Abstract
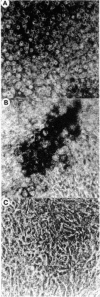
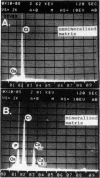
Growth cartilage cells were isolated from the ribs of young rats and cultured at high cell density in Ham's F-12 medium supplemented with 10% fetal calf serum. During 7 days, glycosaminoglycans and proteoglycans were actively synthesized and secreted, forming a metachromatic matrix. When cultured together with growth cartilage cells precultured and biosynthetically prelabeled with 35SO4(2-) in their glycosaminoglycans, bone marrow cells caused release of 35S-labeled material into the culture medium. Glycosaminoglycan was also released by addition of conditioned medium obtained from cultures of bone marrow cells or peritoneal macrophages to the growth cartilage cell cultures. Electron microscopic studies of the extracellular matrix of growth cartilage cells cocultured with bone marrow cells showed that needles of apatite mineral were deposited within and in close apposition to the surfaces of matrix vesicles. These findings suggest that enzymes released from bone marrow cells or macrophages removed glycosaminoglycan or proteoglycans, which may be inhibitors of mineral growth, and consequently mineralization was initiated. From these findings, sequential culture of growth cartilage cells and bone marrow cells is promising as an experimental system for investigating the mechanism of the initial stage of endochondral ossification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol. 1967 Oct;35(1):81–101. doi: 10.1083/jcb.35.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969 Apr;41(1):59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967 Sep;20(1):33–50. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- Eguchi G., Okada T. S. Ultrastructure of the differentiated cell colony derived from a singly isolated chondrocyte in in vitro culture. Dev Growth Differ. 1971 Feb;12(4):297–312. doi: 10.1111/j.1440-169x.1971.00297.x. [DOI] [PubMed] [Google Scholar]
- Hauser P., Vaes G. Degradation of cartilage proteoglycans by a neutral proteinase secreted by rabbit bone-marrow macrophages in culture. Biochem J. 1978 May 15;172(2):275–284. doi: 10.1042/bj1720275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D. S., Pita J. C., Marquez J. F., Madruga J. E. Partition of calcium, phosphate, and protein in the fluid phase aspirated at calcifying sites in epiphyseal cartilage. J Clin Invest. 1968 May;47(5):1121–1132. doi: 10.1172/JCI105801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibril A. O. Proteolytic degradation of ossifying cartilage matrix and the removal of acid mucopolysaccharides prior to bone formation. Biochim Biophys Acta. 1967 Feb 7;136(1):162–165. doi: 10.1016/0304-4165(67)90335-2. [DOI] [PubMed] [Google Scholar]
- Kato Y., Nasu N., Takase T., Daikuhara Y., Suzuki F. A serum-free medium supplemented with multiplication-stimulating activity (MSA) supports both proliferation and differentiation of chondrocytes in primary culture. Exp Cell Res. 1980 Jan;125(1):167–174. doi: 10.1016/0014-4827(80)90200-1. [DOI] [PubMed] [Google Scholar]
- Kato Y., Nasu N., Takase T., Suzuki F. Demonstration of somatomedin activity of "multiplication-stimulating activity" in rabbit costal chondrocytes in culture. J Biochem. 1978 Oct;84(4):1001–1004. doi: 10.1093/oxfordjournals.jbchem.a132180. [DOI] [PubMed] [Google Scholar]
- Kato Y., Nomura Y., Daikuhara Y., Nasu N., Tsuji M., Asada A., Suzuki F. Cartilage-derived factor (CDF) I. Stimulation of proteoglycan synthesis in rat and rabbit costal chondrocytes in culture. Exp Cell Res. 1980 Nov;130(1):73–81. doi: 10.1016/0014-4827(80)90043-9. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Matukas V. J., Krikos G. A. Evidence for changes in protein polysaccharide associated with the onset of calcification in cartilage. J Cell Biol. 1968 Oct;39(1):43–48. doi: 10.1083/jcb.39.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison R. The Possible Significance of Hexosephosphoric Esters in Ossification. Biochem J. 1923;17(2):286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y., Yoneda T., Suzuki F. Osteogenesis by chondrocytes from growth cartilage of rat rib. Calcif Tissue Res. 1975 Dec 22;19(3):179–187. doi: 10.1007/BF02564002. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Yoneda T., Shimomura Y. Calcitonin and parathyroid-hormone stimulation of acid mucopolysaccharide synthesis in cultured chondrocytes isolated from growth cartilage. FEBS Lett. 1976 Nov;70(1):155–158. doi: 10.1016/0014-5793(76)80747-8. [DOI] [PubMed] [Google Scholar]
- Suzuki H. [Vibration syndrome of vibrating tool users in a factory of steel foundry. Part 1. Complained symptoms (author's transl)]. Sangyo Igaku. 1978 Sep;20(5):261–268. doi: 10.1539/joh1959.20.261. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Ishida H., Takano T., Suzuki F. Polyamine and differentiation: induction of ornithine decarboxylase by parathyroid hormone is a good marker of differentiated chondrocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1481–1485. doi: 10.1073/pnas.77.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M., Watanabe R., Ishida H., Asada A., Suzuki F. Induction by parathyroid hormone of ornithine decarboxylase in rabbit costal chondrocytes in culture. J Biochem. 1979 Jan;85(1):311–314. doi: 10.1093/oxfordjournals.jbchem.a132326. [DOI] [PubMed] [Google Scholar]