Abstract
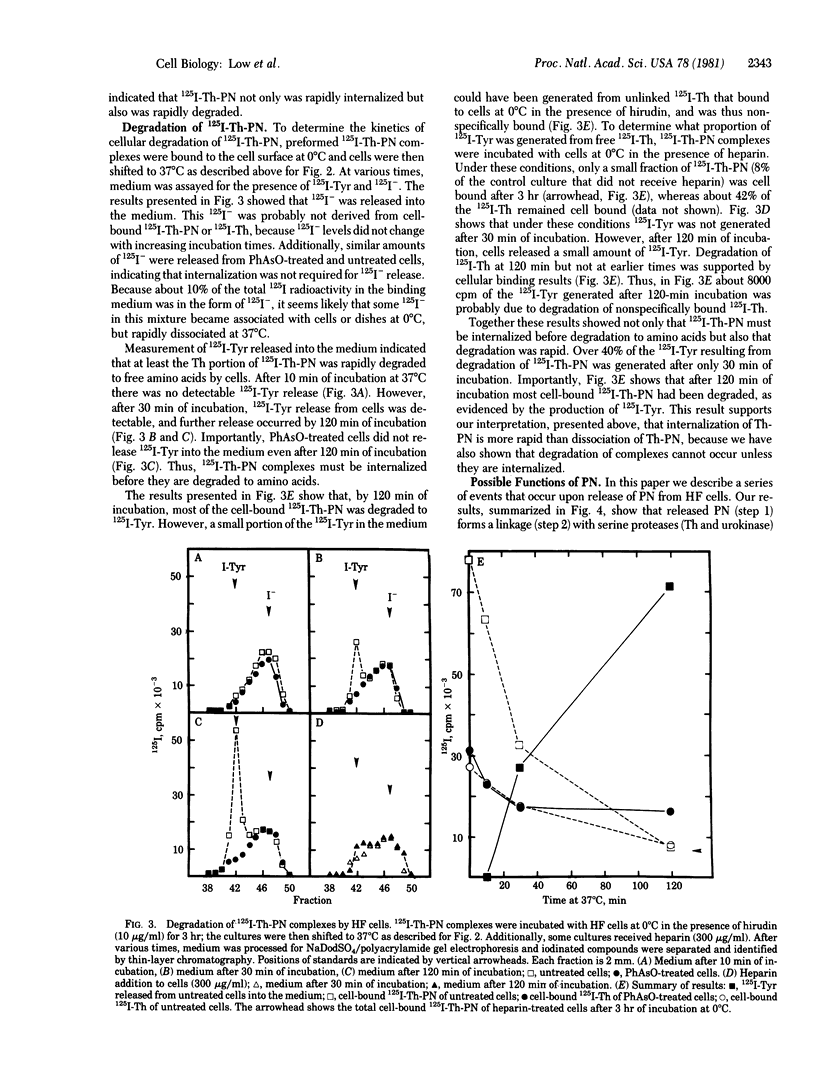
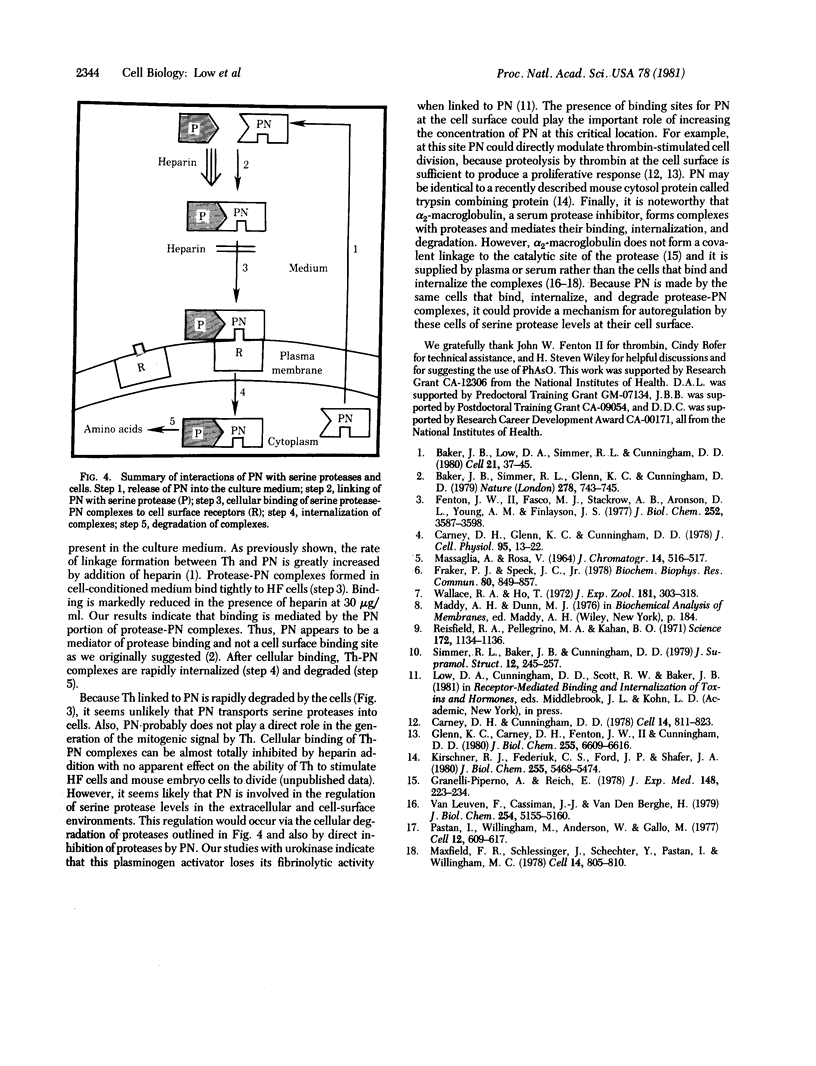
Protease-nexin (PN), a component released by normal human fibroblasts into culture medium, forms covalent linkages with thrombin (Th) and the urinary plasminogen activator urokinase, apparently with their catalytic site serines. The present studies explored the function of PN by examining the interaction of protease-PN complexes with human fibroblasts and the consequences of this interaction. Th-PN and urokinase-PN complexes bind to cells via the PN portion of the complexes. The binding is selectively inhibited by heparin. Because PN has a heparin-binding site, this indicates that protease-PN complexes might bind to a cellular heparin-like site. After binding, the complexes are internalized. By inhibiting endocytosis with phenylarsine oxide, which does not affect cellular binding of Th-PN complexes, we showed that complexes must be internalized before they are degraded. Kinetic analysis of internalization and degradation of Th-PN showed that complexes are internalized more rapidly than they dissociate from the cell surface; by 120 min of incubation at 37 degrees C most cell-bound Th-PN complexes are degraded to amino acids. The results are summarized in a model showing how PN mediates the cellular binding, internalization, and degradation of serine proteases through formation of protease-PN complexes. This series of events may be involved in the regulation of serine protease activity at the cell surface and in the extracellular environment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Simmer R. L., Glenn K. C., Cunningham D. D. Thrombin and epidermal growth factor become linked to cell surface receptors during mitogenic stimulation. Nature. 1979 Apr 19;278(5706):743–745. doi: 10.1038/278743a0. