Dear Editor,
Living cells can change their intramembranous temperature during cell activities such as division, gene expression, enzyme reaction, and metabolism 1, 2. Moreover, under external stimuli, such as drugs or other signals, cells may quickly change their metabolic activities, leading to acute variation of intracellular temperatures from the normal state 3, 4. However, such temperature change inside cells is usually at a small scale and is of transient nature due to the thermo-influence by the extracellular environment, rendering it rather difficult to measure using the conventional temperature detection methods. Thus, a more precise and faster-response thermometer is needed to measure single-cell temperature changes in real time, which may constitute a new layer of cellular information for studies of cellular signaling, and even clinical diagnosis and therapy.
Fluorescent nanogel has been previously applied to detect changes in intracellular temperature 4. Cells were first allowed to take up a fluorescence material and the average intracellular temperature change under a certain treatment was then determined through measuring the distinct fluorescent light intensity before and after the treatment. Such a fluorescent nanogel-based method has a number of disadvantages, including potential toxicity to cells, limit of measurement resolution (generally in the range of 0.29 °C-0.50 °C), and limit of time-scale resolution (at the scale of minutes).
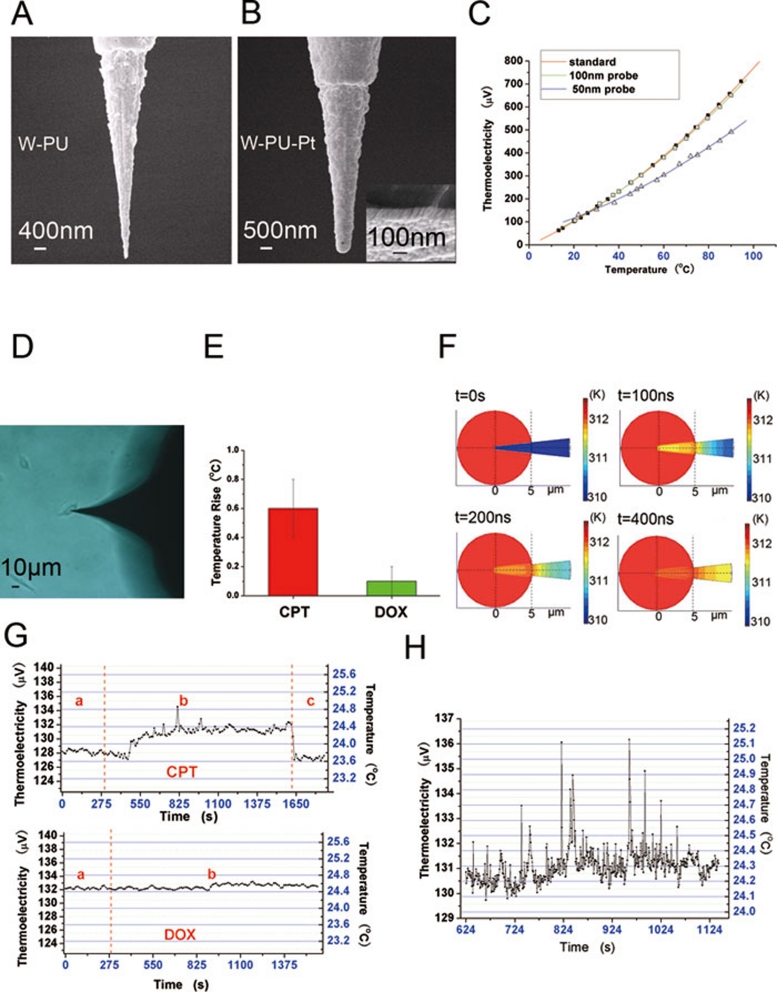
Thermocouple (TC) is widely used in settings that require detection of temperature changes. The TC-based detection method has a number of advantages, including the capacity for achieving high precision and rapid response. To adapt the TC method for temperature measurement at the single-cell level, one would need to develop a micro-sized TC probe (at sub-micrometer scale). The thin film method is a common approach to producing two-dimensional micro- or even nano-TCs for use in electronics industry 5. However, such two-dimensional TCs that rely on the support of silicon chips cannot be readily used for measuring intracellular temperature. In this report, we designed a novel TC device for detecting intracellular temperature (Figure 1A and 1B). Briefly, our TC probe is made of a sandwich structure consisting of the tungsten (W) substrate, an insulating layer made of polyurethane (PU; except at the tip), and a platinum (Pt) film (Supplementary information, Figure S1). We produced two types of TC probes, with different thickness of the Pt film (50 nm and 100 nm). In a calibration experiment with these two types of probes, we found that the 100 nm probe produced a temperature-thermoelectricity curve that showed an almost perfect match with the standard curve produced by a regular macro-sized TC, while the readings from the 50 nm probe showed deviations from the standard curve (Figure 1B and 1C and Supplementary information, Figure S2). This result is consistent with earlier reports that when the thickness of the Pt film decreases beyond the 100 nm range, it will affect the resulting thermoelectric power compared to the macro-sized TC at the same temperature 6, 7. Thus, we have chosen the 100 nm probe in further experiments.
Figure 1.
(A) SEM image of tungsten probe coated by polyurethane (PU; except at the tip which is uncoated). (B) SEM image of the thin platinum film as an outermost layer. (C) Curves of the thermoelectric power from 0 °C to 90 °C. Red, green, and blue curves represent the thermoelectric power of the regular bulk W-Pt thermocouple, TC probe with 100-nm thick Pt film, and TC with 50-nm thick Pt film, respectively. (D) Optical microscopic image of a living U251 cell inserted by the TC probe. (E) A column figure indicating the average temperature changes for total cells after two drug treatment. (F) The simulated results of the TC probe response to a cell 2 °C higher than the environment. (G) Upper panel, a typical curve showing the changes of the intracellular temperature of a single U251 cell after CPT treatment. (a) TC inserted into the cell, (b) addition of CPT, and (c) TC withdrawn from the cell. Lower panel, a typical curve showing no temperature changes after addition of DOX. (a) TC inserted into the cell, and (b) addition of DOX. (H) A higher-resolution plot of part of the curve shown in (G) upper panel.
