074/jbc.M404687200. [DOI] [PubMed] [Google Scholar]
- Lamothe B, Webster WK, Gopinathan A, Besse A, Campos AD, Darnay BG. TRAF6 ubiquitin ligase is essential for RANKL signaling and osteoclast differentiation. Biochem Biophys Res Commun. 2007;359:1044–1049. doi: 10.1016/j.bbrc.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Serrano M, Leitges M, et al. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004;6:303–309. doi: 10.1016/s1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Jin W, Chang M, Paul EM, et al. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J Clin Invest. 2008;118:1858–1866. doi: 10.1172/JCI34257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Carlson L, Story B, et al. Expression of either NF-κB p50 or p52 in osteoclast precursors is required for IL-1-induced bone resorption. J Bone Miner Res. 2003;18:260–269. doi: 10.1359/jbmr.2003.18.2.260. [DOI] [PubMed] [Google Scholar]
- Ruocco MG, Maeda S, Park JM, et al. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005;201:1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero JE, Dai S, Foglia D, et al. Defective osteoclastogenesis by IKKβ-null precursors is a result of receptor activator of NF-κB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation. J Biol Chem. 2008;283:24546–24553. doi: 10.1074/jbc.M800434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Aok K, Saito H, et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IκB Kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- Vaira S, Alhawagri M, Anwisye I, Kitaura H, Faccio R, Novack DV. RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest. 2008;118:2088–2097. doi: 10.1172/JCI33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, et al. Induction of gadd45b by NF-kB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G. Linking JNK signaling to NF-κB: a key to survival. J Cell Sci. 2004;117:5197–5208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- Hauer J, Puschner S, Ramakrishnan P, et al. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-κB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci USA. 2005;102:2874–2879. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack DV, Yin L, Hagen-Stapleton A, et al. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira S, Johnson T, Hirbe AC, et al. RelB is the NF-κB subunit downstream of NIK responsible for osteoclast differentiation. Proc Natl Acad Sci USA. 2008;105:3897–3902. doi: 10.1073/pnas.0708576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson ML, Branstetter DG, Derry JM, et al. Osteoclast differentiation is impaired in the absence of inhibitor of κB kinase α. J Biol Chem. 2004;279:54841–54848. doi: 10.1074/jbc.M406392200. [DOI] [PubMed] [Google Scholar]
- Soysa NS, Alles N, Weih D, et al. The pivotal role of the alternative NF-κB pathway in maintenance of basal bone homeostasis and osteoclastogenesis. J Bone Miner Res. 2010;25:809–818. doi: 10.1359/jbmr.091030. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Fukushima H, Nakao K, et al. Processing of the NF-kappa B2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation. J Bone Miner Res. 2010;25:1058–1067. doi: 10.1359/jbmr.091032. [DOI] [PubMed] [Google Scholar]
- Solan NJ, Miyoshi H, Carmona EM, Bren GD, Paya CV. RelB cellular regulation and trancriptional activity are regulated by p100. J Biol Chem. 2002;277:1405–1418. doi: 10.1074/jbc.M109619200. [DOI] [PubMed] [Google Scholar]
- Otero JE, Dai S, Alhawagri MA, Darwech I, Abu-Amer Y. IKKβ activation is sufficient for RANK-independent osteoclast differentiation and osteolysis. J Bone Miner Res. 2010;25:1282–1294. doi: 10.1002/jbmr.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Hakim I, Tschoep K, Endres S, Bar-Shavit Z. Tumor necrosis factor-alpha mediates RANK ligand stimulation of osteoclast differentiation by an autocrine mechanism. J Cell Biochem. 2001;83:70–83. doi: 10.1002/jcb.1202. [DOI] [PubMed] [Google Scholar]
- Komine M, Kukita A, Kukita T, Ogata Y, Hotokebuchi T, Kohashi O. Tumor necrosis factor-α cooperates with receptor activator of nuclear factor κB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone. 2001;28:474–483. doi: 10.1016/s8756-3282(01)00420-3. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, et al. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–285. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabokbar A, Kudo O, Athanasou NA. Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J Orthop Res. 2003;21:73–80. doi: 10.1016/S0736-0266(02)00106-7. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Yao Z, Li F, et al. NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- Aya K, Alhawagri M, Hagen-Stapleton A, Kitaura H, Kanagawa O, Novack DV. NF-κB-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115:1848–1854. doi: 10.1172/JCI23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derudder E, Dejardin E, Pritchard LL, Green DR, Korner M, Baud V. RelB/p50 dimers are differentially regulated by tumor necrosis factor-{alpha} and lymphotoxin-{beta} receptor activation: CRITICAL ROLES FOR p100. J Biol Chem. 2003;278:23278–23284. doi: 10.1074/jbc.M300106200. [DOI] [PubMed] [Google Scholar]