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Cell surface action of thrombin is sufficient to initiate division of chick cells. Cell. 1978 Aug;14(4):811–823. doi: 10.1016/0092-8674(78)90337-9. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Glenn K. C., Cunningham D. D. Conditions which affect initiation of animal cell division by trypsin and thrombin. J Cell Physiol. 1978 Apr;95(1):13–22. doi: 10.1002/jcp.1040950103. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R. J., Federiuk C. S., Ford J. P., Shafer J. A. Detection and partial characterization of a protein in mouse fibroblasts that binds and inhibits trypsin. J Biol Chem. 1980 Jun 10;255(11):5468–5474. [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Willingham M., Anderson W., Gallo M. Localization of serum-derived alpha 2 macroglobulin in cultured cells and decrease after Moloney sarcoma virus transformation. Cell. 1977 Nov;12(3):609–617. doi: 10.1016/0092-8674(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Pellegrino M. A., Kahan B. D. Salt extraction of soluble HL-A antigens. Science. 1971 Jun 11;172(3988):1134–1136. doi: 10.1126/science.172.3988.1134. [DOI] [PubMed] [Google Scholar]
- Simmer R. L., Baker J. B., Cunningham D. D. Direct linkage of thrombin to its cell surface receptors in different cell types. J Supramol Struct. 1979;12(2):245–257. doi: 10.1002/jss.400120209. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Demonstration of an alpha2-macroglobulin receptor in human fibroblasts, absent in tumor-derived cell lines. J Biol Chem. 1979 Jun 25;254(12):5155–5160. [PubMed] [Google Scholar]
- Wallace R. A., Ho T. Protein incorporation by isolated amphibian oocytes. II. A survey of inhibitors. J Exp Zool. 1972 Sep;181(3):303–317. doi: 10.1002/jez.1401810303. [DOI] [PubMed] [Google Scholar]