To calculate the temperature resolution of our TC-based method, we first derived the thermoelectric power-temperature equation based on the standard curve (Supplementary information, Formula S1). We then calculated the Seebeck coefficient (Supplementary information, Formula S2) from Formula S1. After incorporating the resolution limit of the digital multimeter (Agilent 34410A, 0.1 μV), we determined that the temperature resolution of our measurement was below 0.1 °C. To obtain the thermal response time of the TC, we performed computer simulation (see Supplementary information for details). The simulation results showed that the TC probe inserted into the cell would reach the same temperature as the intracellular environment after about 400 nano-seconds (Figure 1F and Supplementary information, Figure S3). Thus, the rapid response of the TC probe should allow for real-time monitoring of intracellular temperature changes.
We then applied our device in experimental settings. The TC probe was inserted into one U251 cell (Figure 1D) by the micromanipulation system (Supplementary information, Figure S5) and the thermoelectricity readings were recorded. After the reading reached a balanced state (a few minutes after the TC probe insertion into the cell), the drug camptothecin (CPT) was added to the medium (final concentration 12 μM; Figure 1G). CPT is a DNA topoisomerase I inhibitor that can promote tumor cell death 8. In the previous study using the fluorescent nanogel-based method, it was shown that addition of CPT to COS7 cells led to an acute increase in intracellular temperature (from 0.5 °C to 2 °C), which could last for up to two hours. Consistent with this, we found that CPT treatment in our system also led to an acute increase in intracellular temperature (Figure 1E, 1G and Supplementary information, Figure S4). Interestingly, in a separate experiment, we found that another chemotherapeutic drug, doxorubicin (at 50 μM final concentration), which is a topoisomerase II inhibitor 9, did not cause any obvious intracellular temperature change (Figure 1E and 1G). Thus, the distinct effect of these two drugs on cellular temperature likely reflects fundamental differences in their action mechanisms, the details of which remain to be further investigated.
Compared with fluorescent nanogel-based method 4, the TC-based method we developed here can detect intracellular thermal signal with higher accuracy and better time-scale resolution. For instance, a closer inspection revealed that during the initial 20 min after adding CPT, there were violent fluctuations of the intracellular thermal signal in the U251 cell (Figure 1H). Such a fine pattern of thermal signal fluctuations would not have been possible to observe under the minute-level time scale shown in the previous fluorescent nanogel-based measurement 4. We suggest that this fine pattern of thermal signal may contain rich information about intracellular activities, which can only be detected by our method.
Intracellular thermal signal is closely related with disease. For instance, cancerous cells produce more heat than normal cells due to their faster metabolic rate 10. Moreover, we and others have shown that different apoptosis-inducing drugs can elicit distinct intracellular thermal signal changes (Gota et al. 4, and this study). Our study has established a feasible physical approach to measuring single-cell temperature changes in real time, and we suggest that being able to obtain such information will prove useful in studies of diverse areas such as cell signaling, cell metabolism, disease diagnosis, and therapeutic effect evaluations.
Acknowledgments
This work was supported by grants from the National Important Basic Research Program of China (2011CB933500), the National Natural Science Foundation of China (60725101, 50872021 and 30870679) and the US-China International Science and Technology Cooperation Project (2009DFA31990).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Scheme of the thermocouple structure
References
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Yamamura M, Yamakawa A, Fujise T, Nakano S. Microcalorimetric measurements of heat-production in isolated rat brown adipocytes. Biochem Int. 1992;26:873–877. [PubMed] [Google Scholar]
- Tasaki I, Nakaye T. Heat generated by the dark-adapted squid retina in response to light-pulses. Science. 1985;227:654–655. doi: 10.1126/science.3969556. [DOI] [PubMed] [Google Scholar]
- Gota C, Okabe K, Funatsu T, Harada Y, Uchiyama S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J Am Chem Soc. 2009;131:2766–2767. doi: 10.1021/ja807714j. [DOI] [PubMed] [Google Scholar]
- Chu DC, Wong WK, Goodson KE, Pease RFW. Transient temperature measurements of resist heating using nanothermocouples. J Vac Sci Technol B. 2003;21:2985–2989. [Google Scholar]
- Zhang XG, Choi H, Datta A, Li XC. Design, fabrication and characterization of metal embedded thin film thermocouples with various film thicknesses and junction sizes. J Micromech Microeng. 2006;16:900–905. [Google Scholar]
- Salvadori MC, Vaz AR, Teixeira FS, Cattani M, Brown IG. Thermoelectric effect in very thin film Pt/Au thermocouples. Appl Phys Lett. 2006;88:133106. [Google Scholar]
- Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WGJ. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998;279:1504–1513. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- Pigram WJ, Fuller W, Hamilton LD. Stereochemistry of intercalation: interaction of daunomycin with DNA. Nat New Biol. 1972;235:17–19. doi: 10.1038/newbio235017a0. [DOI] [PubMed] [Google Scholar]
- Karnebogen M, Singer D, Kallerhoff M, Ringert RH. Microcalorimetric investigations on isolated tumorous and non-tumorous tissue samples. Thermochim Acta. 1993;229:147–155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scheme of the thermocouple structure