- Yao Z, Xing L, Boyce BF. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009;119:3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaura H, Sands MS, Aya K, et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-α-induced osteoclastogenesis in vivo. J Immunol. 2004;173:4838–4846. doi: 10.4049/jimmunol.173.8.4838. [DOI] [PubMed] [Google Scholar]
- Jimi E, Hirata S, Shin M, Yamazaki M, Fukushima H. Molecular mechanisms of BMP-induced bone formation: cross-talk between BMP and NF-κB signaling pathways in osteoblastogenesis. Jpn Dent Sci Rev. 2010;46:33–42. [Google Scholar]
- Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. Am J Physiol Endocrinol Metab. 2005;288:E1011–1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFα lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-κB. J Bone Miner Res. 2007;22:646–655. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- Eliseev RA, Schwarz EM, Zuscik MJ, O'Keefe RJ, Drissi H, Rosier RN. Smad7 mediates inhibition of Saos2 osteosarcoma cell differentiation by NFkappaB. Exp Cell Res. 2006;312:40–50. doi: 10.1016/j.yexcr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Fukushima H, Shin M, et al. Tumor necrosis factor α represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-κB. J Biol Chem. 2009;284:35987–35995. doi: 10.1074/jbc.M109.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Gilbert L, He X, Rubin J, Nanes MS. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NF kappa B pathways. J Biol Chem. 2006;281:6297–6306. doi: 10.1074/jbc.M507804200. [DOI] [PubMed] [Google Scholar]
- Chang J, Wang Z, Tang E, et al. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Long P, Seyedain A, Piesco N, Shree A, Gassner R. A central role for the nuclear factor-κB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J. 2003;17:899–901. doi: 10.1096/fj.02-0901fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Barron MJ, Tsai CJ, Donahue SW. Mechanical stimulation mediates gene expression in MC3T3 osteoblastic cells differently in 2D and 3D environments. J Biomech Eng. 2010;132:041005. doi: 10.1115/1.4001162. [DOI] [PubMed] [Google Scholar]
- Young SR, Gerard-O'Riley R, Harrington M, Pavalko FM. Activation of NF-κB by fluid shear stress, but not TNF-α, requires focal adhesion kinase in osteoblasts. Bone. 2010;47:74–82. doi: 10.1016/j.bone.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Fukushima H, Nakao K, et al. Processing of the NF-κB2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation. J Bone Miner Res. 2010;25:1058–1067. doi: 10.1359/jbmr.091032. [DOI] [PubMed] [Google Scholar]
- Tatsumi S, Ishii K, Amizuka N, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Litzenberger J, Tang W, Castillo A, Jacobs C. Deletion of β1 integrins from cortical osteocytes reduces load-induced bone formation. Cell Mol Bioeng. 2009;2:416–424. [Google Scholar]
- Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Flint JK, Rezvani G, De Luca F. Nuclear factor-κB p65 facilitates longitudinal bone growth by inducing growth plate chondrocyte proliferation and differentiation and by preventing apoptosis. J Biol Chem. 2007;282:33698–33706. doi: 10.1074/jbc.M702991200. [DOI] [PubMed] [Google Scholar]
- Wu S, Fadoju D, Rezvani G, De Luca F. Stimulatory effects of insulin-like growth factor-I on growth plate chondrogenesis are mediated by nuclear factor-κB p65. J Biol Chem. 2008;283:34037–34044. doi: 10.1074/jbc.M803754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, et al. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Hwang JY, et al. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IκB kinase-α acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-κB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- Clohisy JC, Roy BC, Biondo C, et al. Direct inhibition of NF-κB blocks bone erosion associated with inflammatory arthritis. J Immunol. 2003;171:5547–5553. doi: 10.4049/jimmunol.171.10.5547. [DOI] [PubMed] [Google Scholar]
- McIntyre KW, Shuster DJ, Gillooly KM, et al. A highly selective inhibitor of IκB kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 2003;48:2652–2659. doi: 10.1002/art.11131. [DOI] [PubMed] [Google Scholar]
- Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–3112. doi: 10.1002/art.22117. [DOI] [PubMed] [Google Scholar]
- Vis M, Havaardsholm EA, Haugeberg G, et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFκB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1495–1499. doi: 10.1136/ard.2005.044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NC, Reinwald S, Manning CA, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- Schett G, Middleton S, Bolon B, et al. Additive bone-protective effects of anabolic treatment when used in conjunction with RANKL and tumor necrosis factor inhibition in two rat arthritis models. Arthritis Rheum. 2005;52:1604–1611. doi: 10.1002/art.21021. [DOI] [PubMed] [Google Scholar]
- Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–312. doi: 10.1111/j.0105-2896.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhu F, Lee NH, Konstantopoulos K. Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-kappaB activation. J Biol Chem. 2010;285:24793–24804. doi: 10.1074/jbc.M110.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulai JI, Chen H, Im HJ, et al. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TT, Schulz RM, Rai SS, et al. Biomechanical modulation of collagen fragment-induced anabolic and catabolic activities in chondrocyte/agarose constructs. Arthritis Res Ther. 2010;12:R82. doi: 10.1186/ar3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- Magnano MD, Chakravarty EF, Broudy C, et al. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol. 2007;34:1323–1327. [PubMed] [Google Scholar]
- Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-κB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LJ, Xing X, Franz C, Davies S, Chang LW, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis Cartilage. 2008;16:1560–1571. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene α/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- Chen LX, Lin L, Wang HJ, et al. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-κBp65-specific siRNA. Osteoarthritis Cartilage. 2008;16:174–184. doi: 10.1016/j.joca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Martin-Millan M, Almeida M, Ambrogini E, et al. The estrogen receptor-α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-κB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Frasor J, Weaver A, Pradhan M, et al. Positive cross-talk between estrogen receptor and NF-κB in breast cancer. Cancer Res. 2009;69:8918–8925. doi: 10.1158/0008-5472.CAN-09-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-α and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res. 2007;22:724–729. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode A, Layfield R. Recent advances in understanding the molecular basis of Paget disease of bone. J Clin Pathol. 2010;63:199–203. doi: 10.1136/jcp.2009.064428. [DOI] [PubMed] [Google Scholar]
- Najat D, Garner T, Hagen T, et al. Characterization of a non-UBA domain missense mutation of sequestosome 1 (SQSTM1) in Paget's disease of bone. J Bone Miner Res. 2009;24:632–642. doi: 10.1359/jbmr.081204. [DOI] [PubMed] [Google Scholar]
- Rea SL, Walsh JP, Ward L, et al. Sequestosome 1 mutations in Paget's disease of bone in Australia: prevalence, genotype/phenotype correlation, and a novel non-UBA domain mutation (P364S) associated with increased NF-κB signaling without loss of ubiquitin binding. J Bone Miner Res. 2009;24:1216–1223. doi: 10.1359/jbmr.090214. [DOI] [PubMed] [Google Scholar]
- Chamoux E, Couture J, Bisson M, Morissette J, Brown JP, Roux S. The p62 P392L mutation linked to Paget's disease induces activation of human osteoclasts. Mol Endocrinol. 2009;23:1668–1680. doi: 10.1210/me.2009-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma Y, Kurihara N, Subler MA, et al. A SQSTM1/p62 mutation linked to Paget's disease increases the osteoclastogenic potential of the bone microenvironment. Hum Mol Genet. 2008;17:3708–3719. doi: 10.1093/hmg/ddn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N, Zhou H, Reddy SV, et al. Experimental models of Paget's disease. J Bone Miner Res. 2006;21 Suppl 2:P55–57. doi: 10.1359/jbmr.06s210. [DOI] [PubMed] [Google Scholar]
- Hirbe AC, Uluckan O, Morgan EA, et al. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007;109:3424–3431. doi: 10.1182/blood-2006-09-048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canon JR, Roudier M, Bryant R, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:119–129. doi: 10.1007/s10585-007-9127-1. [DOI] [PubMed] [Google Scholar]
- Lipton A, Theriault RL, Hortobagyi GN. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lipton A, Steger GG, Figueroa J, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- Wang X, Belguise K, Kersual N, et al. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakewell SJ, Nestor P, Prasad S, et al. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbe AC, Roelofs AJ, Floyd DH, et al. The bisphosphonate zoledronic acid decreases tumor growth in bone in mice with defective osteoclasts. Bone. 2009;44:908–916. doi: 10.1016/j.bone.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Metzler I, Krebbel H, Hecht M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21:2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- Hongming H, Jian H. Bortezomib inhibits maturation and function of osteoclasts from PBMCs of patients with multiple myeloma by downregulating TRAF6. Leuk Res. 2009;33:115–122. doi: 10.1016/j.leukres.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Kiziltepe T, et al. Biologic sequelae of IκB kinase (IKK) inhibition in multiple myeloma: therapeutic implications. Blood. 2009;113:5228–5236. doi: 10.1182/blood-2008-06-161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan M, Moreaux J, Vos JD, et al. Targeting NF-κB pathway with an IKK2 inhibitor induces inhibition of multiple myeloma cell growth. Br J Haematol. 2007;138:160–168. doi: 10.1111/j.1365-2141.2007.06629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovina S, Callander NS, O'Connor SL, et al. Bortezomib-resistant nuclear factor-κB activity in multiple myeloma cells. Mol Cancer Res. 2008;6:1356–1364. doi: 10.1158/1541-7786.MCR-08-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]